Introduction
Vitamin A and E are essential micronutrients in
human body, and play very important roles in maternal health and
fetal development (1). Vitamin A is
an essential nutrient needed in small amounts for the normal
functioning of the visual system, it can maintain cell function for
growth, normal immune function, hematopoietic system, epithelial
integrity, red blood cell production, immunity and reproduction
(2), especially during pregnancy.
Vitamin A deficiency (VAD) not only increases the risk of
miscarriage, night blindness, and pregnancy complication, but also
affects the embryonic development. Severe deficiency causes fetal
malformations. Excessive vitamin A increases birth defect risks
(3).
Vitamin E is an essential vitamin for maintaining
the metabolic function of the body and possesses antioxidant and
scavenging free radical activities (1). The deficiency of vitamin E in pregnant
women leads to placental aging, vascular endothelial injury,
incidence of hypertensive disorders of pregnancy, placental
abruption, abortion and premature birth (4). While excessive vitamin E in the body
affects the absorption and function of other fat-soluble vitamins.
However, there are scarce studies analyzing the values of serum
vitamin A and E during pregnancy in China. Therefore, there is an
urgent need to study serum levels of vitamin A and E in
pregnancy.
This study was designed to analyze the serum levels
of vitamin A and E in early, middle and late pregnancy, evaluating
the vitamin nutritional status in pregnancy in order to provide
guidance for pregnant women about vitamin supplements in
pregnancy.
Patients and methods
Sample collection
We conducted a quantitative study on the levels of
vitamin A and E in 12,340 pregnant women from January 2013 to June
2014 at the Beijing Harmony Health Medical Diagnostics Co., Ltd.
(Beijing, China), among which 10,082 cases were primipara and 2,258
cases were pluripara. Those women were 23–40 years of age, healthy,
with no high blood pressure, diabetes, chronic kidney disease,
congenital heart disease or other internal problems, no thrombotic
diseases, such as thrombophilia, antiphospholipid syndrome, no
history of long-term drug use, such as aspirin; and were
transferred to the general hospital for prenatal examination.
Their average age was 29.96±3.77 years, and their
pre-pregnancy BMI ranged from 14.87 to 33.89 kg/m2, with
an average of 21.51±3.06 kg/m2. Serum samples were
collected from each pregnant woman at their first visit (8–12 weeks
of pregnancy), 24–28 weeks and 34–36 weeks of pregnancy. A total of
28,023 serum samples were collected and divided into three groups:
group of early pregnancy (nVA = 3,953, nVE =
3,965), group of middle pregnancy (nVA = 9,014,
nVE = 9,090) and group of late pregnancy (nVA
= 1,002, nVE = 999).
The eligible pregnant women were randomly selected
with a stratified sampling method from the prenatal outpatients at
class A (grade III) and class B (grade II) hospitals in Haidian,
Huairou, Dongcheng, Mentougou, Pinggu, Tongzhou, and Xicheng
Districts in Beijing, China.
This study was approved by the Ethics Committee of
Haidian Maternal and Child Health Hospital (Beijing, China). Signed
informed consents were obtained from the patients or the
guardians.
Sampling detection methods
High performance liquid chromatography (HPLC) was
used to quantitatively determine the concentration of serum vitamin
A and E (HPLC instrument model: Agilent HPLC 1290; Agilent
Technologies, Inc., Santa Clara, CA, USA).
Serum was taken from peripheral venous blood (2 ml),
not anticoagulation, and was stored in dark and cool (0–4°C)
environment. Whole blood samples were centrifuged at 2,500 × g at
4°C for 10 min in a timely manner to obtain serum samples. Protein
and impurities were removed, and hexane was added to extract
effective components from the serum samples. The supernatant
fraction was obtained, dried, and re-dissolved with methanol to
detect effective components. Instrument conditions: i)
chromatographic columns: Agilent CB; ii) column temperature: room
temperature; iii) mobile phase: methanol-pure water; iv) flow rate:
VA, 0.5 ml/min; VE, 1.0 ml/min. v) wavelength: VA, 325 nm; VE, 292
nm.
Sample analysis
A total of 13,969 samples were used to analyze the
serum level of vitamin A, and 14,054 samples were used for serum
vitamin E analysis (Table I).
 | Table I.Serum samples for vitamin A and E
analysis (n). |
Table I.
Serum samples for vitamin A and E
analysis (n).
| Groups | Vitamin A | Vitamin E |
|---|
| Early pregnancy (≤12
weeks) | 3,953 | 3,965 |
| Mid pregnancy (20–24
weeks) | 9,014 | 9,090 |
| Late pregnancy (≥28
weeks) | 1,002 | 999 |
| Total | 13,969 | 14,054 |
Reference value
The normal reference range for serum vitamin A and E
was 0.3–0.7 and 5–20 mg/l, respectively. Low test values for
vitamin A and E were indicative of nutritional deficiency, while
high test values were indicative of nutritional excess.
Statistical analysis
Statistical software CHISS (version 2010; Beijing,
China) was applied for the data analysis. Comparison between
multiple groups was made using one-way ANOVA test followed by a
post hoc test (Least Significant Difference). P<0.05 was
considered to indicate a statistically significant difference. The
standard substances were analyzed to establish the standard curve
equation. Standard deviation was <15%. Vitamin A and E contents
in the quality control and testing samples were calculated based on
the standard curve equation. Quality control range was mean ± 2SD,
Westgard Multirules were used to determine if the quality control
result was qualified, and at least double quality control was used
for each batch of samples.
Results
Serum vitamin A level in
pregnancy
Serum vitamin A level in pregnancy was 0.36±0.10
mg/l, and according to the stage of pregnancy it was 0.33±0.08,
0.37±0.09 and 0.33±0.15 mg/l in early, middle, and late pregnancy,
respectively. The levels in early and late pregnancy were
relatively low. The differences were statistically significant
(P<0.05) (Fig. 1).
There was a total of 25.31% abnormal rate of vitamin
A among these pregnant women and most of them were VAD,
representing 24.98%. The highest abnormal rate was in early
pregnancy, which was 38.35%, the deficiency rate accounted for
38.22% and severe deficiency (<0.2 mg/l) was 2.99%. The second
abnormal rate was observed in late pregnancy, which was 35.93%. The
deficiency rate accounted for 35.1%, and severe deficiency (<0.2
mg/l) was 29.57%. The differences were statistically significant
(P<0.05) (Table II).
 | Table II.Detection of serum levels of vitamin A
in pregnancy. |
Table II.
Detection of serum levels of vitamin A
in pregnancy.
|
| Deficiency, n
(%) |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | (<0.2 mg/l) | (0.2–0.3 mg/l) | Normal, n (%)
(0.3–0.7 mg/l) | Excess, n (%)
(>0.7 mg/l) | Total (n) | Total abnormal rate
(%) |
|---|
| Early | 118 (2.99) | 1,393 (35.24) | 2,437 (61.65) | 5 (0.13) | 3,953 | 38.35 |
| Mid | 95 (1.05) | 1,531 (16.98) | 7,354 (81.58) | 34 (0.38) | 9,014 | 18.42 |
| Late | 296 (29.57) | 56 (5.59) | 642 (64.14) | 8 (0.80) | 1,002 | 35.93 |
| Total | 3,489 (24.98) | 10,433 (74.69) | 47 (0.33) | 13,969 | 25.31 |
Serum vitamin E level in
pregnancy
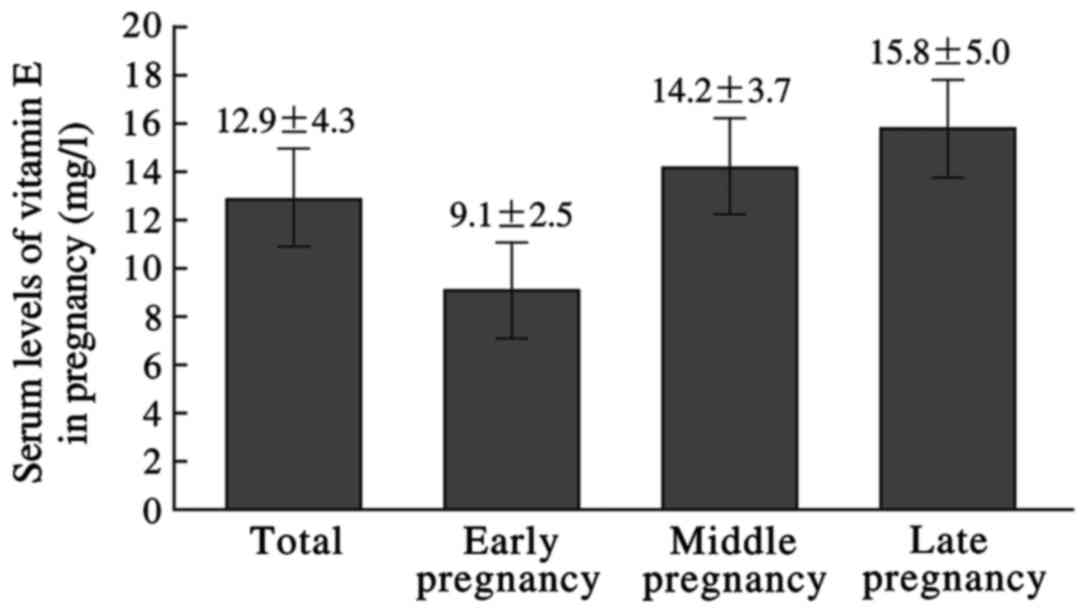
Serum vitamin E level in pregnancy was 12.9±4.30
mg/l, which was respectively, 9.10±2.47, 14.24±3.66, and 15.80±5.01
mg/l in early, middle, and late pregnancy. The levels in early
pregnancy were relatively low. The differences were statistically
significant (P<0.05) (Fig.
2).
There was a total of 5.60% abnormal rate of vitamin
E among these pregnant women and most of them had vitamin E excess,
representing 5.37%. The lowest abnormal rate was among women in
early pregnancy, which was 1.26%. The deficiency rate accounted for
0.76%, and the excess rate was 0.5%. The abnormal rate increased
during pregnancy, and the highest rate was observed among women in
late pregnancy, which was 15.32%. All these abnormal cases were
vitamin E excess. The differences were statistically significant
(P<0.05) (Table III).
 | Table III.Detection of serum levels of vitamin E
in pregnancy. |
Table III.
Detection of serum levels of vitamin E
in pregnancy.
| Groups | Deficiency, n (%)
(<5 mg/l) | Normal, n (%) (5–20
mg/l) | Excess, n (%) (>20
mg/l) | Total (n) | Total abnormal rate
(%) |
|---|
| Early | 30 (0.76) | 3,915 (98.74) | 20 (0.50) | 3,965 | 1.26 |
| Mid | 2 (0.02) | 8,505 (93.56) | 583 (6.41) | 9,090 | 6.43 |
| Late | 0 (0.00) | 846 (84.68) | 153 (15.32) | 999 | 15.32 |
| Total | 32 (0.23) | 13,266 (94.39) | 756 (5.37) | 14,054 | 5.60 |
Discussion
VAD is considered a major nutritional concern in
poor societies, especially in lower income countries. The main
underlying cause of VAD, as a public health problem, is a diet that
is chronically insufficient in vitamin A that can lead to lower
body stores and fail to meet physiological needs (5,6).
Preschool age children and pregnant women are the high-risk groups
of VAD.
Previous studies have found that maternal VAD can
have an adverse effect on embryonic development, resulting in poor
fetal growth and several congenital defects, such as
cryptophthalmos and anophthalmos, heart defects, central nervous
system abnormalities, retardation of skeletal development and
malformation (7,8). Between 1995 and 2005, the global VAD
rate (serum retinol content <0.7 µmol/l) was 15.3%, among which
Asia was the most serious, with a deficiency of Vitamin A of 18.4%,
with that in China being 22.8% (9).
The results of the study on the nutritional status of vitamin A in
Chinese cities from 2010–2012 showed that the deficiency of vitamin
A in pregnant women was 7.4%, and the lack of vitamin A in large
cities was 11.5% (10).
The study revealed that the pregnant women in
Beijing mainly had VAD. The total rate of VAD was 24.98%, which
accounted for 35.13% in late pregnancy, and severe deficiency
(<0.2 mg/l) rate was 29.57%. However, other studies in the
region of developing countries showed that VAD was found among 20%
(in Egypt), 15.8% (in Nigeria) and 18.8% (in Bangladesh) of
pregnant women (11–13). Our study showed that the serum levels
of vitamin A in early and late pregnancy were quite low. This might
be related to the health status of pregnant women, reaction of
pregnancy, hormone levels, regional differences, and many other
factors. Firstly, traditionally, botanical food has higher
proportion (80%) in the diet structure of Chinese women than animal
foods, especially animal liver which is rich in vitamin A. Besides,
progesterone level gradually increases during pregnancy and reaches
the peak in late pregnancy. Progesterone can accelerate vitamin A
in liver and fat tissues to release into blood (14). Since the progesterone level is
relatively low in early pregnancy, there is a lower serum vitamin A
level. Moreover, vitamin A is fat-soluble and its absorption and
utilization is closely associated with that of fat. Because of the
pregnancy reaction, there is a decrease in food intake of vitamin A
and fat, and a limited storage in blood, liver, and fat tissues
leading to less vitamin A releasing to blood. The concentration of
vitamin A is related to the concentration of retinol biding protein
(RBP). In the early stage of pregnancy, RBP concentration is low.
So, the serum level of vitamin A declines accordingly (2). Low levels of vitamin A in late
pregnancy may be affected by the significant growth in fetal weight
and nutrition demand.
Currently, WHO recommends routine vitamin A
supplementation during pregnancy or at any time during lactation in
areas with endemic VAD (where night-blindness occurs). In
pregnancy, extra vitamin A is required for growth and tissue
maintenance in the foetus, for providing it with some reserves and
for maternal metabolism. Pregnant women have a basal requirement of
370 µg/day, maximum dose of 3,000 µg/day and recommended daily
allowance (RDA) of 770 µg/day (15,16).
Based on the findings mentioned above, physicians should pay close
attention to the nutritional status of pregnant women, and monitor
vitamin A serum level in different pregnancy stages, especially in
early and late pregnancy (2). Upon
test reports, physicians can provide personalized dietary guide for
patients. Those pregnant women whose vitamin A is too low should
intake animal food with rich vitamin A, as well as vegetables. For
pregnant women who intake enough food with vitamin A, physicians
should teach them how to convert between different types of food
and to diversify their food recipes (14). For those who intake excessive vitamin
A, physicians should help them to achieve reasonable diets. By
accepting personalized dietary guidance and ensuring an optimized
nutrition intake during pregnancy the possibilities of fetal
abnormalities and congenital defects caused by intaking
insufficient vitamin A can be lower (10).
Vitamin E is the necessary micronutrient for
maintaining the body's normal metabolism and function, with the
features of antioxidant and radical scavenging (1). Pregnant women have a fast metabolism,
an increase of the production of free radicals, and an increase of
lipid peroxidation. So, low vitamin E levels can lead to excessive
free radicals, resulting in aging of placenta, vascular endothelial
injuries, increasing the incidence of hypertensive disorders in
pregnancy (4,17–19). It
may also damage the membrane of fetal membrane cells, increasing
the risk of premature rupture of fetal membranes (6). Vitamin E has anticoagulant activity,
excessive vitamin E can have an impact on blood clotting in the
fetus, increasing the risks of high levels of bilirubin and nuclear
jaundice for newborn babies (20).
In addition, excessive vitamin E has an antagonistic effect on
other fat-soluble vitamins in the blood of pregnant women,
preventing the absorption and functions of other vitamins (21).
The findings of this study showed that women had the
lowest serum level of vitamin E in early pregnancy while the
highest in late pregnancy. Vitamin E was present in excess (5.37%).
As gestational age increased, the excess rate of vitamin E reached
the peak of 15.32% in late pregnancy. Except for some factors
including hormone levels and general health condition, intaking
excessive vitamin E from food and drug supplementation may be the
main reason (22). Therefore,
physicians should pay close attention to monitor the changes in
vitamin E level in pregnancy and provide proper nutrition guidance,
especially emphasizing on rational vitamin E supplement.
In conclusion, good nutritional status during
pregnancy not only can guarantee the normal physiological functions
and keep mothers healthy, but is also crucial for fetal development
and delivery. By detecting the serum levels of vitamin A and E
among pregnant women in Beijing and evaluating their vitamin
nutritional status, the study has value in guiding the vitamin
supplements of pregnant women. In practice, we can reduce the
possibility of fetal abnormalities and congenital defects by
emphasizing perinatal education and nutrition supplement guidance,
strengthening the monitoring of vitamin status, and developing
early prevention and intervention strategies.
Acknowledgements
Not applicable.
Funding
This study was supported by the Development Center
for Medical Science and Technology National Health and Family
Planning Commission of the People's Republic of China Project
(grant no. W2015CAE029).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HC and HJ designed the study and performed the
experiments; HC, NQ, LY and HJ were responsible for the acquisition
of the data; HC and NQ analyzed the data; HC and HJ prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Haidian Maternal and Child Health Hospital (Beijing, China). Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bastani P, Hamdi K, Abasalizadeh F and
Navali N: Effects of vitamin E supplementation on some pregnancy
health indices: A randomized clinical trial. Int J Gen Med.
4:461–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang C, Chen J, Liu Z, Yun C, Piao J and
Yang X: Prevalence and influence factors of vitamin A deficiency of
Chinese pregnant women. Nutr J. 15:122016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buckley GJ, Murray-Kolb LE, Khatry SK,
Leclerq SC, Wu L, West KJ Jr and Christian P: Cognitive and motor
skills in school-aged children following maternal vitamin A
supplementation during pregnancy in rural Nepal: A follow-up of a
placebo-controlled, randomised cohort. BMJ Open. 3:e0020002013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberts JM, Myatt L, Spong CY, Thom EA,
Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM Jr,
et al: Eunice Kennedy Shriver National Institute of Child Health
and Human Development Maternal-Fetal Medicine Units Network:
Vitamins C and E to prevent complications of pregnancy-associated
hypertension. N Engl J Med. 362:1282–1291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clagett-Dame M and Knutson D: Vitamin A in
reproduction and development. Nutrients. 3:385–428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
See AW and Clagett-Dame M: The temporal
requirement for vitamin A in the developing eye: Mechanism of
action in optic fissure closure and new roles for the vitamin in
regulating cell proliferation and adhesion in the embryonic retina.
Dev Biol. 325:94–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu GR, Jing S, Momma K and Nakanishi T:
The effect of vitamin A on contraction of the ductus arteriosus in
fetal rat. Pediatr Res. 49:747–754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chagas CB, Saunders C, Pereira S, Silva J,
Saboya C and Ramalho A: Vitamin a deficiency in pregnancy:
Perspectives after bariatric surgery. Obes Surg. 23:249–254. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang JX, Lin LM, Lian GL and Greiner T:
Vitamin A deficiency and child feeding in Beijing and Guizhou,
China. World J Pediatr. 4:20–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu YC, Chen J, Li M, Wang R, Li WD, Yang
YH, Yang C, Yun CF, Yang LC and Yang XG: Study on anemia and
vitamin A and vitamin D nutritional status of Chinese urban
pregnant women in 2010-2012. Zhonghua Yu Fang Yi Xue Za Zhi.
51:125–131. 2017.(In Chinese). PubMed/NCBI
|
|
11
|
El-Khashab EK, Hamdy AM, Maher KM, Fouad
MA and Abbas GZ: Effect of maternal vitamin A deficiency during
pregnancy on neonatal kidney size. J Perinat Med. 41:199–203. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams IO, Essien EU and Eka OU:
Socioeconomic factors and vitamin A status of pregnant women in
Calabar urban, southeastern Nigeria. Matern Child Health J.
15:943–948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee V, Ahmed F, Wada S, Ahmed T, Ahmed AS,
Parvin Banu C and Akhter N: Extent of vitamin A deficiency among
rural pregnant women in Bangladesh. Public Health Nutr.
11:1326–1331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCauley ME, van den Broek N, Dou L and
Othman M: Vitamin A supplementation during pregnancy for maternal
and newborn outcomes. Cochrane Database Syst Rev.
10:CD0086662015.
|
|
15
|
Trumbo P, Yates AA, Schlicker S and Poos
M: Dietary reference intakes: Vitamin A vitamin K, arsenic, boron,
chromium, copper, iodine, iron, manganese, molybdenum, nickel,
silicon, vanadium, and zinc. J Am Diet Assoc. 101:294–301. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Azaïs-Braesco V and Pascal G: Vitamin A in
pregnancy: Requirements and safety limits. Am J Clin Nutr. 71 Suppl
5:1325S–1333S. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rumbold AR, Maats FH and Crowther CA:
Dietary intake of vitamin C and vitamin E and the development of
hypertensive disorders of pregnancy. Eur J Obstet Gynecol Reprod
Biol. 119:67–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wangkheimayum S, Kumar S and Suri V:
Effect of vitamin E on sP-selectin levels in pre-eclampsia. Indian
J Clin Biochem. 26:169–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banerjee S, Chambers AE and Campbell S:
Vitamin C and vitamin E in pregnant women at risk of pre-eclampsia.
Lancet. 368:1992006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allan KM, Prabhu N, Craig LC, McNeill G,
Kirby B, McLay J, Helms PJ, Ayres JG, Seaton A, Turner SW, et al:
Maternal vitamin D and E intakes during pregnancy are associated
with asthma in children. Eur Respir J. 45:1027–1036. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niki E and Traber MG: A history of vitamin
E. Ann Nutr Metab. 61:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rumbold A, Ota E, Hori H, Miyazaki C and
Crowther CA: Vitamin E supplementation in pregnancy. Cochrane
Database Syst Rev. 9:CD0040692015.
|