Introduction
Necrotizing enterocolitis (NEC) is a serious,
life-threatening disease in premature infants. The incidence of NEC
has increased in spite of clinical developments (1–3).
Additionally, the mortality rate of infants with NEC remains high
(~20–30%), although the clinical outcome for many premature infants
has improved (4). Severe NEC is
often accompanied by the necrosis of the intestinal wall and
perforation, as well as peritonitis, with a very high mortality
rate (5). The severity of NEC varies
from case to case any may affect the entire intestine (6,7). Studies
have demonstrated that various factors are associated with an
increased risk of developing NEC; its pathogenesis has been
explored using animal models in an attempt to develop more
effective therapeutic strategies (8,9). The
latest studies have indicated that inflammatory cascades serve an
important role in the pathogenesis of neonatal NEC (10,11).
Studies have hypothesized that NEC is caused by an uncontrolled
inflammatory response induced by a characteristic intestinal
bacterial colonization in premature infants (12,13).
Various inflammatory mediators, receptors and signal transduction
pathways are involved in the pathophysiological processes of the
disease; however, it remains unclear which factors are most crucial
(1,2). These factors are potential targets for
regulating the prevention and treatment of NEC (14). According to some studies, an
inflammatory cascade involving inflammatory cytokines serve a role
in the onset and progression of NEC (15,16). The
aim of the present study was to develop a method for establishing
an animal model of NEC to screen for effective treatment
agents.
Berberine, an alkaloid widely used in traditional
Chinese medicine to treat gastrointestinal infections, is an
effective antimicrobial treatment with regulatory effects on
glucose and lipid metabolism, as well as insulin resistance
(17,18). The anti-inflammatory and anti-oxidant
effects of berberine and its suppression of gene transcription were
recently reported (17). In
addition, berberine maintains the junctions between intestinal
mucosa (19). Furthermore, the
efflux pump in the intestine mucosa promotes berberine distribution
in the gastrointestinal epithelia (19). Therefore, the current study aimed to
investigate how berberine affects NEC development in a rat model of
NEC.
Materials and methods
NEC model establishment
A total of 60 newborn Sprague-Dawley (SD) rats (6–8
g; 30 Male, 30 Female) were obtained from pregnant SD rats
(Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) on day
21 of gestation. Thereafter, newborn rats were fed with 0.1 ml
artificial milk (Pet-Ag, Inc., Hampshire, IL, USA) twice a day.
Rats were housed at 30°C and 60% humidity with a 12-h light-dark
cycle. NEC models were established via N2 inhalation for
90 sec every 4 h and oral administration of 4 mg/kg/day
lipopolysaccharides (LPS; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) on days 0 and 1 (20). The
present study was approved by the Animal Ethics Committee of The
Second People's Hospital of Liaocheng (Linqing, China).
Animal grouping and berberine
treatment
Newborn were weighed and randomly divided into three
groups (n=20). Rats in the normal control (control) group stayed
with their mothers in the same cage following birth and were fed
breast milk without any intervention. Neonatal rats in the NEC
model (NEC) group were placed in an incubator 48 h after birth and
established as the neonatal NEC rat models. Neonatal rats in the
berberine intervention (NEC + berberine) group were established as
NEC rat models and berberine (Sigma-Aldrich; Merck KGaA) was
administered twice a day for 4 days by gavage at a dose of 0.6
g/kg/day dissolved in artificial milk.
NEC evaluation
Rats that developed distress (lethargy, abdominal
distention and bloody diarrhea) or imminent death prior to 96 h
were sacrificed to acquire the whole intestine. At 96 h later,
remaining rats were sacrificed to obtain the intestines. Intestinal
samples were fixed in 75% ethanol at 4°C for 16 h embedded in
paraffin and sliced into 4–6-µm-thick sections. These sections were
stained using hematoxylin and eosin both for 1 min at room
temperature. Histological evaluation of NEC was performed using
light microscopy (magnification, ×200) (Olympus BX51; Olympus
Corporation, Tokyo, Japan). NEC severity was assessed according to
the NEC scoring system (3).
Histological changes in the intestinal architecture of rats with
NEC were assigned an NEC grade: Grade N (normal), noseparation in
the submucosa or lamina propria; grade L (low), slight submucosal
and lamina propria separation; grade M (moderate), increased
submucosal and lamina propria separation with edema of the
submucosa; grade I (intermediate), severe separation of the
submucosa and lamina propria; and grade S (severe), necrosis and
loss of villi structure. Rats with grade M, I or S were deemed to
have NEC.
ELISA
The rat intestinal tissues were homogenized in NP40
lysis buffer (Sigma-Aldrich; Merck KGaA) on ice and the homogenates
were quantified using a BCA assay (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Total intestinal proteins were assessed using an
ELISA kit to evaluate the levels of IL-6 (cat. no. ab100712; Abcam,
Cambridge, UK), IL-10 (cat. no. ab214566; Abcam), MUC2 (cat. no.
LS-F4717; LifeSpan Biosciences, Inc., Seattle, WA, USA) and SIgA
(cat. no. SE120114; Sigma-Aldrich; Merck KGaA) in the samples.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from intestinal tissues
using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA (1 µg) was used
to generate cDNA with SuperScript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was performed in
triplicate using SYBR Premix Ex Taq (Takara, Dalian, Liaoning,
China) and SsoFast™ Probes Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and a standard thermocycling procedure (35
cycles) was performed on a Bio-Rad CFX96™ Real-time PCR System
(Bio-Rad Laboratories, Inc.). The thermocycling conditions were as
follows: 95°C for 2 min; followed by 35 cycles of 95°C for 1 min,
60°C for 1 min and 72°C for 1 min; then extension was performed at
72°C for 10 min. The 2−ΔΔCq method was used to analyze
the relative changes in gene expression (4). The primers used were as follows: TLR4
forward, 5′-GCATCATCTTCATTGTCCTTGA-3′ and reverse,
5′-CTTGTTCTTCCTCTGCTGTTTG-3′; NF-κB forward,
5′-ATGGCAGACGATGATCCCTAC-3′ and reverse,
5′-CGGAATCGAAATCCCCTCTGTT-3′; iNOS forward,
5′-GAGGCCCAGGAGGAGAGAGATCCG-3′ and reverse,
5′-TCCATGCAGACAACCTTGGTGTTG-3′; TNF-α forward,
5′-CCAGACCCTCACACTCAGATC-3′ and reverse,
5′-CACTTGGTGGTTTGCTACGAC-3′; and β-actin forward,
5′-CTAAGGCCAACCGTGAAAAG-3′ reverse, 5′-TACATGGCTGGGGTGTTGA-3′.
Western blot analysis
Total protein was extracted from ileal tissues with
lysis buffer (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
The protein concentration was determined using a BCA assay.
Proteins (50 µg/lane) were separated by 12% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane and blocked
with 50 g/l skimmed milk at room temperature for 1 h. Primary
antibodies against TLR4 (1:1,000; cat. no. ab13556), iNOS (1:1,000;
cat. no. ab3523) and NF-κB (1:1,000; cat. no. ab32536; all Abcam)
were incubated with the membranes overnight at 4°C, following which
membranes were incubated with an horseradish peroxidase-conjugated
antibody (1:5,000; cat. no. 7071; Cell Signaling Technology, USA)
at room temperature for 1 h. The protein bands were visualized with
a G-BOX imaging system (Syngene Europe, Cambridge, UK) using an ECL
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Western
blotting results were analyzed by ImageJ software 1.8.0 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses and all data are presented as the
mean ± standard deviation. One-way analysis of variance followed by
a Tukey's post-hoc test was used to compare multiple groups and the
least significant difference test was performed for pair-wise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Berberine treatment decreases the
incidence and severity of NEC
In the control, NEC and NEC + berberine groups, the
incidence of NEC was 0, 65 and 25%, respectively (Fig. 1). A significant difference in the
incidence rate of NEC between the NEC group and NEC + berberine
group was observed (Fig. 2).
Fig. 2B presents the representative
microscopic morphology of the intestinal samples. All rats in grade
M or I exhibited bloody diarrhea (data not shown).
Berberine treatment decreases TLR4
expression in ileal tissues
In the NEC group, the expression of TLR4 mRNA and
protein was significantly and markedly increased, respectively, at
48 h compared with the control group (Fig. 3). However, in the NEC + berberine
group, TLR4 mRNA and protein were significantly and markedly
suppressed, respectively, compared with the NEC group (Fig. 3).
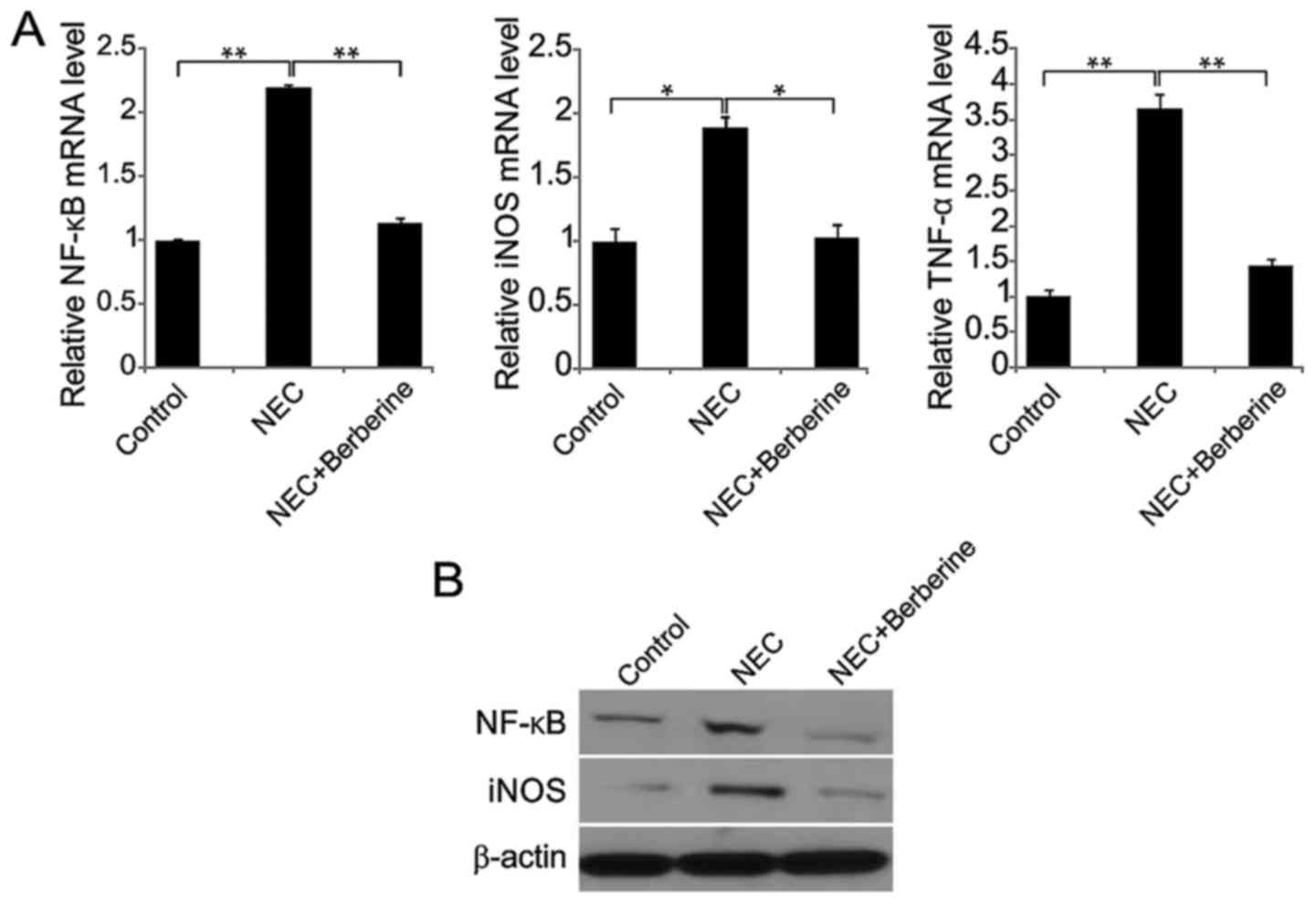
Berberine treatment decreases cytokine
expression in ileal tissues
In the NEC group, NF-κB, iNOS and TNF-α mRNA
expression was significantly increased at 48 h compared with the
control group (Fig. 4A). However, in
the NEC + berberine group, NF-κB, iNOS and TNF-α mRNA expression
was significantly decreased compared with the NEC group. Similarly,
NF-κB and iNOS protein levels were increased in the NEC group,
while these levels were decreased following berberine treatment
(Fig. 4B).
Berberine treatment decreases IL-6 and
IL-10 expression in the ileum and serum
In the NEC group, the ileal expression of IL-6 and
IL-10 was significantly increased at 48 h compared with the control
group (Fig. 5A). However, in the NEC
+ berberine group, ileal IL-6 and IL-10 were significantly lower
compared with the NEC model group. Similar results were observed in
serum samples (Fig. 5B).
Berberine treatment increases the
expression of MUC2 and SIgA in ileal tissues
Compared with the control group, the expression
level of MUC2 and SIgA protein was significantly lower in the NEC
group at 48 h (Fig. 6). However, in
the NEC + berberine group, the expression of MUC2 and SIgA were
significantly greater compared with the NEC group.
Discussion
NEC is a common disorder that affects newborns,
primarily occurring in preterm infants of very low birth weight
(14). In the present study, NEC
symptoms were evident 2 days after the model was established. There
are differences in the clinical manifestation of NEC; mild
symptoms, including a swollen abdomen and diarrhea, can rapidly
develop into necrosis of the intestinal wall, perforation or
peritonitis (2,8). Therefore, prevention is particularly
important. Studies have suggested several preventive measures,
including the use of probiotics to regulate intestinal
microecology, intestinal supplementation with arginine and
glutamine and treatment with glucocorticoids (21,22).
However, the efficacy of these treatments has not yet been proven
and their use remains controversial. Therefore, the discovery of
safe and effective preventive methods has become a research
hotspot.
Symbiotic bacteria often colonize the sterile gut of
neonates, which can increase the incidence of NEC (23). TLRs, cell transmembrane receptors of
the natural immune system, are critical for pathogen resistance
(24). Berberine has been
demonstrated to effectively reduce inflammatory cytokine levels
(25) and to attenuate NEC by
inhibiting inflammation and apoptosis via the phosphatidylinositol
4,5-bisphosphate 3-kinase/RAC-α serine/threonine-protein kinase
signaling pathway (26). The authors
of the present study inferred that berberine may be effective at
reducing the incidence and severity of NEC through its
anti-inflammatory effects.
NEC is caused by multiple factors that primarily act
through the inflammatory cascade (10). A possible target for NEC treatment is
the regulation of key components of the inflammatory response
(6). More importance has been
attached to the effects of TLR4 in the pathogenesis of neonatal NEC
(27). Different TLRs, recognize
their respective pathogens, triggering signaling pathways, thereby
activating a series of immune responses (28). The p65/p50 dimer of NF-κB was
identified in nearly all nucleated eukaryotic cells (29). TLR2 and TLR4 are normally lowly
expressed in intestinal epithelial cells, and so they have been
used to monitor the state of the intestinal flora (24). NF-κB remains inactive in the
cytoplasm until intestinal epithelial cells are stimulated by
bacteria acting on the TLRs, triggering the activation of
downstream signaling pathways and causing NF-κB to migrate into the
nuclei (30). Thereafter, NF-κB has
been demonstrated to further promote the overexpression of
cytokines associated with immune responses (5). In an LPS-induced animal model of NEC,
activated TLR4 triggered an inflammatory cascade, thereby
regulating intestinal injury repair in neonates and accelerating
the development and progression of NEC (4). IL-6 causes neutrophils to recruit and
release a large number of active oxygen radicals in lesion areas by
virtue of chemotaxis and coordinates with other inflammatory cells
to give a cytotoxic effect (31). A
positive feedback loop is formed by the stimulation of inflammatory
mediators, which can lead to excessive inflammatory responses,
injury and necrosis of the intestinal tissues, as well as
destruction of the mucosal barrier, thereby developing into NEC
(32).
Mucin is a major component of the intestinal mucus
layer, covering the surface of the intestinal epithelial cells and
acting as the first line of defense (33). Among the 20 known types of mucin,
secretory mucin MUC2 was the first to be identified and exists in
the highest concentration in the human intestinal cavity (34). MUC2 covers the surface of the
intestinal cavity and forms a gelatinous mucus layer to preserve
complete barrier function (34).
Previous studies have demonstrated that MUC2 is involved in the
pathogenesis of NEC (6). SIgA is an
immunoglobulin on the surface of intestinal mucosa, which serves an
important role in the defense of gastrointestinal mucosa (35). A Previous study has demonstrated that
the expression of SIgA was reduced in NEC rats, compared with rats,
and that this effect was weakened by Insulin-like growth factor I
(7). In the present study, changes
were observed in the ileal expression of MUC2. The results
indicated that MUC2 and SIgA expression was lower in the NEC group.
In the NEC + berberine group, MUC2 and SIgA expression was similar
to that observed in the control group.
In summary, the results of the present study
demonstrated that enteral administration of berberine ameliorates
the clinical symptoms and decreases the incidence of NEC in a
neonatal rat model. This may be achieved via berberine-induced TLR4
downregulation, which in turn inhibits the production of
inflammatory mediators, and the upregulated expression of MUC2 and
SIgA. Together, these expression changes may protect the mechanical
and immuno-barrier functions of the intestinal mucosa. Therefore,
berberine may be a potential therapeutic agent for the treatment of
NEC.
Acknowledgments
Not applicable.
Funding
The current study was supported by the Natural
Science Foundation of China (grant no. 81460249) and Guizhou
Province Joint Fund [qianKehe LH (2015); grant no. 7478].
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ designed and planned the study. YJ and FP
collected the data. YJ, FP and YS analyzed the data. JJ interpreted
the data. YJ and JJ analysed the literature and wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of The Second People's Hospital of Liaocheng.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hackam DJ and Sodhi CP: Toll-like
receptor-mediated intestinal inflammatory imbalance in the
pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol
Hepatol. 6:229–238.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halpern MD and Denning PW: The role of
intestinal epithelial barrier function in the development of NEC.
Tissue Barriers. 3:e10007072015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dvorak B, Halpern MD, Holubec H, Williams
CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM and McCuskey
RS: Epidermal growth factor reduces the development of necrotizing
enterocolitis in a neonatal rat model. Am J Physiol Gastrointest
Liver Physiol. 282:G156–G164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:2017.
|
|
6
|
Martin NA, Mount Patrick SK, Estrada TE,
Frisk HA, Rogan DT, Dvorak B and Halpern MD: Active transport of
bile acids decreases mucin 2 in neonatal ileum: Implications for
development of necrotizing enterocolitis. PLoS One. 6:e271912011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian F, Liu GR, Li N and Yuan G: YUAN4
insulin-like growth factor I reduces the occurrence of necrotizing
enterocolitis by reducing inflammatory response and protecting
intestinal mucosal barrier in neonatal rats model. Eur Rev Med
Pharmacol Sci. 21:4711–4719. 2017.PubMed/NCBI
|
|
8
|
Garg BD, Sharma D and Bansal A: Biomarkers
of necrotizing enterocolitis: A review of literature. J Matern
Fetal Neonatal Med. 31:3051–3064. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rai SE, Sidhu AK and Krishnan RJ:
Transfusion-associated necrotizing enterocolitis re-evaluated: A
systematic review and meta-analysis. J Perinat Med. 46:665–676.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Plaen IG: Inflammatory signaling in
necrotizing enterocolitis. Clin Perinatol. 40:109–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frost BL, Jilling T and Caplan MS: The
importance of pro-inflammatory signaling in neonatal necrotizing
enterocolitis. Semin Perinatol. 32:100–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
MohanKumar K, Namachivayam K,
Chapalamadugu KC, Garzon SA, Premkumar MH, Tipparaju SM and
Maheshwari A: Smad7 interrupts TGF-β signaling in intestinal
macrophages and promotes inflammatory activation of these cells
during necrotizing enterocolitis. Pediatr Res. 79:951–961. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Claud EC: Neonatal necrotizing
enterocolitis-inflammation and intestinal immaturity. Antiinflamm
Antiallergy Agents Med Chem. 8:248–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zani A and Pierro A: Necrotizing
enterocolitis: Controversies and challenges. F1000Res. 4:F1000
Faculty Rev. –1373. 2015.PubMed/NCBI
|
|
15
|
Maheshwari A, Schelonka RL, Dimmitt RA,
Carlo WA, Munoz-Hernandez B, Das A, McDonald SA, Thorsen P,
Skogstrand K, Hougaard DM, et al: Cytokines associated with
necrotizing enterocolitis in extremely-low-birth-weight infants.
Pediatr Res. 76:100–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benkoe T, Baumann S, Weninger M, Pones M,
Reck C, Rebhandl W and Oehler R: Comprehensive evaluation of 11
cytokines in premature infants with surgical necrotizing
enterocolitis. PLoS One. 8:e587202013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Habtemariam S: Berberine and inflammatory
bowel disease: A concise review. Pharmacol Res. 113:592–599. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cicero AF and Baggioni A: Berberine and
its role in chronic disease. Adv Exp Med Biol. 928:27–45. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Ren F, Wei H, Liu L, Shen T, Xu S,
Wei J, Ren J and Ni H: Combination of berberine and evodiamine
inhibits intestinal cholesterol absorption in high fat diet induced
hyperlipidemic rats. Lipids Health Dis. 16:2392017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shinyama S, Kaji T, Mukai M, Nakame K,
Matsufuji H, Takamatsu H and Ieiri S: The novel preventive effect
of Daikenchuto (TJ-100), a Japanese herbal drug, against neonatal
necrotizing enterocolitis in rats. Pediatr Surg Int. 33:1109–1114.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel RM and Underwood MA: Probiotics and
necrotizing enterocolitis. Semin Pediatr Surg. 27:39–46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harpavat S, Pammi M and Gilger M: Novel
treatments for NEC: Keeping IBD in mind. Curr Gastroenterol Rep.
14:373–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang F, Meng D, Weng M, Zhu W, Wu W,
Kasper D and Walker WA: The symbiotic bacterial surface factor
polysaccharide A on Bacteroides fragilis inhibits IL-1beta-induced
inflammation in human fetal enterocytes via toll receptors 2 and 4.
PLoS One. 12:e01727382017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dolasia K, Bisht MK, Pradhan G, Udgata A
and Mukhopadhyay S: TLRs/NLRs: Shaping the landscape of host
immunity. Int Rev Immunol. 37:3–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohammadi S, Seyedhoseini FS, Asadi J and
Yazdani Y: Effects of berberine on the secretion of cytokines and
expression of genes involved in cell cycle regulation in THP-1
monocytic cell line. Iran J Basic Med Sci. 20:530–537.
2017.PubMed/NCBI
|
|
26
|
Fang C, Xie L, Liu C, Fu C, Ye W, Liu H
and Zhang B: Berberine ameliorates neonatal necrotizing
enterocolitis by activating the phosphoinositide 3-kinase/protein
kinase B signaling pathway. Exp Ther Med. 15:3530–3536.
2018.PubMed/NCBI
|
|
27
|
Nanthakumar N, Meng D, Goldstein AM, Zhu
W, Lu L, Uauy R, Llanos A, Claud EC and Walker WA: The mechanism of
excessive intestinal inflammation in necrotizing enterocolitis: An
immature innate immune response. PLoS One. 6:e177762011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong XS, Xu XY, Sun YQ, Wei-Liu, Jiang ZH
and Liu Z: Toll-like receptor 4 is involved in myocardial damage
following paraquat poisoning in mice. Toxicology. 312:115–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong JT: NF-kB as a mediator of brain
inflammation in AD. CNS Neurol Disord Drug Targets. 2017.
|
|
30
|
Liu Q, Xu D, Jiang S, Huang J, Zhou F,
Yang Q, Jiang S and Yang L: Toll-receptor 9 gene in the black tiger
shrimp (Penaeus monodon) induced the activation of the
TLR-NF-κB signaling pathway. Gene. 639:27–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi W, Shen Q, Zhang L, Han LP and Wang S:
Study on the inflammatory intervention of erythropoietin on NEC.
Exp Ther Med. 11:2221–2224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Satoh T, Izumi H, Iwabuchi N, Odamaki T,
Namba K, Abe F and Xiao JZ: Bifidobacterium breve prevents
necrotising enterocolitis by suppressing inflammatory responses in
a preterm rat model. Benef Microbes. 7:75–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sicard JF, Le Bihan G, Vogeleer P, Jacques
M and Harel J: Interactions of intestinal bacteria with components
of the intestinal mucus. Front Cell Infect Microbiol. 7:3872017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Betge J, Schneider NI, Harbaum L,
Pollheimer MJ, Lindtner RA, Kornprat P, Ebert MP and Langner C:
MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: Expression
profiles and clinical significance. Virchows Arch. 469:255–265.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chatterton DE, Nguyen DN, Bering SB and
Sangild PT: Anti-inflammatory mechanisms of bioactive milk proteins
in the intestine of newborns. Int J Biochem Cell Biol.
45:1730–1747. 2013. View Article : Google Scholar : PubMed/NCBI
|