Introduction
Osteoarthritis (OA) is a common chronic joint
disease in elderly and middle-aged people and in China, the
incidence of OA is increasing rapidly due to the ageing population.
Performing physically strenuous jobs, which is common for people in
less developed areas of China, is closely associated with the
development of OA (1). Indeed, the
incidence of knee osteoarthritis in elderly females is higher in
China (30–45%) than in the US (20–35%) (2). Degenerative changes of the articular
cartilage, cartilage destruction and secondary subchondral bone
hyperplasia are the primary pathological features of OA, and the
clinical manifestations of this disease include joint pain, joint
deformity and dysfunction (1).
Several studies have been performed to investigate
the roles of cytokines in pathogenesis of OA. It has been
demonstrated that interleukin (IL)-1β serves an important role
during the inflammatory response to cause cartilage tissue injury
in the body (3,4). Chondrocytes respond to IL-1β by
secreting various inflammatory mediators, including cytokines,
chemokines, matrix metalloproteinases (MMPs), nitric oxide (NO) and
prostaglandin E2 (PGE2) (5–7). These factors serve crucial roles in the
degeneration of cartilage matrix and destruction of articular
cartilage during the development of OA and have therefore been
selected as potential targets to treat OA (8). Emodin is a type of anthraquinone
isolated from the Chinese herb Radix et Rhizoma Rhei and has been
reported to exhibit anti-inflammatory, antibacterial and antitumor
action (9). Emodin has been reported
to exhibit therapeutic effects in various diseases. For example,
emodin inhibits the effect of glucocorticoids, which may in turn
alleviate insulin resistance and reduce the severity of type 2
diabetes (9). Furthermore, emodin
has been reported to have an anti-cancer effect in human pancreatic
cancer (10) and exhibit
neuroprotective effects against glutamate toxicity (11). In addition, it has been reported that
emodin may suppress lipopolysaccharide (LPS)-induced
pro-inflammatory responses and the activation of nuclear factor
(NF)-κB (12), meaning that it may
be used to treat inflammatory disorders.
The aim of the current study was to investigate the
effect of emodin on the inflammatory mediators involved in the
development of osteoarthritis. The results may enable the
development of novel diagnostic and therapeutic strategies to treat
osteoarthritis.
Materials and methods
Isolation and culture of rat
chondrocytes
A total of 8 Sprague Dawley rats (8 weeks old,
250–300 g; 4 males and 4 females) were purchased from Guangdong
Medical Laboratory Animal Center [Foshan, China; Certificate of
Quality no. SCXK (Guangdong) 2015-0019]. Rats were kept in a quiet
environment at 25°C, in strict compliance with their circadian
rhythm (30–70% relative humidity; 12 h light-dark cycle) for 1 week
before operation. All rats were had ad libitum access to
food and water. Rats were sacrificed and knee joints were cut in
the middle following disinfection with iodine tincture and 75%
ethanol. The articular cartilage of the femoral condyle, trochlear
and patella were collected and placed in phosphate-buffered saline
(PBS) containing double antibiotics (100 U/ml penicillin and 100
g/ml streptomycin). Tissues were washed 3 times with PBS containing
double antibiotics and blood were removed using sterile surgical
instruments. Cartilage tissue was cut using ophthalmic scissors and
placed in a sterile bottle. Following magnetic stirring, 0.25%
trypsin was added to completely digest cartilage tissue.
Subsequently, centrifugation was performed at 300 × g for 8 min at
room temperature and the supernatant was discarded. Then, 4 ml
Dulbecco's Modified Eagle's medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) was mixed with cells. Cells were inoculated into
a 25-cm2 flask and maintained at 37°C in an incubator
containing 5% CO2. The culture medium was replenished
every three days and cells were observed using an inverted
microscope. In the test group, chondrocytes collected following 3
subcultures (each, 37°C for 24) were treated with 10, 20 or 30
µg/ml emodin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2
h. Subsequently, 10 ng/ml IL-1β (Sigma-Aldrich; Merck KGaA) was
added to the cells and they were incubated for 24 h (13). Treatment with LPS (1 µ/m;
Sigma-Aldrich; Merck KGaA) and the ERK inhibitor SCH772984 (10 nM,
Sigma-Aldrich; Merck KGaA) was administered at room temperature for
2 h prior to subsequent experiments.
All experiments on animals were performed in
accordance with the Declaration of Helsinki and the present study
was approved by the Animal Ethics Committee of the Chinese Medicine
Hospital of Xinjiang Uygur Autonomous Region (Urumqi, China).
The detection of NO and PGE2
Chondrocyte culture medium was centrifuged at 1,800
× g (room temperature) for 15 min to collect supernatant, and
levels of NO and PGE2 were determined using ELISA kits (cat. no.
KGE001 and KGE004B, respectively; R&D Systems, Inc.,
Minneapolis, MN, USA). Positive control, negative control and blank
control wells were all used. The positive and negative controls
were included in the kits, and diluent buffer was used as the blank
control. Optical density (OD) values were measured at 450 nM using
a microplate reader (Thermo Fisher Scientific, Inc.). Serum levels
of NO and PGE2 were then calculated using a standard curve plotted
using standard samples produced from the aforementioned ELISA
kits.
MTT assay
A total of 2×103 cells/well were
inoculated in a 96-well plate following incubation with the
different concentrations of emodin (0, 10, 20 and 30 µg/ml) and
incubation at 37°C in 5% CO2 for 96 h. Subsequently, 20
µl MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added.
Following incubation for a further 4 h, the supernatant was then
removed and 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA)
was added. OD values at 490 nm were measured using a microplate
reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following cell culture for 7 days, cells were
digested with trypsin and collected following centrifugation (1,800
× g for 10 min at room temperature). TRIzol reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from cells. RNA
purity and concentration were detected using a NanoDrop ND-2000
UV-Vis Spectrophotometer (Thermo Fisher Scientific, Inc.) and
reverse transcription was performed using SuperScript III Reverse
Transcriptase kit (Thermo Fisher Scientific, Inc.). Reverse
transcription conditions were as follows: 25°C for 5 min, 55°C for
20 min and 80°C for 20 min. The SYBR®-Green Real-Time
PCR Master mix (Thermo Fisher Scientific, Inc.) and cDNA were then
used in the qPCR reaction system. The following primers were used
for qPCR: MMP-3 forward, 5′-GCATTGGCTGAGTGAAAGAGACTGTATC-3′ and
reverse, 5′-ATGATGAACGATGGACAGATGA-3′; MMP-13 forward,
5′-AGTAGTTCCAAAGGCTACAACTTGTTT-3′ and reverse,
5′-GGAGTGGTCAAGCCCTAAGGA-3′; and GAPDH forward,
5′-ATGACAACTCCCTCAAGAT-3′ and reverse, 5′-GATCCACAACGGATACATT-3′.
qPCR was performed on an CFX96 Touch™ Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The thermocycling conditions were as follows: 95°C for 50 sec,
followed by 40 cycles of 95°C for 12 sec and 60°C for 35 sec, and
72°C for 5 min. Cq values were processed using the
2−ΔΔCq method and the relative expression level of each
gene was normalized to the endogenous control GAPDH.
Western blotting
Total protein was extracted from chondrocytes using
RIPA Lysis and Extraction buffer (Thermo Fisher Scientific, Inc.)
and protein quantification was performed using a bicinchoninic
assay kit. A total of 30 µg protein/lane was subjected to 10%
SDS-PAGE and were then transferred to PVDF membranes. Membranes
were blocked with 5% skimmed milk at room temperature for 3 h.
Following washing, membranes were incubated with the following
primary antibodies overnight at 4°C of MMP3 (1:1,000; cat. no.
ab53015; Abcam, Cambridge, UK), MMP13 (1:1,000; cat. no. ab39012;
Abcam), ERK1/2 (1:1,000; cat. no. ab196883; Abcam), p-ERK1/2
(1:1,000; cat. no. ab214362; Abcam), β catenin (1:1,000; cat. no.
ab16051; Abcam) and GAPDH (1:1,000; cat. no. ab9485; Abcam).
Following three washes with TBST (15 min/wash), membranes were
incubated with horseradish peroxidase conjugated anti-rabbit
immunoglobulin G (1:1,000; cat. no. ab6721; Abcam) for 1 h at room
temperature. Following three washes with TBST (15 min/wash),
enhanced chemiluminescence solution (Thermo Fisher Scientific,
Inc.) was added to detect the signals. The relative expression
level of each protein was normalized to the expression of the
endogenous control GAPDH using Image-J V1.49 software (National
Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation and
were analyzed by SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA).
Comparisons among multiple groups were conducted using one-way
analysis of variance followed by a least-significant difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Emodin reduces the cytotoxicity of
IL-1β in rat chondrocytes
The cytotoxicity of emodin was determined using an
MTT assay. The results demonstrated that 10, 20 and 30 µg/ml emodin
was not toxic to rat chondrocytes (Fig.
1A). The treatment of chondrocytes with IL-1β significantly
reduced cell viability (P<0.05; Fig.
1B). However, this reduction in chondrocyte viability was
significantly reversed following pretreatment with emodin
(P<0.05) in a dose-dependent manner.
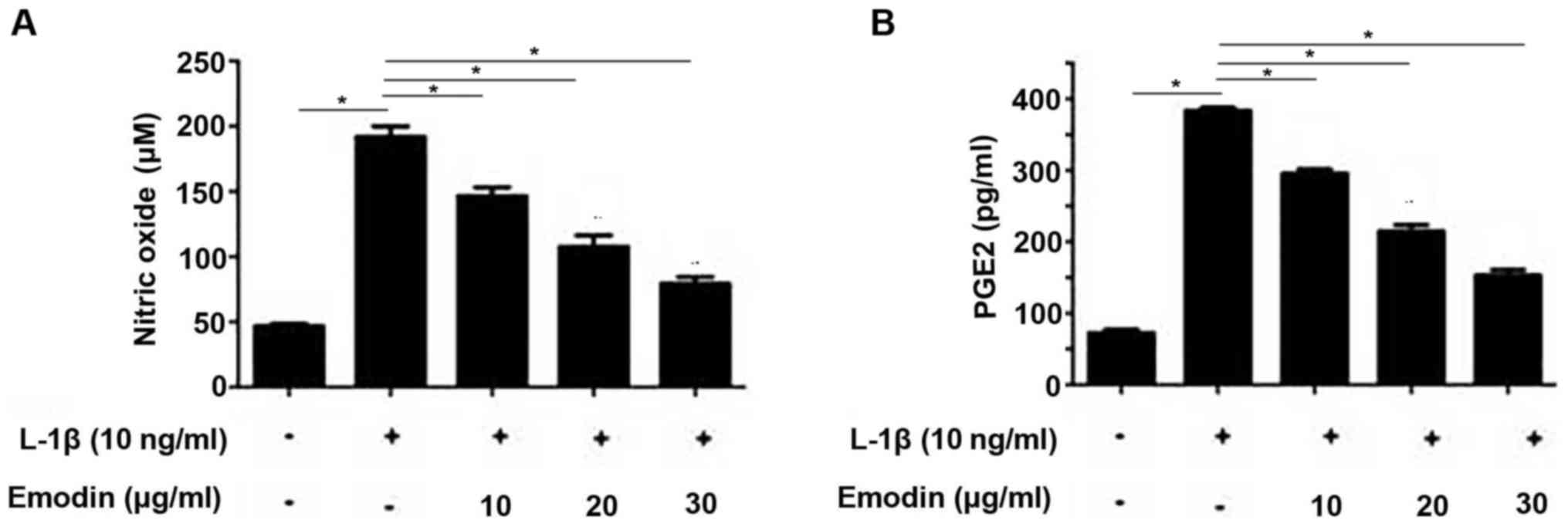
Emodin inhibits NO and PGE2
NO and PGE2 are closely associated with IL-1β
signaling in chondrocytes (14). The
inhibitory effect of emodin on IL-1β expression is may determined
by measuring NO and PGE2 levels. Following the addition of IL-1β,
NO and PGE2 levels were significantly increased compared with the
control group (P<0.05; Fig. 2A and
B). However, pretreatment with emodin significantly decreased
NO and PGE2 levels in a dose-dependent manner (P<0.05).
Emodin inhibits the expression of MMP1
and MMP13
High expression levels of MMP proteins stimulate
cartilage degradation (15). In the
present study, MMP-3 and MMP13 levels in rat chondrocytes were
determined by RT-qPCR and western blotting. It has been
demonstrated that IL-1β is able to induce the expression of MMP3
and MMP13 (6) and this was also
identified in the present study. The expression of MMP3 and MMP13
were significantly increased following the addition IL-1β
(P<0.05; Fig. 3). However, cells
the expression of MMP3 and MMP13 significantly decreased in cells
pretreated with emodin compared with those treated with IL-1β
alone. These decreases occurred in a dose-dependent manner.
Emodin inhibits the activation of the
extracellular-signal regulatory kinase (ERK) pathway induced by
LPS
The ERK signaling pathway regulates the
proliferation and differentiation chondrocytes and serves a key
role in the growth and repair of articular cartilage (16). It has also been demonstrated that LPS
is able to induce the phosphorylation of ERK1/2 to activate ERK
signaling pathway (16) and the
results of the current study confirmed that this is the case. As
shown in Fig. 4, Compared with
untreated cells, the phosphorylation levels of ERK1/2 were
significantly increased in cells treated with LPS (P<0.05).
Treatment with the ERK inhibitor SCH772984 significantly reversed
the increase in phosphorylation levels of ERK1/2 induced by LPS.
Furthermore, treatment with different concentrations of emodin also
significantly reduced the increased phosphorylation levels of
ERK1/2 induced by LPS in a dose-dependent manner, indicating that
emodin inhibits the signal transduction of the ERK pathway.
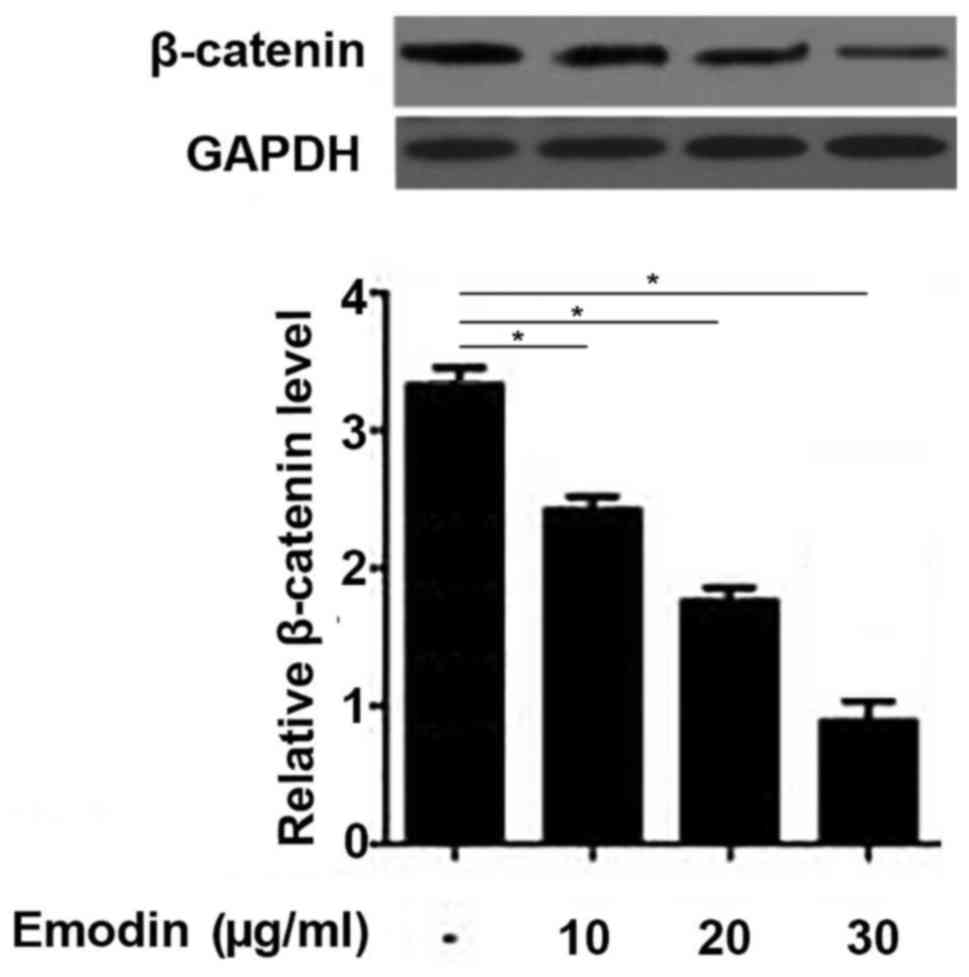
Emodin inhibits the Wnt/β-catenin
pathway in rat chondrocytes
The Wnt/β-catenin pathway serves a pivotal role
during the development of OA (17);
therefore, the current study investigated the effects of emodin on
β-catenin expression. The results demonstrated that the expression
of β-catenin in the chondrocytes was significantly downregulated
following treatment with emodin in a dose-dependent manner
(Fig. 5). These data suggest that
emodin may treat OA by inhibiting the Wnt/β-catenin pathway.
Discussion
It has been demonstrated that emodin not only
exhibits neuroprotective activity (11) but also inhibits LPS-induced
pro-inflammatory responses and the activation of NF-κB (12). However, its underlying mechanism of
action remains unknown. In the current study, the effects of emodin
on inflammatory mediators in the chondrocytes of rats were
investigated. The results demonstrated that emodin reduced the
cytotoxicity of IL-1β in rat chondrocytes in a
concentration-dependent manner. These results are consistent with
the results of a previous study demonstrating that emodin inhibited
the IL-1β-induced inflammatory response by suppressing the NF-kB
signaling pathway (12).
MMP genes are highly expressed in the articular
cartilage and various cytokines, including IL-1 and tumor necrosis
factor (TNF)-α are also produced in the articular cartilage. It has
been demonstrated that excessive production of NO in OA may be
associated with the IL-1 receptor (IL-1Ra) (18). Autocrine IL-1β released by the
OA-affected cartilage is able to regulate NO and PGE2 production
(19). The results of the current
study demonstrated that emodin decreases NO and PGE2 levels.
Furthermore, NO and PGE2 levels decreased following treatment with
emodin in a dose-dependent manner. The results are consistent with
those of a previous study, which reported that emodin treatment
could inhibit the expression of TNF-α and IL-6 in plasma and the
production of PGE2 in the synovial tissues of mice (20). As the major enzymes involved in the
degradation of the extracellular matrix, MMP family members serve
pivotal roles in resisting compressive forces and maintaining the
tensile properties of tissue (21,22).
Previous studies determined that MMP-3 and MMP-13 are important
genes that may be targeted to treat OA (22,23).
Furthermore, it has been demonstrated that the expression of MMP-3
and MMP-13 are significantly increased in response to IL-1 and
TNF-α in OA (23,24). The results of the current study are
in accordance with those of previous studies, which demonstrated
that emodin inhibited MMP-3 and MMP-13 expression and that this
degree of inhibition was positively associated with the
concentration of emodin (25,26).
The ERK pathway serves a critical function in
regulating chondrocyte proliferation by regulating the expression
of associated genes (27) and
Wnt/β-catenin is a key factor in the development of OA. In the
present study, emodin significantly reduced the phosphorylation
level of ERK1/2 and the expression of β-catenin in chondrocytes in
a dose-dependent manner. These data suggest that emodin may inhibit
the development of OA by inhibiting the ERK and Wnt/β-catenin
pathways.
In conclusion, the results of the current study
suggest that emodin may improve OA by reducing the cytotoxicity of
IL-1β, downregulating the expression of MMP-3 and MMP-13 and
inhibiting the ERK and Wnt/β-catenin pathways. The results indicate
that emodin may be used as a novel therapeutic strategy to treat
patients with OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZFL, RF and QM designed the experiments, whilst ZFL
and YL performed them. LL, ZQL, YD and RF analyzed the data. RF
wrote the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Animal experiments were performed in accordance with
the Declaration of Helsinki and the present study was approved by
the Animal Ethics Committee of the Chinese Medicine Hospital of
Xinjiang Uygur Autonomous Region (Urumqi, China).
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
References
|
1
|
Kang X, Fransen M, Zhang Y, Li H, Ke Y, Lu
M, Su S, Song X, Guo Y, Chen J, et al: The high prevalence of knee
osteoarthritis in a rural Chinese population: The Wuchuan
osteoarthritis study. Arthritis Rheum. 61:641–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu
W, Qin M, Lui LY and Felson DT: Comparison of the prevalence of
knee osteoarthritis between the elderly Chinese population in
Beijing and whites in the United States: The Beijing osteoarthritis
study. Arthritis Rheum. 44:2065–2071. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
4
|
Lotz M: Cytokines in cartilage injury and
repair. Clin Orthop Relat Res. (391 Suppl):S108–S115. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wojdasiewicz P and Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:ID 561459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Y, Zhou L and Wang C: Effects of
platycodin D on IL-1β-induced inflammatory response in human
osteoarthritis chondrocytes. Int Immunopharmacol. 40:474–479. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mobasheri A: The future of osteoarthritis
therapeutics: Targeted pharmacological therapy. Curr Rheumatol Rep.
15:3642013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He K, Ye X, Wu H, Wang Y, Zou Z, Ning N,
Hu Y, Chen B, Fang X and Li X: The safety and
anti-hypercholesterolemic effect of emodin in Syrian golden
hamsters. Lipids. 50:185–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin S, Chen H, Wei W, Ye S, Liao W, Gong
J, Jiang Z, Wang L and Lin S: Antiproliferative and antimetastatic
effects of emodin on human pancreatic cancer. Oncol Rep. 26:81–89.
2011.PubMed/NCBI
|
|
11
|
Gu JW, Hasuo H, Takeya M and Akasu T:
Effects of emodin on synaptic transmission in rat hippocampal CA1
pyramidal neurons in vitro. Neuropharmacology. 49:103–111. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng G, Liu Y, Lou C and Yang H: Emodin
suppresses lipopolysaccharide-induced pro-inflammatory responses
and NF-κB activation by disrupting lipid rafts in CD14-negative
endothelial cells. Br J Pharmacol. 161:1628–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou PH, Liu SQ and Peng H: The effect of
hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat
model of osteoarthritis. J Orthop Res. 26:1643–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chowdhury TT, Bader DL and Lee DA: Dynamic
compression inhibits the synthesis of nitric oxide and PGE(2) by
IL-1beta-stimulated chondrocytes cultured in agarose constructs.
Biochem Biophys Res Commun. 285:1168–1174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
Development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corr M: Wnt-beta-catenin signaling in the
pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol.
4:550–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vincenti MP: The matrix metalloproteinase
(MMP) and tissue inhibitor of metalloproteinase (TIMP) genes.
Transcriptional and posttranscriptional regulation, signal
transduction and cell-type-specific expression. Methods Mol Biol.
151:121–148. 2001.
|
|
19
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Zeng K, Qiu Y, Yan F and Lin C:
Therapeutic effect of emodin on collagen-induced arthritis in mice.
Inflammation. 36:1253–1259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galasso O, Familiari F, De Gori M and
Gasparini G: Recent findings on the role of gelatinases (matrix
metalloproteinase-2 and −9) in osteoarthritis. Adv Orthop 2012.
Article ID 834208. 2012. View Article : Google Scholar
|
|
22
|
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im
HJ and Chen D: MMP13 is a critical target gene during the
progression of osteoarthritis. Arthritis Res Ther. 15:R52013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vincenti MP and Brinckerhoff CE:
Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in
arthritis: Integration of complex signaling pathways for the
recruitment of gene-specific transcription factors. Arthritis Res.
4:157–164. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobayashi M, Squires GR, Mousa A, Tanzer
M, Zukor DJ, Antoniou J, Feige U and Poole AR: Role of
interleukin-1 and tumor necrosis factor alpha in matrix degradation
of human osteoarthritic cartilage. Arthritis Rheum. 52:128–135.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ha MK, Song YH, Jeong SJ, Lee HJ, Jung JH,
Kim B, Song HS, Huh JE and Kim SH: Emodin inhibits proinflammatory
responses and inactivates histone deacetylase 1 in hypoxic
rheumatoid synoviocytes. Biol Pharm Bull. 34:1432–1437. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang JK, Noh EM, Moon SJ, Kim JM, Kwon
KB, Park BH, You YO, Hwang BM, Kim HJ, Kim BS, et al: Emodin
suppresses inflammatory responses and joint destruction in
collagen-induced arthritic mice. Rheumatology (Oxford).
52:1583–1591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krens SF, Spaink HP and Snaar-Jagalska BE:
Functions of the MAPK family in vertebrate-development. FEBS Lett.
580:4984–4990. 2006. View Article : Google Scholar : PubMed/NCBI
|