Introduction
Cardiovascular disease is one of the main causes of
human death. Viral heart disease (VHD) is a cardiovascular disease
caused by viral infection (1). Viral
myocarditis (VMC) is the main cause of serious cardiac arrhythmias,
acute heart failure, and cardiogenic shock in patients with heart
disease. Some patients will also develop DCM and eventually require
heart transplantation (2). Studies
have shown that ~25–50% of VMCs are associated with viral
infections. A total of >20 viral infections can induce VMC, and
coxsackievirus B3 (CVB3) is the most common source of VMC
infection. (3). There are many
clinical manifestations of acute VMC, such as arrhythmia, atrial
fibrillation and atrioventricular block, and severe sudden death
can occur; the prognosis of most acute myocarditis is better, but
there is a small section that of disease that undergoes transition
to chronic VMC. With progress of the disease, acute and chronic VMC
will have different degrees of myocardial fibrosis at all stages,
and myocardial fibrosis is the main pathological basis for the
development of multiple complications of VMC and myocarditis to
cardiomyopathy (4).
Myocardial fibrosis is the change of collagen fiber
composition in the normal tissue structure of cardiac myocardium
fibers, and changes in the proportion of different collagen
components, resulting in a large number of deposition of collagen
fibers, resulting in disordered collagen metabolism, causing
pathological changes in myocardium (5). Transforming growth factor-β1 (TGF-β1)
is a recognized profibrotic cytokine, a growth factor most closely
related to myocardial fibrosis, and plays an important role in the
process of fibrosis in various organs (6). ADAMTS-1 is a newly discovered
metalloprotease with proteolytic activity in recent years, and is
one of the major members of the metalloprotease family (7). ADAMTS-1 is widely distributed and
expressed in mammalian tissues and organs. It participates in the
pathological process of atherosclerosis and neuropathic injury, and
it is associated with arterial remodeling (8).
At present, the relationship between ADAMTS-1 and
VMC has not been studied. This study investigated the role of
ADAMTS-1 in myocardial fibrosis of VMC by observing the expression
of ADAMTS-1 in myocardial tissue of VMC mouse model in order to
provide clinical reference.
Materials and methods
Experimental animals
A total of 150 purebred inbred Balb/c mice, 3–7
weeks of age, body weight 15–23 g were purchased from Model
Organisms [license no. SCXK (Shanghai) 2005-0002; Shanghai, China].
The mice were kept in a clean environment with an indoor
temperature of 22–26°C and a humidity of 52–58%. Standard cages
were used, with 5 mice in each cage. The mice were maintained in a
12 h light/dark cycle, and were allowed free access to food and
water. This animal study was approved by the Yidu Central Hospital
of Weifang Ethics Committee (Weifang, China).
Virus preparation
The CVB3 Nancy strain (Virus Research Institute of
Wuhan University Medical College, Wuhan, China) was passaged in
HeLa cells (Shanghai Gefan Biotechnology Co. Ltd., Shanghai,
China). After the cells grew to form a confluent monolayer, the
culture fluid was discarded and washed twice with D-Hank's
solution, and the virus was diluted and added to the cells, and the
supernatant was discarded after adsorption at 37°C for 1 h. The
supernatant was then supplemented with 2% calf serum cell culture
medium, and cultured in an incubator at 37°C to establish a normal
cell control to observe the cytopathogenic condition. Diseased and
normal cells were harvested at 24–48 h with cell lesions >50%
(++), and were frozen at −20°C. Diseased and normal cells were
separated by repeated freezing and thawing twice to completely
release the virus particles. The samples were centrifuged at 4°C
and 1,006.2 × g for 30 min. The supernatant was obtained and stored
at −40°C. Selected sensitive cell lines were placed in tissue
culture wells for culture, and maintenance solutions were diluted
and added to culture wells, incubated at 37°C, and cytopathological
conditions were observed by using a microscope (Olympus
Corporation, Tokyo, Japan). One week later, Reed and Muench method
was used to calculate the concentration of 105
TCID50 CVB3, and the sample was restored for further
use.
Animal model establishment
Of the total 150 Balb/c mice, based on the principle
of similar body weight, 50 mice were selected to establish an acute
VMC animal model (acute VMC group), 50 mice were selected to
establish chronic VMC animal model (chronic VMC group), and the
remaining 50 mice were selected as control group. The mice in the
acute and chronic VMC groups were sterilized at the injection site,
and the asepsis inoculation concentration was 105
TCID50/0.1 ml CVB3 0.1 ml. After being fed for 7 days,
an acute VMC animal model was established. In the chronic VMC
group, the same CVB3 was inoculated intraperitoneally every month
after the first vaccination. The monthly dose was gradually
increased by 0.07 ml. After 3 months, a chronic VMC animal model
was established. The control group was injected with virus-free
EMEM at the same time. The number of the mice that died was
counted. The mice were sacrificed by decapitation 7 days and 3
months after the completion of the modeling. A portion of the
myocardium was removed and quickly placed in liquid nitrogen, and
stored at −80°C until use.
Determination of myocardial collagen
in mice
Myocardial tissue was taken to prepare paraffin
sections and refer to the staining methods reported by Rittié
(9) to perform picrosirius red
collagen stain to observe myocardial fibrosis changes. Collagen
volume fraction (CVF, %): myocardial tissue microscopy was
performed by using a T1-T2-200V-200VS optical microscope (Olympus
Corporation). Each section was randomly selected from 10 fields for
microscopy. The myocardium was yellow, and the collagen tissue was
red. Pictures were taken with the Leica camera system (Leica
Microsystems, Ltd., Shanghai, China), and QWin image analysis
system [Keyence (China) Co., Ltd., Shanghai, China] was used for
calculating the percentage of collagen in each field of view, and
the mean number represented CVF (%), which was the severity of
myocardial interstitial fibrosis.
RT-qPCR detection
Total RNA extraction: the myocardial tissue
cryopreserved at −80°C was obtained, and TRIzol reagent [Thermo
Fisher Scientific (China) Co. Ltd.] was used to lyse the solution
after shaking for 30 min at room temperature. The extraction
process was strictly in accordance with the manufacturer's
protocol. The absorption value of RNA was measured by using an
Ultrospec III ultraviolet spectrophotometer (Eppendorf, Hauppauge,
NY, USA), and the purity of the total RNA was measured on a 1%
agarose gel power supply. RT-qPCR assay was performed by using
TGF-β1, ADAMTS-1 fluorescence quantitative PCR kit (Biomiga, Inc.,
San Diego, CA, USA). RT reaction (reaction volume 18.6 µl): RNA 10
µl and oligo(dT) 1.3 µl was added to thin-walled tubes to mix well,
and heated at 65°C for 30 min; 7.3 µl Mix liquid (with 2.3 µl dNTP,
4.0 µl buffer and 1.0 µl M-MLV) was added and mixed, reacted at
37°C for 2.5 h, heated at 65°C for 30 min, and 20 µl cDNA was
diluted to 100 µl with ionized water and stored at −20°C. PCR
reaction: GAPDH as an internal reference. Primer sequences are
shown in Table I. All the primers
were synthesized by Suzhou Hongxun Biotechnologies Co., Ltd.
(Suzhou, China). PCR reaction conditions: pre-denaturation at 94°C
for 3 min, denaturation at 94°C for 30 sec, annealing at 60°C for
30 min, extension at 72°C for 1 min, elongation at 72°C for 10 min,
and a total of 35 cycles of amplification reaction. The
manufacturer's software was used for amplification analysis, and
GAPDH was used as an internal reference gene. The result was
processed using 2−ΔΔCq (10).
 | Table I.Primers for TGF-β1, ADAMTS-1 and GAPDH
gene sequences. |
Table I.
Primers for TGF-β1, ADAMTS-1 and GAPDH
gene sequences.
| Gene name | Upstream primer
sequences | Downstream primer
sequences |
|---|
|
TGF-β1 |
5′-ACCTGCAAGAC-TATCGACATGGAGCTGGTG-3′ |
5′-ACCTGCAAGAC-TATCGACATGGAGCTGGTG-3′ |
| ADAMTS-1 |
5′-AGCATCCCAGCATTAGGA-3′ |
5′-CATGTAGGCACTGCAAGG-3′ |
| GAPDH |
5′-GGTGAAGGTCGGTGTGAACG-3′ |
5′-CTCGCTCCTGGAAGATGGTG-3′ |
Statistical analysis
SPSS 17.0 (Softnet Technology Co., Ltd., Tianjin,
China) was used for statistical analysis. Measured data were
expressed as mean ± standard deviation (means ± SD). The t-test was
used to compare measurement data between two groups. Chi-square
test was used for comparison of enumeration data between the
groups. ANOVA was used to compare multiple groups of means.
Dunnetts test was used for the post-hoc pairwise comparisons.
Pearson's correlation test was used to analyze the correlation
between ADAMTS-1 mRNA and CVF and TGF-β1 mRNA in mice with chronic
VMC. P<0.05 for the difference was considered statistically
significant.
Results
General conditions of mice and
myocardial fibrosis
Nine mice died in the acute VMC group, and the
mortality rate was 18.00% (9/50). Thirteen mice died in the chronic
VMC group, and the mortality rate was 26.00% (13/50). None of the
mice in the control group died. The sex, age, body weight, indoor
temperature and indoor humidity of the three groups of mice had no
effect on this experiment (p>0.05) (Table II). The myocardial tissue of mice
was stained with picrosirius acid red collagen after the
calculation. It was observed that compared with the control group,
the CVF of the myocardial tissue in the acute VMC group was
significantly increased, and the increase of CVF in the myocardial
tissue of the chronic VMC group was the most obvious (p<0.001)
(Fig. 1).
 | Table II.General information of the three
groups of mice. |
Table II.
General information of the three
groups of mice.
| Category | Acute VMC group
(n=41) | Chronic VMC group
(n=37) | Control group
(n=50) | F/χ2 | P-value |
|---|
| Sex, n (%) |
| Male | 26 (63.41) | 19 (51.35) | 34 (68.00) | 2.568 | 0.277 |
|
Female | 15 (36.59) | 18 (48.65) | 16 (32.00) |
|
|
| Age, n (%) |
| ≤5
weeks | 29 (70.73) | 23 (62.16) | 31 (62.00) | 1.040 | 0.595 |
| >5
weeks | 12 (29.27) | 14 (37.84) | 19 (38.00) |
|
|
| Body mass, n (%) |
| ≤19
g | 25 (60.98) | 26 (70.27) | 29 (58.00) | 1.426 | 0.490 |
| <19
g | 16 (39.02) | 11 (29.73) | 21 (42.00) |
|
|
| Indoor temperature
(°C) | 24.09±1.23 | 24.16±1.29 | 24.56±1.46 | 1.642 | 0.197 |
| Indoor humidity
(%) | 55.29±2.15 | 56.16±2.31 | 55.28±1.94 | 2.245 | 0.110 |
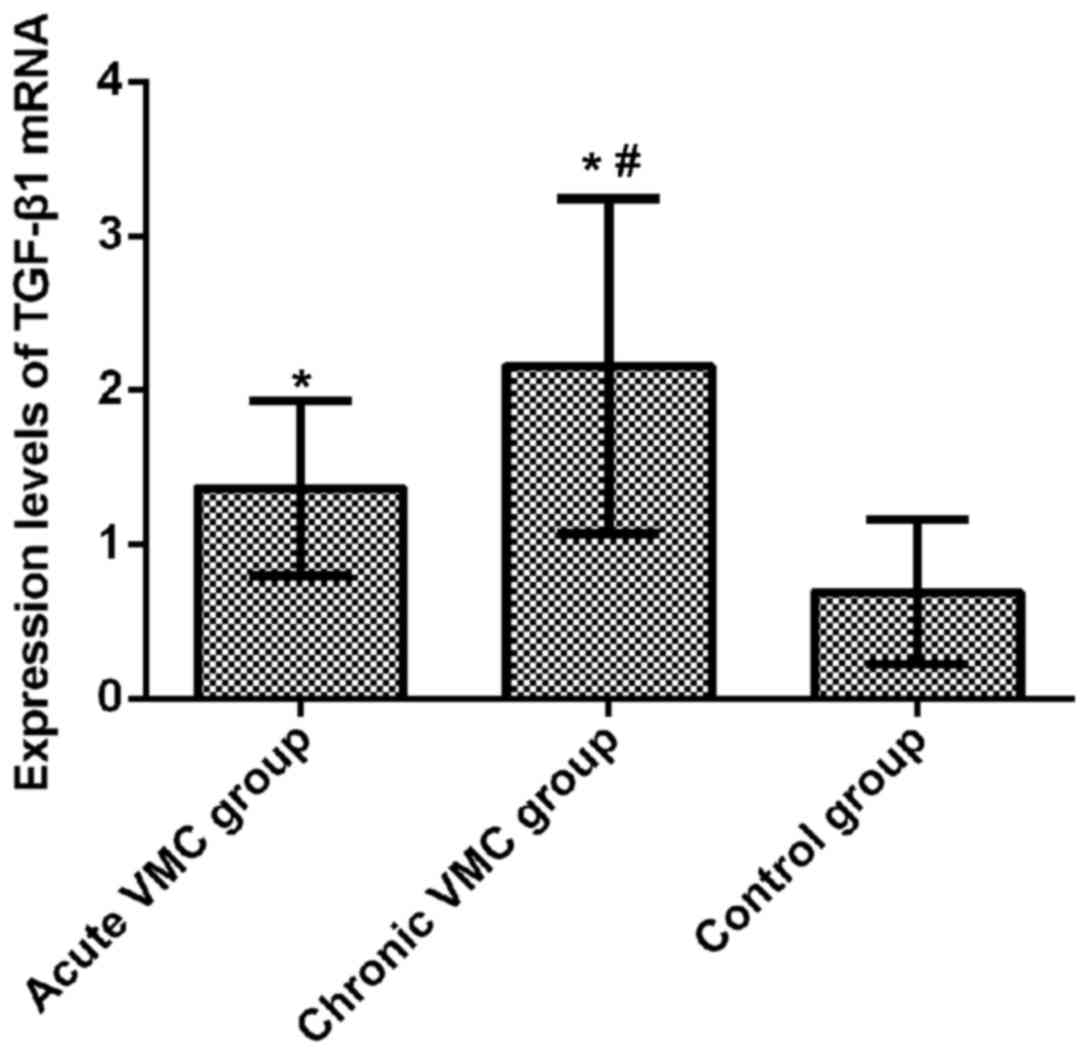
Relative expression of TGF-β1 mRNA in
mouse myocardium
The relative expression levels of TGF-β1 mRNA in
myocardial tissue of the acute and chronic VMC and control groups
were 1.364±0.564, 2.158±1.086, and 0.692±0.470, respectively.
Compared with the control group, the relative expression of TGF-β1
mRNA in myocardial tissue of mice in the acute VMC group was
significantly increased (t=6.201, p<0.001). The relative
expression of TGF-β1 mRNA in myocardial tissue of the chronic VMC
group was significantly increased (t=8.538, p<0.001); the
relative expression of TGF-β1 mRNA in myocardial tissue of the
chronic VMC group was significantly higher than that of the acute
VMC group (t=4.109, p<0.001). The difference between the three
groups was statistically significant (F=43.340, p<0.001)
(Fig. 2).
Relative expression of ADAMTS-1 mRNA
in mouse myocardium
The relative expression levels of ADAMTS-1 mRNA in
myocardial tissue of the acute and chronic VMC and control groups
were 2.697±1.323, 3.781±1.587, and 1.349±0.757, respectively.
Compared with the control group, the relative expression of
ADAMTS-1 mRNA in the myocardial tissue of the acute VMC group was
significantly increased (t=6.094, p<0.001). The relative
expression of ADAMTS-1 mRNA in the myocardial tissue of the chronic
VMC group was significantly increased (t=9.488, p<0.001); the
relative expression of ADAMTS-1 mRNA in myocardial tissue of the
chronic VMC group was significantly higher than that of the acute
VMC group (t=3.288, p<0.001). The difference between the three
groups was statistically significant (F=42.550, p<0.001)
(Fig. 3).
Correlation of ADAMTS-1 mRNA with CVF
and TGF-β1 mRNA in myocardium of the chronic VMC group
Pearson's correlation test results showed that the
relative expression of ADAMTS-1 mRNA was positively correlated with
CVF in the myocardial tissue of mice (r=0.351, p<0.01), and
positively correlated with TGF-β1 mRNA in the mouse myocardium
(r=0.401, p<0.01) (Figs. 4 and
5).
Discussion
There will be a common pathological change in a
variety of heart diseases when they develop to a certain extent,
namely myocardial fibrosis (11).
Myocardial fibrosis is an adaptive reaction in the body, which is
the pathological and physiological reaction process of ventricular
whole body compensation and lesion repair. It is mainly
characterized by the cardiac fibroblast hyperproliferation,
abnormal distribution and arrangement disorder of various types of
collagen (12). Myocardial fibrosis
in the normal tissue of the myocardium showed a large number of
deposition of collagen fibers, and myocardial collagen composition
changed, increasing collagen concentration, which is closely
related to collagen metabolism abnormalities and other factors of
extracellular matrix components of the heart (13). Myocardial fibrosis is one of the
manifestations of myocardial remodeling, and studies have shown
that cell-level damage can alter the original material and heart
morphology, resulting in myocardial cell remodeling and myocardial
collagen network remodeling (14).
Excessive myocardial fibrosis is the pathological
basis of many complications and disease evolution of VHD. In the
acute phase of viral infection, collagen hyperplasia is a
manifestation of compensatory fibrosis. The simultaneous presence
of reactive fibrosis and repair fibrosis in chronic and
convalescent VHD is a major factor affecting cardiac function
(15,16). TGF-β1 is one of the cytokines
involved in the regulation of myocardial fibrosis, and can
stimulate the ECM protein gene to induce myocardial fibrosis
(17). TGF-β1 can promote the
transformation of myocardial tissue collagen gene and cardiac
fibroblasts, promote myocardial synthesis of extracellular matrix,
increase myocardial collagen deposition, resulting in myocardial
fibrosis, which can be used as a growth factor to predict
myocardial fibrosis (18,19). ADAMTS-1 is mainly derived from colon
cancer cells, and is a tumor malignant metamorphosis specific gene.
Its protein molecules have a secretory function, and can be
secreted into the ECM to participate in the regulation of ECM
proteins. It plays an important role in human growth and
development, urogenital organ function, and physiological and
pathological processes of inflammation (20). Dubey et al (21) showed that ADAMTS-1 may activate and
cleave MMP13 and MMP14, thereby reducing the degradation of ECM
proteins by matrix metalloproteinases, and ultimately causing
abnormal follicle excretion. The results of this study showed that
compared with the control group, the relative expression of TGF-β1
and ADAMTS-1 in the myocardial tissue of mice with acute VMC was
significantly increased. With the progress of the disease, the
TGF-β1 in the myocardial tissue of mice with chronic VMC was
significantly increased. The relative expression levels of ADAMTS-1
and ADAMTS-1 were significantly higher than those of the acute VMC
group, suggesting that both of them may be involved in the
occurrence and development of myocardial fibrosis. RT-qPCR was used
to detect TGF-β1 mRNA and ADAMTS-1 mRNA in acute and chronic
phases. The expression of TGF-β1 mRNA and ADAMTS-1 mRNA were
gradually increased with the progress of the disease. The Pearson's
correlation test showed that ADAMTS-1 mRNA was positively
correlated with CVF and TGF-β1 mRNA, suggesting that ADAMTS-1 mRNA
may play a role in promoting myocardial fibrosis during the
development of VHD. This is similar to the study of Mittaz et
al (22). ADAMTS-1 plays an
important role in the occurrence and development of renal
interstitial fibrosis by participating in the regulation of ECM,
promoting renal interstitial fibrosis. Ng et al (23) showed that TGF-β1 can differentially
regulate the expression of ADAMTS-1 mRNA in primary cultures of
decidual stromal cells, but this study failed to explore the
regulatory mechanism between them, which still needs further
verification.
In this study, purebred inbred Balb/c mice were
selected as animal models, and the modeling was simple and
reliable. The injection of CVB3 Nancy strain into the mouse
infection model was able to better simulate the environmental
changes in the body and the clinical pathology of human
myocarditis. The performance is similar, ensuring the reliability
of the experiment. This study did not analyze the regulation
mechanism of ADAMTS-1 in myocardial tissue of the acute and chronic
VMC groups, and did not do in vitro experiments to provide
more theoretical support. Therefore, it is hoped that in the next
study, the regulation mechanism of ADAMTS-1 in myocardium of VMC
myofibrotic animal model can be further discussed.
In summary, ADAMTS-1 is involved in the occurrence
and development of myocardial fibrosis, and is positively
correlated with CVF and TGF-β1. It may play a role in promoting
myocardial fibrosis during the development of VHD, and can be used
as a predictor of myocardial fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and YZ wrote the manuscript and established the
animal model. WS and JL determined the myocardial collagen in mice.
HL, YZ and JX were responsible for RT-qPCR. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang (Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yuan J, Liao YH, Jin XJ, Wang ZH, Yu M,
Chen RZ, Xu DJ, Wei J, Wan J, Zhao DC, et al: Continued elevation
of plasma IL-4 and IL-17 predicts the progression from viral
myocarditis to dilated cardiomyopathy. Eur Heart J. 38 (suppl
1):P51432017. View Article : Google Scholar
|
|
2
|
Clemente-Casares X, Hosseinzadeh S, Barbu
I, Dick SA, Macklin JA, Wang Y, Momen A, Kantores C, Aronoff L,
Farno M, et al: A CD103+ conventional dendritic cell
surveillance system prevents development of overt heart failure
during subclinical viral myocarditis. Immunity. 47:974–989.e8.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Becher PM, Gotzhein F, Klingel K, Escher
F, Blankenberg S, Westermann D and Lindner D: Cardiac function
remains impaired despite reversible cardiac remodeling after acute
experimental viral myocarditis. J Immunol Res. 2017:65906092017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin SA, Lim BK, Kim SK, Seo HJ, Seo BS,
Kwon HJ, Seong SW, Jeong JO and Seong IW: Identification of serum
apurinic/apyrimidinic endonuclease 1/redox effector factor-1
(APE1/Ref-1) in mouse model of viral myocarditis; implication of a
novel biomarker for myocarditis. Eur Heart J. 38 (suppl
1):35152017. View Article : Google Scholar
|
|
5
|
Lurz JA, Luecke C, Lang D, Besler C,
Rommel KP, Klingel K, Kandolf R, Adams V, Schöne K, Hindricks G, et
al: CMR-derived extracellular volume fraction as a marker for
myocardial fibrosis: The importance of coexisting myocardial
inflammation. JACC Cardiovasc Imaging. 11:38–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou B and Yu JW: A novel identified
circular RNA, circRNA_010567, promotes myocardial fibrosis via
suppressing miR-141 by targeting TGF-β1. Biochem Biophys Res
Commun. 487:769–775. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tola EN, Karatopuk DU, Koroglu N, Ergin M
and Oral HB: Follicular ADAMTS-1 and aggrecan levels in polycystic
ovary syndrome. J Assist Reprod Genet. 34:811–816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang H, Lee M, Kim EH, Bishop D and
Rodgers GM: siRNA-knockdown of ADAMTS-13 modulates endothelial cell
angiogenesis. Microvasc Res. 113:65–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rittié L: Method for picrosirius
red-polarization detection of collagen fibers in tissue sections.
Methods Mol Biol. 1627:395–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schelbert EB, Fridman Y, Wong TC, Abu Daya
H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman
P, et al: Temporal relation between myocardial fibrosis and heart
failure with preserved ejection fraction: Association with baseline
disease severity and subsequent outcome. JAMA Cardiol. 2:995–1006.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Francone M: Role of cardiac magnetic
resonance in the evaluation of dilated cardiomyopathy: Diagnostic
contribution and prognostic significance. ISRN Radiol.
2014:3654042014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CY, Su MY, Lin LY, Tsai CT, Wang YC,
Lin YH, Lee JK, Wu CK, Juang JM, Huang JJ, et al: Congenital
hypertrophic cardiomyopathy with genetic mutations is highly
associated with left ventricular myocardial fibrosis and larger
right ventricle (TW-HCM study). Eur Heart J. 38 (suppl_1):40972017.
View Article : Google Scholar
|
|
14
|
Fang L, Ellims AH, Beale AL, Taylor AJ,
Murphy A and Dart AM: Systemic inflammation is associated with
myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy
in patients with hypertrophic cardiomyopathy. Am J Transl Res.
9:5063–5073. 2017.PubMed/NCBI
|
|
15
|
Spartera M, Damascelli A, Mozes F, De
Cobelli F and La Canna G: Three-dimensional speckle tracking
longitudinal strain is related to myocardial fibrosis determined by
late-gadolinium enhancement. Int J Cardiovasc Imaging.
33:1351–1360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arbustini E, Favalli V and Narula N:
Extracellular volume in dilated cardiomyopathy: Interstitial
fibrosis and more? JACC Cardiovasc Imaging. 11:60–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Y, Gupte M, Umbarkar P, Singh AP, Sui
JY, Force T and Lal H: Entanglement of GSK-3β, β-catenin and TGF-β1
signaling network to regulate myocardial fibrosis. J Mol Cell
Cardiol. 110:109–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong SK, Choo EH, Ihm SH, Chang K and
Seung KB: Dipeptidyl peptidase 4 inhibitor attenuates
obesity-induced myocardial fibrosis by inhibiting transforming
growth factor-βl and Smad2/3 pathways in high-fat diet-induced
obesity rat model. Metabolism. 76:42–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dzeshka MS, Appadoo K, Shantsila E,
Snezhitskiy VA and Lip GYH: Increased evidence of left ventricular
myocardial fibrosis in patients with paroxysmal atrial fibrillation
and sinus node dysfunction. Eur Heart J. 38:P17202017. View Article : Google Scholar
|
|
20
|
Salvarani N, Maguy A, De Simone SA,
Miragoli M, Jousset F and Rohr S: TGF-β1 (transforming growth
factor-β1) plays a pivotal role in cardiac myofibroblast
arrhythmogenicity. Circ Arrhythm Electrophysiol. 10:e0045672017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dubey D, McRae PA, Rankin-Gee EK, Baranov
E, Wandrey L, Rogers S and Porter BE: Increased metalloproteinase
activity in the hippocampus following status epilepticus. Epilepsy
Res. 132:50–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mittaz L, Ricardo S, Martinez G, Kola I,
Kelly DJ, Little MH, Hertzog PJ and Pritchard MA: Neonatal calyceal
dilation and renal fibrosis resulting from loss of Adamts-1 in
mouse kidney is due to a developmental dysgenesis. Nephrol Dial
Transplant. 20:419–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ng YH, Zhu H, Pallen CJ, Leung PC and
MacCalman CD: Differential effects of interleukin-1beta and
transforming growth factor-beta1 on the expression of the
inflammation-associated protein, ADAMTS-1, in human decidual
stromal cells in vitro. Hum Reprod. 21:1990–1999. 2006. View Article : Google Scholar : PubMed/NCBI
|