Introduction
Acute respiratory distress syndrome (ARDS) refers to
diffuse alveolar inflammation and damage to the capillary wall
secondary to severe trauma, shock, infection and other factors,
resulting in pulmonary edema and ultimately leading to severe
hyoxemia and carbon dioxide emission disorder (1). The main clinical manifestations of ARDS
are extreme difficulty in breathing, cyanosis, increased heart
rate, and diffuse infiltrated shadow shown in pulmonary X-ray
(2). In recent years, the incidence
rate of ARDS has increased significantly with an annual incidence
of approximately 59/100,000, but the prognosis is still poor, and
the mortality rate is still as high as 30% (3,4).
Clinical diagnosis and treatment of ARDS are very challenging
(5). With the constant research on
ARDS, the current clinical treatment methods of ARDS include
protective pulmonary ventilation therapy, prone position
ventilation, high positive end expiratory pressure (PEEP) and high
frequency ventilation, but they cannot effectively reduce the
fatality rate of ARDS, and some patients eventually suffer from
pulmonary fibrosis, leading to permanent lung dysfunction (6). The pathogenesis of ARDS is complex, one
of which is excessive and extensive inflammatory response of lung
tissues after the attack of various pathogenic factors (7). Glucocorticoids have a potent
anti-inflammatory effect, which can interfere in inflammatory
signaling pathways mediated by various cytokines (8), thereby hindering the occurrence and
development of pulmonary fibrosis. In this study, therefore, the
ARDS rat model was established via oleic acid combined with
lipopolysaccharide (LPS) of Escherichia coli, changes in
tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-10 and
vascular endothelial growth factor (VEGF) in ARDS rats were
observed at different time points, and the effects of dexamethasone
on them were also observed, so as to preliminarily investigate the
pathogenesis of ARDS, and provide a theoretical basis for the
clinical application of dexamethasone in ARDS.
Materials and methods
Materials
Dexamethasone injection (Shandong LukangCisen
Pharmaceutical Co., Ltd., Jining, China, NMPN H37021969), oleic
acid and LPS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
urethane (Shandong Qilu Xinghua Pharmaceutical Co. Ltd., Jining,
China), TNF-α, IL-6, IL-10 and VEGF enzyme-linked immunosorbent
assay (ELISA) kits (Sigma-Aldrich; Merck KGaA), clean bench
(Shanghai Boxun Industrial Co., Ltd., Shanghai, China), electronic
balance (Sartorius AG, Göttingen Germany), and continuous
wavelength multifunctional microplate reader (Tecan Austria GmbH,
Grödje, Austria). This study was approved by the Animal Ethics
Committee of Shihezi University Animal Center (Shihezi, China).
Experimental animal grouping and
preparation
A total of 72 specific pathogen-free (SPF)
Sprague-Dawley (SD) rats (equal number of males and females) aged
12–13 weeks weighing 200–220 g were purchased from Shanghai SLAC
Laboratory Animal Center, Shanghai, China [license no: SCXK
(Shanghai) 2012–0002], and divided into 4 groups using a random
number table: Normal control group (N, n=24), ARDS model group (L,
n=24) and dexamethasone group (D, n=24). Rats in group N were
injected with 5 ml/kg normal saline via the caudal vein, rats in L
were injected with 0.05 ml/kg oleic acid via the caudal vein and
2.5 mg/kg LPS after 30 min, and rats in D were injected with 0.05
ml/kg oleic acid via the caudal vein and 2.5 mg/kg LPS and 6 mg/kg
dexamethasone after 30 min. Rats were kept in cage with controlled
temperature and light cycles (24°C and 12/12 light cycles) and free
access to food and water, humidity was 60±10%.
Observation time points
After rats in the three groups were injected twice,
they were observed at three different time points (4, 8 and 12 h),
8 rats at each time point.
Specimen collection
At corresponding time points, rats were anesthetized
via intraperitoneal injection of 1 g/kg 20% urethane. The blood was
collected from the abdominal aorta and centrifuged at 1,610 × g at
4°C for 15 min to separate the serum, and the serum was stored at
−80°C. The chest was quickly opened, the right hilus of the lung
was ligated, right lung tissues were removed, and the wet weight of
right lung was measured. Then the right lung was baked in a
constant-temperature drying box at 80°C until constant weight, and
the dry weight of right lung was measured. The wet/dry weight ratio
of right lung tissues was calculated. The front end of catheter
with 1.8 mm in external diameter was inserted into the bifurcation
of the lower left principal bronchus, and 2.5 ml normal saline at
37°C was slowly injected via catheter for bronchoalveolar lavage.
Bronchoalveolar lavage fluid (BALF) was recycled, and the lavage
was repeated 5 times. The recycled BALF was collected into a
centrifuge tube and centrifuged at 402 × g at 4°C for 10 min. The
supernatant was separated and stored at −80°C for standby
application.
Detection of inflammatory factors in
serum and BALF
An appropriate amount of serum and BALF was taken,
followed by loading and treatment in strict accordance with
instructions of the ELISA kit. The optical density (OD) value was
detected using the continuous wavelength multifunctional microplate
reader (Tecan Austria GmbH, Grödje, Austria), and the standard
curves were drawn. TNF-α, IL-6, IL-10 and VEGF protein levels in
each sample were calculated.
Statistical analysis
Experimental results are presented as (mean ± SD),
and SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was used for
statistical analysis of data. Independent-samples t-test was used
for the comparison between two groups, and one-way analysis of
variance was used for the comparison among groups and the post hoc
test was Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Wet/dry weight ratio of lung
tissues
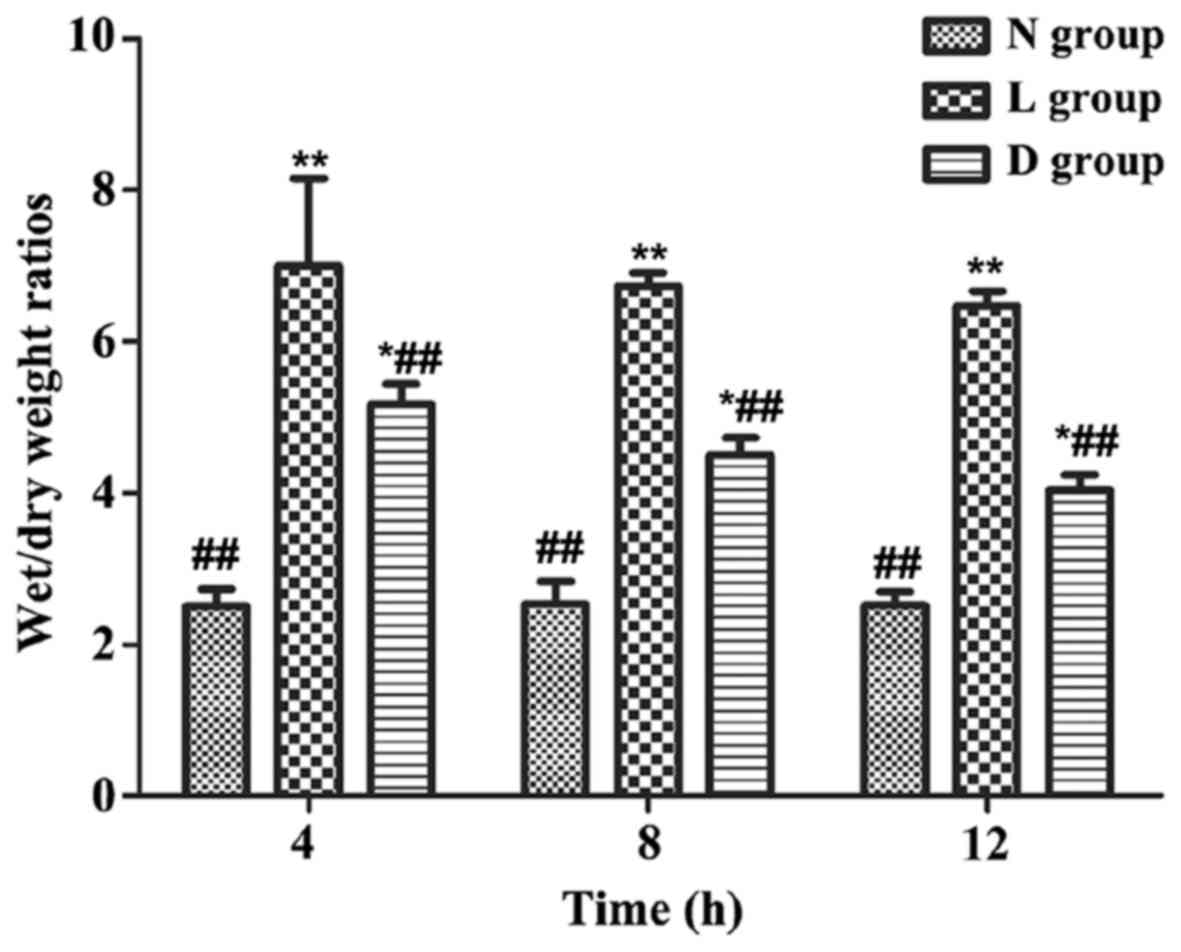
The wet/dry weight ratio of lung tissues of rats in
group L was significantly increased compared with that in group N
(P<0.01). The wet/dry weight ratio of lung tissues of rats in
group D was significantly decreased compared with that in L
(P<0.05 or P<0.01), and the decrease was the most significant
at 12 h, but it was still significantly higher than that in N
(P<0.05 or P<0.01) (Fig.
1).
Changes in serum TNF-α, IL-6, IL-10
and VEGF levels
The levels of serum TNF-α, IL-6 and VEGF in rats in
group L and D were obviously increased compared with those in group
N at each time point (P<0.01), but the levels of IL-10 were
obviously decreased compared with that in group N (P<0.01). The
levels of serum TNF-α, IL-6 and VEGF in rats in group D were
significantly decreased compared with those in L (P<0.01), but
the level of IL-10 was significantly increased compared with that
in group L (P<0.01) (Tables
I–IV).
 | Table I.Changes in serum TNF-α content in rats
(ng/l, n=8). |
Table I.
Changes in serum TNF-α content in rats
(ng/l, n=8).
| Groups | 4 h | 8 h | 12 h |
|---|
| N | 58.39±3.02 | 57.64±3.33 | 58.91±3.87 |
| L |
375.52±8.63a |
358.40±9.19a |
337.65±10.24a |
| D |
261.07±9.40a,b |
228.75±8.66a,b |
202.87±9.91a,b |
 | Table IV.Changes in serum VEGF content in rats
(pg/ml, n=8). |
Table IV.
Changes in serum VEGF content in rats
(pg/ml, n=8).
| Groups | 4 h | 8 h | 12 h |
|---|
| N | 22.05±4.01 | 23.19±3.77 | 22.81±3.64 |
| L |
73.42±4.54a |
70.30±3.68a |
66.99±4.82a |
| D |
47.53±4.22a,b |
38.51±3.80a,b |
34.06±3.56a,b |
Changes in TNF-α, IL-6, IL-10 and VEGF
levels in BALF
The levels of TNF-α, IL-6 and VEGF in BALF of rats
in group L and D were obviously increased compared with those in
group N at each time point (P<0.01), but the levels of IL-10
were obviously decreased compared with that in group N (P<0.01).
The levels of TNF-α, IL-6 and VEGF in BALF of rats in group D were
significantly decreased compared with those in L (P<0.01), but
the level of IL-10 was significantly increased compared with that
in group L (P<0.01) (Tables
V–VIII).
 | Table V.Changes in TNF-α content in BALF of
rats (ng/l, n=8). |
Table V.
Changes in TNF-α content in BALF of
rats (ng/l, n=8).
| Groups | 4 h | 8 h | 12 h |
|---|
| N | 92.37±4.02 | 91.55±3.73 | 91.81±2.96 |
| L |
566.07±10.24a |
528.79±12.11a |
495.38±11.45a |
| D |
420.05±12.55a,b |
406.67±13.20a,b |
387.73±12.97a,b |
 | Table VIII.Changes in VEGF content in BALF of
rats (pg/ml, n=8). |
Table VIII.
Changes in VEGF content in BALF of
rats (pg/ml, n=8).
| Groups | 4 h | 8 h | 12 h |
|---|
| N | 20.36±2.87 | 20.52±3.19 | 19.85±3.74 |
| L |
81.17±4.26a |
76.45±5.08a |
72.63±4.00a |
| D |
56.54±3.81a,b |
48.02±3.44a,b |
29.76±4.62a,b |
Discussion
At present, ARDS animal models include hydrochloric
acid aspiration-type, whole lung lavage-type, endotoxin-type and
oleic acid-type models, among which endotoxin-type and oleic
acid-type models are the most widely used (9). Studies have shown that oleic acid can
directly contract pulmonary arterial vessels, damage the alveolar
capillary endothelium, and cause increased permeability of
pulmonary capillary, and a large amount of fluid and protein
exudation, thus leading to the occurrence of ARDS (10). However, LPS is the major component of
endotoxin, which has less effect on the direct damage to lung
tissues (11). Besides, LPS mainly
activates macrophages, releases a large number of pro-inflammatory
factors, and activates polymorphonuclear leukocytes, thus making
the body enter systemic inflammatory response state. Systemic
inflammatory response can further damage the lung tissues,
eventually resulting in ARDS (12).
Therefore, oleic acid mainly destroys the pulmonary vascular
endothelial cells, causing increased vascular permeability and a
large amount of fluid exudation, and leading to pulmonary edema.
LPS leads to inflammatory response of lung tissues mainly through
the inflammatory cascade reaction. Studies suggest that in
pathogenesis of ARDS, in addition to direct lung damage caused by
trauma, infection and other primary causes, there are also
‘second-strike’ factors due to intestinal bacterial toxins into the
blood and surgical trauma (13).
Therefore, in this study, the ‘second-strike’ ARDS animal model was
established using oleic acid combined with LPS to better simulate
the pathogenetic process of human ARDS. It was observed and found
in this study that after injection of oleic acid and LPS into rats
in group L and group D, cyanosis in four limbs, respiratory
distress, decreased activity, piloerection, pulmonary edema and
rise in inflammatory indexes occurred, indicating successful
modeling.
The pathogenesis of ARDS is very complicated and has
not been fully elucidated so far. In recent years, the research of
a large number of scholars worldwide on its pathogenesis mainly
focuses on the regulatory mechanism of lung tissue inflammation and
pulmonary edema (14). Early ARDS is
characterized by increased permeability of alveolar epithelial
cells and pulmonary capillary endothelial cells, and a large amount
of fluid exudation between alveoli and lung interstitium, while the
exudate mainly contains a variety of inflammatory cells dominated
by neutrophils. Neutrophils can further adhere and gather on the
surface of damaged vascular endothelial cells, and migrate to the
interstitium and alveolar space, promoting the release of a large
number of inflammatory mediators and ultimately exacerbating the
inflammatory response in lung tissues (15).
In trauma, infection occurs in the body, macrophages
are activated, producing and releasing a large amount of TNF-α.
TNF-α can damage the vascular endothelium, destroy its barrier
function, and cause increased capillary permeability and a large
amount of fluid exudation, forming pulmonary edema, and thus
inducing ARDS (16). At the same
time, in the entire course of ARDS, TNF-α can reduce the production
of antioxidants, leading to reduced scavenging of oxygen free
radicals, thereby aggravating tissue damage, and causing disease
exacerbation and progression (17).
At the same time, TNF-α, as the most important pro-inflammatory
factor in early inflammatory response, can promote the formation of
a large number of IL family members, thereby exacerbating the
body's inflammatory response (18).
IL-6 is a key inflammatory factor that induces inflammatory cascade
reaction in ARDS, which is mainly secreted by T cells,
monocytes-macrophages and endothelial cells. Besides, it activates
neutrophils to mediate the secretion of a large number of
acute-phase proteins in the liver, ultimately promoting acute
inflammatory response (19). VEGF is
the strongest vascular permeability factor in the body, which can
increase vascular permeability in multiple organ systems, causing
local exudation and inflammatory response (20). VEGF can increase the permeability of
pulmonary vascular endothelial cells in ARDS, aggravate pulmonary
edema, enlarge and continue the body's inflammatory response,
promoting the occurrence and development of ARDS (21). In the whole course of ARDS, the
degree of interaction between anti-inflammatory factors and
pro-inflammatory factors determines the development direction of
inflammatory response in ARDS. When the secretion of
anti-inflammatory factors is insufficient in the body, it is not
enough to resist the body's inflammatory response, which will lead
to apoptosis, and exacerbate the damage of organ function, thus
increasing the mortality rate of patients (22). Moreover, IL-10 is secreted mainly by
helper T lymphocytes and mononuclear macrophages, and exerts an
anti-inflammatory effect through T cells (23). IL-10 restores the balance between
anti-inflammatory response and inflammatory response via inhibiting
the body's inflammatory response. Studies have found (24) that in the early ARDS patients, the
level of IL-10 in BALF is reduced, so it is not enough to exert an
anti-inflammatory effect, aggravating inflammatory response in the
lung. In this study, it was found that levels of TNF-α, IL-6 and
VEGF in serum and BALF of ARDS rats were significantly increased,
but the IL-10 levels were significantly decreased. It can be seen
that there is an imbalance between anti-inflammatory response and
inflammatory response in ARDS rats, and serious inflammatory
response occurs.
Glucocorticoids have been applied for a long time in
the treatment of ARDS, but their roles in ARDS are still
controversial. Some scholars believe that the long-term application
of high-dose glucocorticoids has side effects, such as
osteoporosis, elevated blood glucose and aggravated infection.
However, the application of glucocorticoids indeed has a positive
effect in the treatment of ARDS in general (25). In this experimental study, it was
found that injecting dexamethasone into ARDS rats via the caudal
vein could significantly alleviate the body's inflammatory response
in ARDS rats, and maintain the balance between anti-inflammatory
response and inflammatory response, thereby reducing inflammatory
exudation of lung tissues and pulmonary edema, and protecting the
lung. Glucocorticoids have a wide range of mechanisms of action in
the treatment of ARDS, which can directly or indirectly act on
inflammatory cells, reduce myeloperoxidase activity, and inhibit
neutrophil activation, thereby alleviating tissue damage (26). At the same time, glucocorticoids can
promote the expression of mitogen-activated protein kinase
phosphatase-1 in lung tissues, and inhibit expression levels of
neutrophil chemokine, monocyte chemoattractant protein-1 and
P-selectin, thereby reducing the inflammatory response in lung
tissues (27). In addition,
glucocorticoids can improve ARDS by alleviating disorders of the
alveolus superficial active substance (7). In case of hypoxia in lung tissues,
glucocorticoids can maintain the activity of some sodium ion
channels, thereby reducing pulmonary edema (28).
In conclusion, this study suggests that there is a
serious imbalance between anti-inflammatory response and
inflammatory response in rats with ARDS induced by oleic acid
combined with LPS of Escherichia coli, whereas dexamethasone
can maintain the balance between anti-inflammatory response and
inflammatory response through inhibiting expression levels of
inflammatory factors (TNF-α, IL-6 and VEGF) and promoting the
expression of anti-inflammatory factor (IL-10) in serum and BALF,
thus alleviating lung tissue injury. The inflammatory response
process of ARDS is very complex, involving a variety of cytokines,
and other related cytokines remain to be further studied. At the
same time, the occurrence mechanism of ARDS is complicated, and
whether glucocorticoids can improve ARDS through other signaling
pathways also needs to be further studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published manuscript.
Authors' contributions
MQ designed the study and prepared the manuscript,
ZQ was responsible for data collection and analysis, and MQ for
fund collection. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Shihezi University Animal Center (Shihezi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van den Steen PE, Deroost K, Deckers J,
Van Herck E, Struyf S and Opdenakker G: Pathogenesis of
malaria-associated acute respiratory distress syndrome. Trends
Parasitol. 29:346–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kong MY, Li Y, Oster R, Gaggar A and
Clancy JP: Early elevation of matrix metalloproteinase-8 and −9 in
pediatric ARDS is associated with an increased risk of prolonged
mechanical ventilation. PLoS One. 6:e225962011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Del Sorbo L and Slutsky AS: Ventilatory
support for acute respiratory failure: New and ongoing
pathophysiological, diagnostic and therapeutic developments. Curr
Opin Crit Care. 16:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grasso S, Stripoli T, De Michele M, Bruno
F, Moschetta M, Angelelli G, Munno I, Ruggiero V, Anaclerio R,
Cafarelli A, et al: ARDS net ventilatory protocol and alveolar
hyperinflation: Role of positive end-expiratory pressure. Am J
Respir Crit Care Med. 176:761–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janz DR and Ware LB: Biomarkers of
ALI/ARDS: Pathogenesis, discovery, and relevance to clinical
trials. Semin Respir Crit Care Med. 34:537–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CT, Lin HH, Ruan SY, Lee MS, Tsai YJ
and Yu CJ: Efficacy and adverse events of high-frequency
oscillatory ventilation in adult patients with acute respiratory
distress syndrome: A meta-analysis. Crit Care. 18:R1022014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mokra D, Drgova A, Kopincova J, Pullmann R
and Calkovska A: Anti-inflammatory treatment in dysfunction of
pulmonary surfactant in meconium-induced acute lung injury. Adv Exp
Med Biol. 756:189–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HA, Park JH, Lee S, Choi JS, Rhim T
and Lee M: Combined delivery of dexamethasone and plasmid DNA in an
animal model of LPS-induced acute lung injury. J Control Release.
156:60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matute-Bello G, Frevert CW and Martin TR:
Animal models of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L379–L399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pierrakos C, Karanikolas M, Scolletta S,
Karamouzos V and Velissaris D: Acute respiratory distress syndrome:
Pathophysiology and therapeutic options. J Clin Med Res. 4:7–16.
2012.PubMed/NCBI
|
|
11
|
Ghosh S, Wilson MR, Choudhury S, Yamamoto
H, Goddard ME, Falusi B, Marczin N and Takata M: Effects of inhaled
carbon monoxide on acute lung injury in mice. Am J Physiol Lung
Cell Mol Physiol. 288:L1003–L1009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Z, Kozlowski J and Schuster DP:
Physiologic, biochemical, and imaging characterization of acute
lung injury in mice. Am J Respir Crit Care Med. 172:344–351. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matthay MA, Zimmerman GA, Esmon C,
Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr,
Hoffman E, Hubmayr RD, et al: Future research directions in acute
lung injury: Summary of a National Heart, Lung, and Blood Institute
working group. Am J Respir Crit Care Med. 167:1027–1035. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prabhakaran P: Acute respiratory distress
syndrome. Indian Pediatr. 47:861–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson BT and Matthay MA: The Berlin
definition of ARDS versus pathological evidence of diffuse alveolar
damage. Am J Respir Crit Care Med. 187:675–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seybold J, Thomas D, Witzenrath M, Boral
S, Hocke AC, Bürger A, Hatzelmann A, Tenor H, Schudt C, Krüll M, et
al: Tumor necrosis factor-alpha-dependent expression of
phosphodiesterase 2: Role in endothelial hyperpermeability. Blood.
105:3569–3576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YD, Liu W and Liu Z: Influence of
long-term drinking alcohol on the cytokines in the rats with
endogenous and exogenous lung injury. Eur Rev Med Pharmacol Sci.
17:403–409. 2013.PubMed/NCBI
|
|
18
|
Li T, Luo N, Du L, Liu J, Gong L and Zhou
J: Early and marked up-regulation of TNF-α in acute respiratory
distress syndrome after cardiopulmonary bypass. Front Med.
6:296–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MacLaren R and Stringer KA: Emerging role
of anticoagulants and fibrinolytics in the treatment of acute
respiratory distress syndrome. Pharmacotherapy. 27:860–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mura M, dos Santos CC, Stewart D and Liu
M: Vascular endothelial growth factor and related molecules in
acute lung injury. J Appl Physiol (1985). 97:1605–1617. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cabebe E and Wakelee H: Sunitinib: A newly
approved small-molecule inhibitor of angiogenesis. Drugs Today
(Barc). 42:387–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhatia M and Moochhala S: Role of
inflammatory mediators in the pathophysiology of acute respiratory
distress syndrome. J Pathol. 202:145–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miura Y, Nishimura Y, Katsuyama H, Maeda
M, Hayashi H, Dong M, Hyodoh F, Tomita M, Matsuo Y, Uesaka A, et
al: Involvement of IL-10 and Bcl-2 in resistance against an
asbestos-induced apoptosis of T cells. Apoptosis. 11:1825–1835.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fumeaux T and Pugin J: Role of
interleukin-10 in the intracellular sequestration of human
leukocyte antigen-DR in monocytes during septic shock. Am J Respir
Crit Care Med. 166:1475–1482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meduri GU, Marik PE, Chrousos GP, Pastores
SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G and Annane D: Steroid
treatment in ARDS: A critical appraisal of the ARDS network trial
and the recent literature. Intensive Care Med. 34:61–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sombra MA, Vasconcelos MP, Guimarães SB,
Escalante RD, Garcia JH and Vasconcelos PR: Acute pulmonary injury
induced by experimental muscle trauma. Acta Cir Bras. 26 Suppl
1:43–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yubero S, Manso MA, Ramudo L, Vicente S
and De Dios I: Dexamethasone down-regulates the inflammatory
mediators but fails to reduce the tissue injury in the lung of
acute pancreatitis rat models. Pulm Pharmacol Ther. 25:319–324.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Urner M, Herrmann IK, Booy C,
Roth-Z'Graggen B, Maggiorini M and Beck-Schimmer B: Effect of
hypoxia and dexamethasone on inflammation and ion transporter
function in pulmonary cells. Clin Exp Immunol. 169:119–128. 2012.
View Article : Google Scholar : PubMed/NCBI
|