Introduction
Chronic heart failure (CHF) is one of the
manifestations and the main causes of death of various
cardiovascular diseases in the terminal stage (1). Prevention and treatment for CHF in
coronary heart disease are one of the impotant research issues
world-wide. The pathogenesis of CHF is very complicated, and it is
considered that the cardiac overload, mainly characterized by
pathological remodeling of the left ventricle, involving various
factors and primarily manifested as neuroendocrinology, is the main
cause of CHF (2). Traditional
Chinese medicine has certain advantages in the treatment of CHF,
such as minor side effects, improvement of symptoms, whole
recuperative medical care and improvement of the patient's quality
of life. The main components of Qishen Yiqi dropping pills are
Danshen (Radix Salviae Miltiorrhizae), Huangqi (radix astragali),
Sanqi (radix notoginseng) and Jiangxiang oil (Lignum Dalbergiae
Odoriferae Oil), and the pills have effects of activating blood
circulation, relieving pain, and promoting circulation of
qi. Qishen Yiqi dropping pills have been proved to have
certain curative effects in the treatment of CHF with few adverse
reactions (3). However, there is
still no report on the exact mechanism of CHF treatment with Qishen
Yiqi dropping pills. This study aims to analyze the protective
effect of Qishen Yiqi dropping pills on the myocardium of rats by
establishing the rat model of CHF with Sprague-Dawley (SD) rats as
subjects.
Materials and methods
Experimental animals and grouping
A total of 60 specific-pathogen-free (SPF) SD male
rats weighing 250–360 g, with an average weight of 280±15 g were
purchased from Shanghai SLAC Laboratory Animal Center Co., Ltd
(Shanghai, China). The rats were fed to acclimate for 1 week. They
were kept in cages in the dark at 25°C with enough millet and cold
boiled water. A total of 60 rats were randomly divided into the
sham operation (n=20), the model (n=20) and Qishen Yiqi dropping
pill treatment (n=20) groups.
The study was approved by the Ethics Committee of
Zhengzhou Central Hospital Affiliated to Zhengzhou University
(Zhengzhou, China).
Main reagents
Qishen Yiqi dropping pills (Tasly Pharmaceutical
Group Co., Ltd., Shanghai, China; NMPN Z20030139), hydrated chloral
mixture (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) detection kit (Shanghai Ming Bo Biological Technology Co.,
Ltd., Shanghai, China) were used in the study. Other reagents were
analytically pure and made in China.
Main instruments
Instruments used in the study were: Clean bench
(Suzhou Purification Equipment Co., Ltd., Suzhou, China), desk-type
cryogenic high-speed refrigerated centrifuge (Taicang Medical
Appliance Factory, Jiangsu, China), ultraviolet spectrophotometer
and electrophoresis apparatus (Shanghai No. 3 Analytical Instrument
Factory, Shanghai, China), BS223S type electronic balance
(Sartorius Scientific Instruments Co., Ltd., Beijing, China), and
iChem-530 automatic biochemical analyzer (Shenzhen ICUBIO
Biotechnology Co., Ltd., Shenzhen, China).
Establishment of the rat model of
CHF
The CHF model was established via coronary artery
ligation. Hydrated chloral mixture was intraperitoneally injected
to anaesthetize the potruncus of rats for skin preparation, rats
were connected to small animal ventilator. Τhe skin at the
pulsating cardiac apex was cut to expose the heart, and the
anterior descending coronary artery was cut with a 0-gauge suture.
The myocardial ischemic changes for 30 min displayed in an
electrocardiogram indicated a successful surgery. After the model
was successfully established, the heart was quickly laid back, and
the chest wall was sutured. Intramuscular injection of penicillin
was conducted to prevent infection for 7 consecutive days. In the
sham operation group, the left anterior descending coronary artery
was only occluded with sutures but not ligated after thoracotomy.
The other procedures were the same as above.
Administration methods
On the 7th day after modeling, the treatment group
was intragastrically administered with Qishen Yiqi dropping pills
(0.135 g/kg) once a day for 4 consecutive weeks. The model and the
sham operation groups were given the same amount of normal
saline.
Observational indexes
Material drawing
Within 24 h after the last administration, the rats
were sacrificed, the heart was isolated, the bilateral atria and
right ventricle were removed, the left ventricle was taken, and
some of the fresh left ventricular myocardia were taken. Part of
the myocardia were applied for reverse transcription-polymerase
chain reaction (RT-PCR) and western blot analysis, and part of the
left ventricle tissues were fixed with 4% paraformaldehyde for
histological examination.
Histopathological detection
Myocardial tissues were taken, routinely embedded in
paraffin and sectioned. In a staining jar, paraffin sections were
washed with xylene and eluted with gradient alcohol. Hematoxylin
and eosin (H&E) staining was performed, followed by observation
under an optical microscope (Shanghai Yongke Optical Instrument
Co., Ltd., Shanghai, China).
Detection of cell apoptosis via
TUNEL
Myocardial tissues were taken and routinely embedded
in paraffin and sectioned. In a staining jar, paraffin sections
were rinsed in xylene and eluted by using gradient alcohol. The
tissues were treated with proteinase K for 15–30 min and washed
with phosphate-buffered saline (PBS), followed by addition of TUNEL
reaction mixture. After the glass slide was air-dried, 50 µl TUNEL
reaction mixtures were added to samples for reaction in a wet box
at 37°C for 1 h, followed by rinsing with PBS three times. Then
50–100 µl diaminobenzidine (DAB) substrates were added for reaction
at 15–25°C for 10 min, followed by washing with PBS three times.
Subsequently, the tissues were counterstained with hematoxylin, and
dehydrated with gradient alcohol and made transparent with xylene,
followed by mounting using neutral gums. Apoptotic cells (200–500
cells in total) were observed under an optical microscope. The
apoptosis index was calculated according to apoptosis index = the
number of apoptotic cells × 100%/total cell number.
Detection of myocardial infarction
area
Heart samples were taken and immediately frozen in
liquid nitrogen. The samples were continuously sectioned along the
long axis of the heart from the ligation point to the cardiac apex
and stained with triphenyltetrazolium chloride (TTC). Through
observation by naked eye, normal tissues were brick-red, and
tissues in the ischemic area were gray-white. Infarction area was
calculated by using image processing software. Infarction area =
ischemic area/total area of the left ventricle × 100%.
Detection of the expression levels of
transforming growth factor-β1 (TGF-β1), mothers against
decapentaplegic homolog 2 (Smad2), Smad3, and caspase-3 messenger
ribonucleic acids (mRNAs) via RT-PCR
The total RNA was extracted from thoracic aorta
tissues with TRIzol, and RT-PCR amplification was performed with
RT-PCR kit (Thermo fisher, Waltham, MA, USA). Pre-denaturation at
94°C for 10 min, denaturation at 94°C for 15 sec and renaturation
at 60°C for 60 sec for 45 cycles. Primer sequences: Smad2-forward:
5′-CTTGACGCAGGGACTGTCCA-3′ and Smad2-reverse:
5′-ACCTCTTTGAGCGCCACTAC-3′, with the product length of 129 bp,
Smad3-forward: 5′-CTTGGTGCAGAGACTGTCA-3′ and Smad3-reverse:
5′-TTCTCTGTGATTGCCACTGC-3′, with the product length of 129 bp,
caspase-3-forward: 5′-CCAACTGCAGACTGTCCAGA-3′ and
caspase-3-reverse: 5′-CAGGCTCCAGAAGAAGTTGG-3′, TGF-β1-forward:
5′-TGAGTGGCTGTCTTTTGACG-3′ and TGF-β1-reverse:
5′-ACTGAAGCGAAAGCCCTGTA-3′, and β-actin-forward:
5′-GTCAGGTCATCACTATCGGCAAT-3′ and β-actin-reverse:
5′-AGAGGTCTTTACGGATGTCAACGT-3′.
Detection of the expression levels of
TGF-β1, Smad2, Smad3 and caspase-3 proteins via western blot
analysis
Thoracic aorta tissues were cut up with scissors,
and the total protein was extracted by using pre-cooled tissue
lysates. Bradford assay was applied to determine the protein
content of samples, and 12% gels were used for protein separation.
Proteins on the gels were transferred onto a polyvinylidene
fluoride (PVDF) membrane by using membrane transfer equipment (wet
transfer) at 100 V for 1.5 h and blocked with skim milk powder for
2 h. After membrane washing, the proteins bound to TGF-β1, Smad2,
Smad3 and caspase-3 monoclonal antibodies (1:1,000) overnight,
followed by color development using DAB. The gel imaging and
chemiluminescence analysis system were employed to collect
chromogenic bands. The chemiluminescence analysis kit was purchased
from Shanghai Xin Yu Biological Technology Co., Ltd. (Shanghai,
China). Quantity One software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was applied for protein band data analysis.
Statistical analysis
In statistical analysis, all data were analyzed and
processed by using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). The
χ2 test was conducted for enumeration data. Measurement
data are expressed as (mean ± SD), and the t-test was performed at
the same time. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histopathological results
In the sham operation group, cardiomyocytes were
stained evenly and arranged neatly and densely with clear
structures. In the model group, the cell morphology was fuzzy, the
myocytes were hypertrophied, the nuclear pyknosis was fragmented,
the arrangement was disordered, the intercellular space was
narrowed, and the cytoplasm was missing. In the Qishen Yiqi
dropping pill treatment group, the cell morphology tended to be
normal (Fig. 1).
Cell apoptosis degree
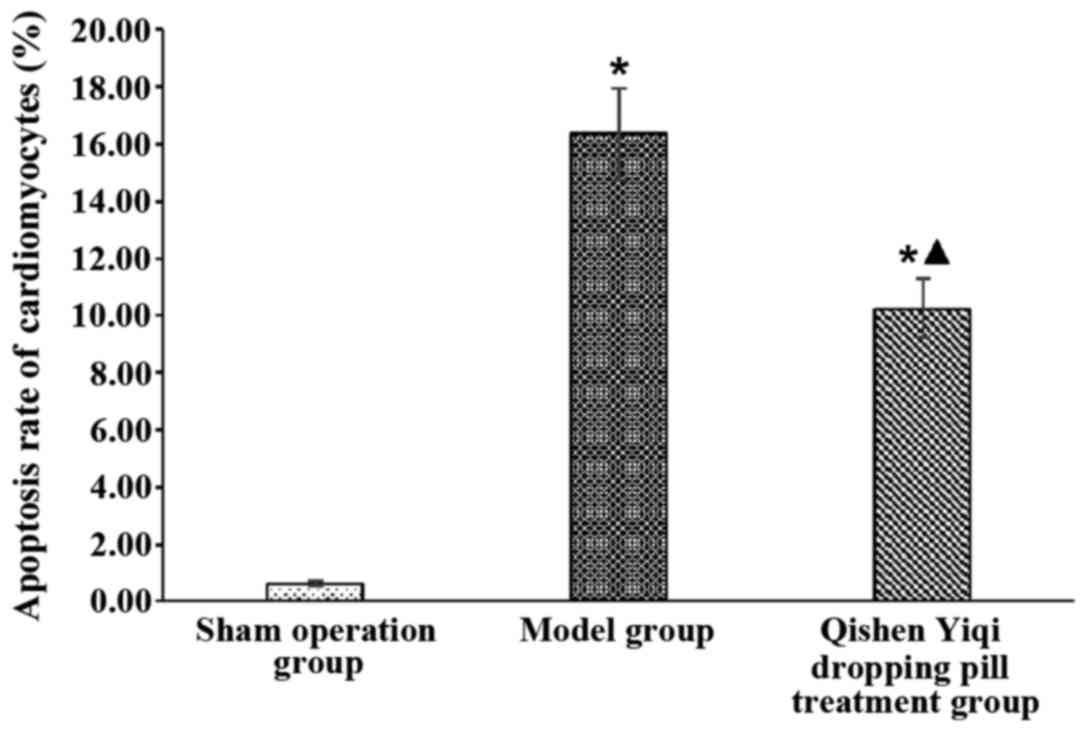
The apoptotic rates of cardiomyocytes in the sham
operation, the model and the Qishen Yiqi dropping pill treatment
group were 0.68±0.22%, 16.35±3.58% and 10.25±2.28%, respectively.
The apoptosis rates of cardiomyocytes in the model group and the
Qishen Yiqi dropping pill treatment were significantly higher than
that in the sham operation group (P<0.05), and there was a
significant difference in the comparison between the model and the
Qishen Yiqi dropping pill treatment group (P<0.05) (Fig. 2).
Myocardial infarction area
The apoptosis rates of cardiomyocytes in the sham
operation, the model, and the Qishen Yiqi dropping pill treatment
group were 0.72±0.25%, 38.56±6.89% and 22.15±5.15%, respectively.
The myocardial infarction areas in the model group and the Qishen
Yiqi dropping pill treatment were remarkably larger than that in
the sham operation group (P<0.05), and there was an obvious
difference in the comparison between the model and the Qishen Yiqi
dropping pill treatment group (P<0.05) (Fig. 3).
RT-PCR and western blot analysis
detection results
The expression levels of TGF-β1, Smad2, Smad3, and
caspase-3 mRNAs and proteins in the model group and the Qishen Yiqi
dropping pill treatment group were obviously higher than those in
the sham operation group (P<0.05), and there were significant
differences in the comparison between the model and the Qishen Yiqi
dropping pill treatment group (P<0.05) (Tables I and II).
 | Table I.Comparison of the expression levels of
TGF-β1, Smad2, Smad3 and caspase-3 mRNAs at different time points
in each group of rats (mean ± SD, n=5). |
Table I.
Comparison of the expression levels of
TGF-β1, Smad2, Smad3 and caspase-3 mRNAs at different time points
in each group of rats (mean ± SD, n=5).
| Groups | TGF-β1 | Smad2 | Smad3 | Caspase-3 |
|---|
| Sham operation | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| Model |
6.23±1.28a |
7.25±1.22a |
7.87±1.44a |
5.33±0.85a |
| Qishen Yiqi dropping
pill treatment |
2.53±0.85a,b |
4.60±0.62a,b |
4.46±0.55a,b |
2.85±0.85a,b |
 | Table II.Comparison of the expression levels of
TGF-β1, Smad2, Smad3 and caspase-3 proteins at different time
points in each group of rats (mean ± SD, n=5). |
Table II.
Comparison of the expression levels of
TGF-β1, Smad2, Smad3 and caspase-3 proteins at different time
points in each group of rats (mean ± SD, n=5).
| Groups | TGF-β1 | Smad2 | Smad3 | Caspase-3 |
|---|
| Sham operation | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| Model |
5.11±0.83a |
4.39±0.83a |
5.89±0.35a |
2.83±0.51a |
| Qishen Yiqi dropping
pill treatment |
3.51±0.53a,b |
3.29±0.75a,b |
3.58±0.55a,b |
1.80±0.26a,b |
Discussion
Chronic heart failure (CHF) is a complex clinical
syndrome of ventricular ejection or filling disorders due to
structural or functional abnormalities in the heart. CHF not only
has a high prevalence rate but also has a poor prognosis, and it
has become one of the leading causes of death among the elderly
(4,5). Most scholars agree that the
pathological remodeling of the left ventricle is the most important
pathophysiological mechanism of the occurrence of CHF. Decreased
cardiac function and left ventricular hypertrophy are the main
signs of left ventricular remodeling. In current clinical treatment
for CHF, β-blockers and angiotensin-converting enzyme inhibitors
are used to inhibit neuroendocrine activation during heart failure
generally based on the symptomatic treatments such as vasodilation,
cardiac arrest and diuresis so as to delay and prevent myocardial
remodeling, thereby reducing the patient's hospitalization and
mortality rates (6,7). However, in general, the overall
efficacy of western medicine in the treatment of CHF is
unsatisfactory.
The treatment of CHF with traditional Chinese
medicine has a long history, which can alleviate the symptoms of
patients, improve their quality of life, and delay the occurrence
and development of CHF. In the theory of Traditional Chinese
medicine, CHF has been included in the category of syndromes such
as ‘edema’, ‘thoracic obstruction’, ‘dyspnea with cough’ and
‘palpitation’. It is believed that due to multiple etiologies, the
body's qi, blood, yin and yang are impaired. Zang-Fu
imbalance leads to internal retention of water with extravasated
blood. The pathogenesis of the disease is blood stasis, the
heart-yang, water-rheum collecting internally and heart-qi
deficiency, and its treatment is based on the principles of
promoting blood circulation and collaterals and replenishing yang.
Qishen Yiqi dropping pills are a traditional Chinese medicine
preparation composed of Danshen, Huangqi, Jiangxiang and Sanqi,
which have the functions of activating blood circulation, relieving
pain and promoting circulation of qi. With a stable dosage
form and reliable clinical efficacy, it is one of the
representative drugs in the treatment of heart disease with
traditional Chinese medicine (8–10).
Previous studies have revealed that Qishen Yiqi
dropping pills play roles in expanding coronary blood vessels,
increasing coronary sinus blood oxygen content and coronary blood
flow volume, improving myocardial blood and oxygen supply, reducing
myocardial oxygen consumption index, elevating cardiac stroke
output and cardiac output, increasing the maximum rate of increase
of left ventricular pressure, and adjusting cardiac compliance
(11,12). At the same time, Qishen Yiqi dropping
pills can lower the platelet aggregation rate, reduce the thickness
of aortic plaques, tend to reduce the area of aortic plaques, and
have the effects of preventing and treating atherosclerosis and
anti-lipid peroxidation (13). The
myocardial protective effect of Qishen Yiqi dropping pills on CHF
rats was investigated in this study. The results indicated that
after treatment with Qishen Yiqi dropping pills, the morphology of
myocardial cells tended to be normal, and the rate of apoptosis and
myocardial infarction area were decreased, suggesting that Qishen
Yiqi dropping pills can protect the myocardium, and its possible
mechanism is to reduce the degree of myocardial cell apoptosis and
inhibit the fibrosis of myocardial cells.
Increased expression level of TGF-β1 in the vascular
wall can promote vascular smooth muscle cell proliferation,
migration, extracellular matrix deposition, lipid accumulation in
the arterial wall and inflammatory cell infiltration, and these
factors are all crucial steps in myocardial cell fibrosis (14). As the main downstream mediators of
TGF-β1, Smad2 and Smad3 play important roles in the process of
myocardial fibrosis (15). After
phosphorylation, Smad2/3 can bind to Smad4 to form a complex
involved in the regulation of gene transcription, so as to promote
the expression of collagens as well as the formation and
progression of myocardial fibrosis. Caspase-3 is one of the
important genes for apoptosis. The results of RT-PCR and western
blot analysis in this study manifested that Qishen Yiqi dropping
pills might improve the degree of myocardial fibrosis by
suppressing the TGF-β1/Smads pathway and inhibit apoptosis of
cardiomyocytes by impeding the caspase-3 signaling pathway, thus
protecting the myocardium.
In summary, Qishen Yiqi dropping pills obviously
protect the myocardium of CHF rats, which may improve the degree of
myocardial fibrosis by impeding the TGF-β1/Smads pathway and
improve cardiomyocyte apoptosis by inhibiting the caspase-3
signaling pathway, so as to play a role in protecting the
myocardium. However, since traditional Chinese medicine is
characterized by multiple components, multiple targets and
comprehensive treatments, the conclusions of this study have to be
further verified.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and DW wrote the manuscript. YL, DW and XY
established the rat model of CHF. MW and ZL helped with TUNEL. XB
and CZ performed PCR. HJ was responsible for western blot analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhengzhou Central Hospital Affiliated to Zhengzhou University
(Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing inetrests
The authors declare they have no competing
interests.
References
|
1
|
Abraham WT, Stevenson LW, Bourge RC,
Lindenfeld JA, Bauman JG and Adamson PB: CHAMPION Trial Study
Group: Sustained efficacy of pulmonary artery pressure to guide
adjustment of chronic heart failure therapy: Complete follow-up
results from the CHAMPION randomised trial. Lancet. 387:453–461.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma A, Lavie CJ, Borer JS, Vallakati A,
Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D and Lazar JM:
Meta-analysis of the relation of body mass index to all-cause and
cardiovascular mortality and hospitalization in patients with
chronic heart failure. Am J Cardiol. 115:1428–1434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
JianXin C, Xue X, ZhongFeng L, Kuo G,
FeiLong Z, ZhiHong L, Xian W and HongCai S: Qishen Yiqi Drop Pill
improves cardiac function after myocardial ischemia. Sci Rep.
6:243832016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Satake H, Fukuda K, Sakata Y, Miyata S,
Nakano M, Kondo M, Hasebe Y, Segawa M and Shimokawa H: CHART-2
Investigators: Current status of primary prevention of sudden
cardiac death with implantable cardioverter defibrillator in
patients with chronic heart failure - A report from the CHART-2
Study. Circ J. 79:381–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Troughton RW, Frampton CM, Brunner-La
Rocca HP, Pfisterer M, Eurlings LW, Erntell H, Persson H, O'Connor
CM, Moertl D, Karlström P, et al: Effect of B-type natriuretic
peptide-guided treatment of chronic heart failure on total
mortality and hospitalization: An individual patient meta-analysis.
Eur Heart J. 35:1559–1567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaggin HK, Szymonifka J, Bhardwaj A,
Belcher A, De Berardinis B, Motiwala S, Wang TJ and Januzzi JL Jr:
Head-to-head comparison of serial soluble ST2, growth
differentiation factor-15, and highly-sensitive troponin T
measurements in patients with chronic heart failure. JACC Heart
Fail. 2:65–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arzt M, Woehrle H, Oldenburg O, Graml A,
Suling A, Erdmann E, Teschler H and Wegscheider K: SchlaHF
Investigators: prevalence and predictors of sleep-disordered
breathing in patients with stable chronic heart failure: The
SchlaHF Registry. JACC Heart Fail. 4:116–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Q and Cao Y: Study on mechanisms and
myocardial protective effect of Qishen Yiqi dropping pills on rats
with myocardial infarction. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
29:501–505. 2017.(In Chinese). PubMed/NCBI
|
|
9
|
Yu FC, Xu YJ, Tong JY, Lu ZZ and Zhang XH:
Therapeutic effects of Qishen Yiqi Dropping Pill on myocardial
injury induced by chronic hypoxia in rats. Chin J Nat Med.
13:776–780. 2015.PubMed/NCBI
|
|
10
|
Cui ZT, Wei WL, Liu M and Wang WJ: Effect
of pretreatment with qishen yiqi dropping pills on right cardiac
function of patients undergoing valve replacement. Zhongguo Zhong
Yao Za Zhi. 39:916–919. 2014.(In Chinese). PubMed/NCBI
|
|
11
|
Liu W, Gao FF, Li Q, Lv JW, Wang Y, Hu PC,
Xiang QM and Wei L: Protective effect of astragalus polysaccharides
on liver injury induced by several different chemotherapeutics in
mice. Asian Pac J Cancer Prev. 15:10413–10420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Lu L, Wang Y, Wu Y, Han J, Wang W,
Li C and Tu P: Qishenyiqi Dropping Pill attenuates myocardial
fibrosis in rats by inhibiting RAAS-mediated arachidonic acid
inflammation. J Ethnopharmacol. 176:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Wang Q, Li C, Lu L, Zhang Q, Zhu R
and Wang W: A review of Chinese herbal medicine for the treatment
of chronic heart failure. Curr Pharm Des. 23:5115–5124.
2017.PubMed/NCBI
|
|
14
|
Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong
X, Li W and Xuan J: Suppression of TGF-β1/Smad signaling pathway by
sesamin contributes to the attenuation of myocardial fibrosis in
spontaneously hypertensive rats. PLoS One. 10:e01213122015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Fan Q, He S, Tang T, Liao Y and Xie
J: MicroRNA-21 negatively regulates Treg cells through a
TGF-β1/Smad-independent pathway in patients with coronary heart
disease. Cell Physiol Biochem. 37:866–878. 2015. View Article : Google Scholar : PubMed/NCBI
|