Introduction
Liver fibrosis is an important stage in the
development of various liver diseases, which is associated with the
activation of hepatic stellate cells (HSCs) (1), the promotion of extracellular matrix
synthesis and degradation, the induction of inflammatory and
fibrous correlation factor expression and the mediation of liver
cell apoptosis (2). Transforming
growth factor (TGF)-β1 serves a vital role in the activation and
transformation of HSCs, and promotes the expression of
extracellular matrix molecules in these cells (3,4). In this
process, TGF-β1 combines with the intracellular receptor,
drosophila mothers against decapentaplegic protein (Smad), and
mediates TGF-β1 signaling pathways (5).
A previous study has suggested that the activated
TGF-β1 signaling pathway may induce epithelial-mesenchymal
transition (EMT) of HSCs (6). During
this process, the expression of the epithelial component,
E-cadherin, is downregulated, while that of the mesenchymal
component, α-smooth muscle actin (α-SMA)/vitamin E, is upregulated
(7,8). The inhibition of epithelial-mesenchymal
changes in HSCs may prevent the development of hepatic fibrosis
(9,10).
microRNAs (miRNAs) are 20–24 nucleotides long,
endogenous, highly conserved, non-coding small single-stranded RNAs
that inhibit the regulation of gene expression through an
interpreter and participate in the metabolism of liver cells and
physiological processes, including the stress response (11). miRNA-122 accounts for 70% of all
identified miRNAs in the liver and serves a pivotal role in liver
physiology (12). miRNA-122 is
associated with various pathological changes in the liver,
including chronic inflammation of the liver (13), liver fibrosis (14,15),
cirrhosis (16) and liver cancer,
and is considered a non-invasive diagnostic predictor of liver
fibrosis (14). In addition,
miRNA-122 can act as a tumor suppressor miRNA and regulate
metastasis of liver cancer (17,18).
Previous findings have indicated that the decrease in miRA-122
expression is negatively correlated with proliferation, migration
and hepatocellular carcinoma cell invasion (19). The Wnt/β-catenin signaling pathway
regulates miRNA-122-mediated regulation of the proliferation and
apoptosis of liver cancer cells (20) and transformation of the epithelium of
liver cancer (21). However, it is
not known whether microRNA may inhibit the transformation of the
epithelium of HSCs and suppress the development of hepatic
fibrosis. Therefore, the effect of miRNA-122 on EMT activation in
HSCs was evaluated to explore its therapeutic potential for the
treatment of liver fibrosis.
Materials and methods
Cell lines and culture
A rat HSC-T6 cell line (cat. no. FS-0060; American
Type Culture Collection, Manassas, VA, USA) was maintained in
Dulbeccos minimum essential medium (DMEM; Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone) and 1%
penicillin at 37°C in a humidified atmosphere containing 5%
CO2.
MTT assay
The MTT method was used to determine the optimal
concentration of TGF-β1 required to induce the logarithmic growth
phase in rat HSCs. Cells were treated with 0.25% trypsin and seeded
at a density of 104 cells after vaccination in 96-well
plates. Cells were cultured at 37°C in a humidified atmosphere
containing 5% CO2 for 12 h and treated with 0, 5, 10 and
20 ng/ml of TGF-β1 (PeproTech, Inc., Rocky Hill, NJ, USA). The
cells were observed for a total of 4, 8, 24, 48 and 72 h, and
incubated with 20 µl/ml MTT solution at 37°C in a humidified
atmosphere containing 5% CO2. Cells were treated with
150 µl dimethyl sulfoxide and incubated at room temperature for 10
min under constant shaking. The absorbance was measured at a
wavelength of 490 nm. The survival rate of the control group was
considered as 100% and used to obtain the survival rate of other
groups as follows after 12 h TGF-β1 treatment: Cell survival rate
(%)=(experimental group OD-blank group OD)/(control group OD-blank
group OD) ×100. This experiment was repeated four times and the
results were indicated as the mean ± standard deviation.
Immunofluorescence staining
Cells from the logarithmic phase were washed with
phosphate-buffered saline (PBS) and treated with 0.25%
ethylenediaminetetraacetic acid-trypsin. Cells were seeded at a
density of 2×104 cells, following vaccination in 6-well
plates and cultured for 24 h at 37°C in a humidified atmosphere
containing 5% CO2. Subsequently, cells were fixed with
4% paraformaldehyde for 20 min, then washed three times with PBS
containing 0.1% Triton X-100 for 20 min. Samples were further
washed three times with PBS and incubated with 7.5% bovine serum
albumin (cat. no. A3912-10; Sigma-Aldrich; Merck KGaA; Darmstadt,
Germany) at room temperature for 1 h. Following blocking, cells
were treated with mouse anti- F-actin (cat. no. ab-205; 1:100;
Abcam, Cambridge, UK) antibody overnight at 4°C. Cells were washed
three times with PBS and subjected to treatment with
FITC-conjugated IgG (1:50; cat. no. AS001; ABclonal Biotech Co.,
Ltd., Wuhan, China) for 1h at 37°C. Following this,
4′,6-diamidino-2-phenylindole nuclear staining was performed for 15
min and cells were observed using a fluorescence microscope
(magnification, ×100).
Transfection of miRNA-122 slow virus
vector
miRNA-122 slow virus vector (cat. no. 15993-1;
vector: Ubi-MCS-SV40-EGFP-IRES-puromycin) was designed and
synthesized by the Shanghai Jikai Medical Laboratory (Shanghai
Genechem Co., Ltd.; sequence:
5′-ACCGGTCCACGGAGGAGTCTGTGACAAAGAAGGAGGGTGAAGGGGAGGTTAGCACCCTTGTGCCTACAGACTCTCCTTAGCAGAGCTCTGGAGTGTGACAATGGTGTTTGTGTCCAAAACATCAAACGCCATCATCACACTAAACAGCTACTGCTAGGCTATCCGTCTACTCCGTGCGCGACTTGACGTCTGCCCTCTCAGAGCAAGAAGTTTTGTCTTATGTACTCTCCGTCATAGGTAATTTATGGCTAGC-3).
CON238 (Ubi-MCS-SV40-EGFP-IRES-puromycin) was used as a negetive
virus vector. In this part, HSC cells treated with 10 µg/l TGF-β1
only and HSC cells treated with 10 µg/l TGF-β1 and CON238 were
utilized as control groups. HSC cells treated with 10 µg/l TGF-β1
and micro-122 slow virus vector were utilized as experimental
groups. First, HSCs were diluted to obtain
3×104−3×106 cells/ml. Following this, a total
of 2 ml cell suspension was added to each well of a 6-well plate.
Cells were allowed to adhere to the surface of the plate. HSC cells
were then cultivated with 2 ml fresh culture medium (DMEM
containing 10% fetal bovine serum) containing 5 ug/ml polybrene
(cat. no. REVG0001; Shanghai Genechem Co., Ltd.) Preliminary
experiments revealed an MOI of 10 (Drop in the infection by degree
of × = virus volume/cell number; data not shown), the micro-122
slow virus vector and CON238 negative virus vector were diluted to
1×108 tu/ml using Enhanced Infection Solution (cat. no.
ENI.S. REVG0002; Shanghai Genechem Co., Ltd.). Subsequently, a
total of 20 µl virus solution was added to the three groups and
incubated for 6 h at 37°C. HSC Cells were then washed with PBS and
further incubated with DMEM with 10% fetal bovine serum for 48 h at
37°C. Cells were observed using a fluorescence microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from 1×107
cells/ml using the TRIzol reagent (cat. no. 15596-026; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to
manufacturers instructions. The concentration of total RNA was
measured using an Eppendorf Bio Photometer. First-strand cDNA was
synthesized from 5 µg total RNA per sample in a 20-µl system
including 1 µl random 6-mers, 1 µl dNTP mixture, 0.5 µl PrimeScript
RTase, 0.5 µl Rnase and 17 µl ddH20 using an RT-qPCR kit
(cat. no. DRRO14A; Takara Biotechnology Co., Ltd., Dalian, China).
The following gene-specific primers were used: α-SMA forward,
5′-CCACTGCTGCTTCCTCTTC-3′ and reverse, 5′-CGCCGACTCCATTCCAAT-3′;
N-cadherin forward, 5′-TATGGTGGTGGTGATGACTGA-3′ and reverse,
5′-CGGTGCTAGTGGACTACAGA-3′; E-cadherin forward,
5′-GCTCGCTGAACTCCTCTGA-3′ and reverse, 5′-TCGCCGCCACCATACATA-3′;
Smad4 forward, 5′-ACTTCCCCAACATTCCTGTG-3′ and reverse,
5′-ATCCATTCTGCTGCTGTCCT-3′; GAPDH forward,
5′-ACGGCAAGTTCAACGGCACAG-3′ and reverse,
5′-GAAGACGCCAGTAGACTCCACGAC-3′; miRNA-122,
5′-GCTGTGGAGTGTGACAATGGTG-3′; U6 forward,
5′-CGCTTCGGCAGCACATATACT-3′ and reverse,
5′-GAATTTGCGTGTCATCCTTGC-3′. The reaction steps were as follows:
Stage 1 Prevariant: 95°C for 5 min; Stage 2 Cyclic reaction: 95°C
for 10 min and 60°C for 30 sec for 40 cycles. Stage 3 dissociation
curve: 95°C for 15 sec, 60°C for 60 sec and 90°C for 15 sec for one
cycle. The 2−ΔΔCq method (22) was used to quantify the relative gene
expression levels. U6 and GAPDH were used as reference genes.
Western blot analysis
Total protein was extracted from 1×107/ml
HSC cells using radioimmunoprecipitation assay buffer (cat. no.
P0013; Beyotime Institute of Biotechnology, Haimen, China).
Subsequently, protein concentration was determined using a BCA
protein kit (Beyotime Institute of Biotechnology). Protein (100
µg/lane) was separated on 12% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Following blocking with 5%
skimmed milk at room temperature for 2 h, the membrane was
incubated with the following primary antibodies overnight at 4°C:
ianti-α-SMA (cat. no. ab5694; 1:500), anti-N-cadherin (cat. no.
ab76011; 1:500), anti E-cadherin (cat. no. ab76055; 1:1,000; all
Abcam), anti-Smad4 (cat. no. 51144-1-AP; 1:1,000; Proteintech
Group, Inc., Chicago, IL, USA) and anti-GAPDH (cat. no.
EM31010-011: 1,000; Beijing Emarbio Science & Technology Co.,
Ltd., Beijing, China) overnight at 4°C. The membrane was then
incubated with horseradish peroxidase conjugated goat anti-mouse
Immunoglobulin G (1:2,000; cat. no. AS003; ABclonal Biotech Co.,
Ltd.) for a further 2 h at room temperature. An ECL Plus
ultra-sensitive luminescent liquid (Applygen Technologies, Inc.,
Beijing, China) was utilized to develop the membrane. Protein bands
were analyzed using Quantity One v4.6.2 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The density of each target
band was calculated relative to GAPDH to determine relative
expression levels.
Statistical analysis
Results were reported as the mean ± standard
deviation and analyzed using SPSS software (version 21.0 for
Windows; IBM Corp., Armonk, NY, USA) was used for all statistical
analyses. One-way analysis of variance followed by the least
significant difference test. Two-group comparisons were performed
using the Student t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TGF-â1 at 10 ìg/l promotes the
viability and survival of HSCs
For the effective activation of HSCs, the most
suitable concentration of TGF-â1 was determined. Cells were treated
with different concentrations (0, 5, 10 and 20 ìg/l) of TGF-â1. MTT
assay results revealed that treatment with 10 ìg/l TGF-â1 for 72 h
resulted in maximum cell viability (Fig.
1). Using 0 ìg/l TGF-â1 treatment as the control (100%
survival), it was revealed that 10 ìg/l TGF-â1 demonstrated the
highest survival rate after 48 h (Table
I).
 | Table I.Effect of different concentration of
TGF-β1 on the cell survival rate on 48 h. |
Table I.
Effect of different concentration of
TGF-β1 on the cell survival rate on 48 h.
| Groups | Concentration
(µg/l) | Absorption value | Survival rate
(%) |
|---|
| Control | 0 | 0.615±0.020 | 100.00 |
| TGF-β1 | 5 |
0.692±0.021a | 112.52 |
|
| 10 |
0.714±0.022b | 116.15 |
|
| 20 |
0.668±0.002a | 108.56 |
Upregulation and morphological changes
in F-actin indicate the activation of HSCs
Cell migration is associated with cytoskeleton
remodeling of actin, including F-actin upregulation and
morphological changes, indicating the activation of HSCs (23). Results indicated an increase in the
expression of F-actin in HSCs following treatment with 10 µg/l
TGF-β1. Notably, increased expression of the stress fiber in the
cell is indicative of the activation and proliferation of HSCs
(24). In the control group, the
cytoskeleton exhibited diffused distribution, irregular and uneven
length of actin filaments and bright nuclei fluorescence (Fig. 2A-C; magnification, ×100). In the
TGF-β1 group, F-actin green fluorescence was concentrated and
clustered, and the fluorescence intensity was homogenous within the
cell membrane and cytoplasm (Fig.
2D-F; magnification, ×100); F-actin was relatively thick and
long along the longitudinal axis of the cells and arranged parallel
to the thin strips of stress fibers (Fig. 2G and H; magnification, ×100).
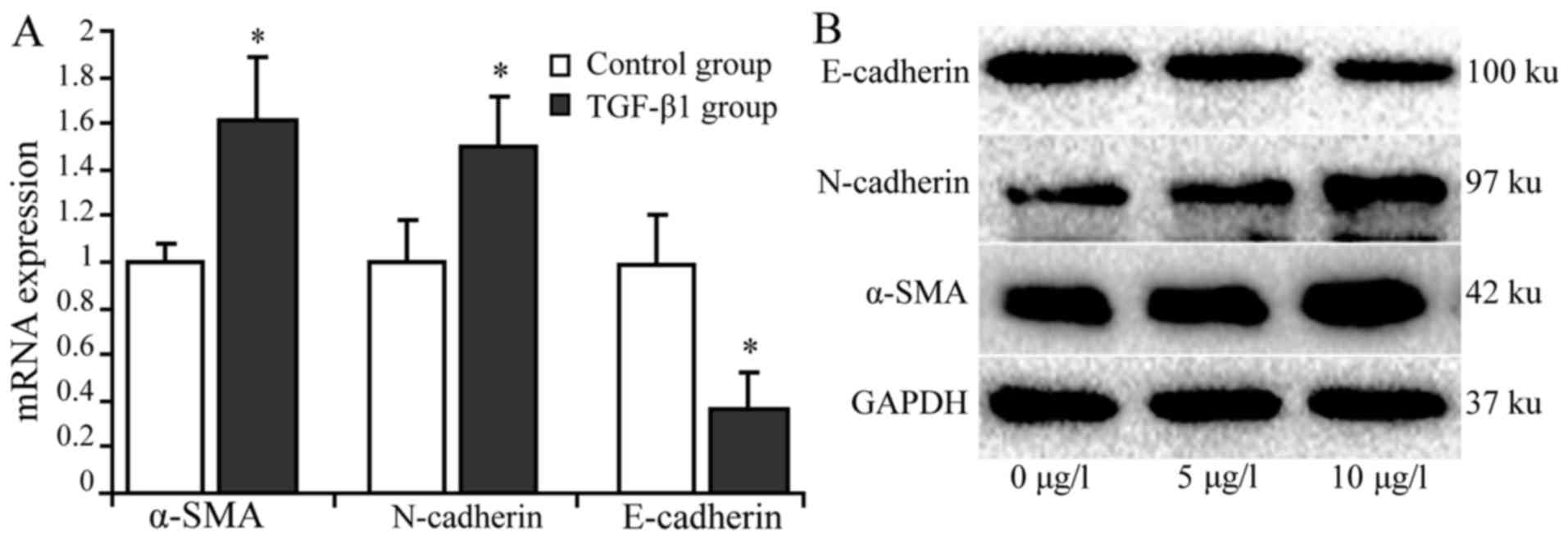
TGF-β1 upregulates á-SMA but
downregulates E-cadherin expression in activated HSCs
RT-qPCR and western blot analysis were used to
detect the expression of α-SMA, N-cadherin and E-cadherin in HSCs
treated with 10 µg/l TGF-β1. The results indicated that the
relative expression levels of α-SMA mRNA and protein was
significantly higher in the treatment group compared with the
control group. The relative expression of N-cadherin mRNA was
marginally higher in the treatment group compared with the control
group. N-cadherin protein expression was markedly higher in the
treatment group compared with the control group. The mRNA
expression levels of E-cadherin were significantly lower in the
treatment group compared with the control group (P<0.05), and
similar results were indicated with regard to E-cadherin protein
expression (Fig. 3).
Expression of miRNA-122 is upregulated
in HSCs transfected with miRNA-122 lentiviral vector
Fluorescence microscopy revealed green fluorescence
in HSCs transfected with miRNA-122 lentiviral vectors into the cell
cytoplasm. Nuclei exhibited bright fluorescence, and the
fluorescence intensity was stronger than that of the cytoplasm. The
expression level of miRNA-122 in the transfected cells (4.92±0.09)
was significantly higher (P<0.05) than that observed in cells
transfected with the negative control vector (1.21±0.08) and the
blank control group (1.00±0.11; Fig.
4).
Smad4 serves as the key protein in the
TGF-â1 signaling pathway
The expression of Smad4 may result in the inhibition
of the activation and biological activity of HSCs induced by the
TGF-â1 signaling pathway. Therefore, the expression of Smad4 was
studied following miRNA-122 transfection in HSCs. According to the
results in Fig. 5, Smad4 expression
was significantly (P<0.05) higher in cells treated with TGF-β1
compared with cells treated with TGF-β1 and miRNA-122. Thus,
miRNA-122 inhibited the activation of HSCs induced by TGF-β1.
Downregulation of N-cadherin
expression and overexpression of E-cadherin in HSCs treated with
miRNA-122 and TGF-â1 indicates that miRNA-122 may inhibit EMT of
HSCs induced by TGF-â1
TGF-β1 and empty plasmid groups were used as control
groups. The results suggested that the mRNA expression of
E-cadherin was significantly higher (P<0.05) in the treatment
group (2.73±0.16) compared with the negative vector transfection
group (1.17±0.12) and blank control group (1.01±0.20). In addition,
E-cadherin protein expression was higher in the treatment group
(0.87±0.07) compared with the control groups (0.36±0.11 and
0.41±0.130). The mRNA and protein expression of N-cadherin was
lower in the treatment group (0.21±0.13 and 0.32±0.07,
respectively) compared with the two control groups (0.92±0.12 and
0.88±0.14, respectively, for negative vector transfection group)
and (1.02±0.20 and 0.21±0.07, respectively, for blank control
group; Fig. 6). Notably the
difference regarding mRNA N-cadherin expression was statistically
significant (P<0.05).
Discussion
The activation of HSCs is a major cause of liver
fibrosis and cirrhosis, and effective inhibition of the
proliferation and activation of HSCs is a key step in the
prevention of liver fibrosis development (25). EMT in differentiated cells is caused
by external factors that impart mature epithelial characteristics
to cells, characterized with the loss of cell polarity and change
in the cytoskeleton structure and migration ability (26). EMT has long been determined a
characteristic of tumor cells. Previous findings on renal fibrosis,
pulmonary fibrosis and peritoneal fibrosis have focused on EMT
(27). A study in the mouse model of
carbon tetrachloride-induced liver fibrosis indicated that the
lower expression of E-cadherin and higher expression of α-SMA
directly affected EMT in liver fibrosis (6). However, to the best of our knowledge,
no report has described the series of processes that lead to the
activation of HSCs.
The present study investigated whether the
activation of HSCs induced by TGF-β1 causes the transformation of
the epithelium. It was first explored whether TGF-β1 may activate
HSCs, and results indicated that the activity and proliferation of
HSCs increased with an increase in the concentration of TGF-β1;
however, concentrations higher than 10 µg/l failed to induce a
significant increase in HSC activity and proliferation. Therefore,
the concentration of 10 µg/l TGF-β1 was used for further
experiments. The HSC activation by TGF-β1 was studied, and it was
observed that myofibroid cells with stellate caused reconstruction
of the cytoskeleton. F-actin is an important skeletal protein
involved in the formation of the cytoskeleton (28). In addition to maintaining the normal
morphology of the cells, F-actin also participates in biological
processes through stress regulation (29). An increase in the expression of
F-actin and stretching of the muscle fiber filament was observed in
a previous study (30). In addition,
the effects of TGF-β1 on the expression of E-cadherin and
N-cadherin were evaluated in HSCs. N-cadherin gene and protein
expression levels increased, but the gene and protein expression
levels of the epithelial component E-cadherin were downregulated.
Thus, TGF-β1 induced HSC activation and caused EMT.
The inhibition of the TGF-β1 signaling pathway may
suppress the activation of HSCs and reduce the development of
hepatic fibrosis. To identify the target gene or protein that
effectively inhibited this regulation, miRNA-122, which is
expressed specifically in the liver and accounts for 70% of all
hepatic microRNAs, was used. miRNA-122 serves an important role in
liver development, differentiation and maintenance under normal
physiological processes and may cause a series of liver diseases,
including inflammation, cirrhosis/liver fibrosis, fat metabolism
and cancer (13,16). The present study indicated miRNA-122
may inhibit the regulation of the TGF-β1 signaling pathway in
HSCs.
A previous study suggested that the expression of
miRNA-122 was associated with the inflammatory response of the
liver (31). In addition, miRNA-122
expression is significantly lower in chronic inflammation than in
normal conditions and early inflammation (32). Previous findings have indicated that
miRNA-122 is considered a marker of liver fibrosis (14). An miRNA-122 lentiviral vector was
constructed and successfully transfected into HSCs in the present
study. Successful transformants were selected using the green
fluorescent protein expression method.
The expression of E-cadherin/N-cadherin was studied
in HSCs treated with miRNA-122 lentiviral vector alone or in
combination with TGF-β1. It was revealed that the increase in the
expression of miRNA-122 resulted in a significant increase in
E-cadherin expression in cells transfected with miRNA-122
lentiviral vector when compared with those treated with empty
plasmid group or TGF-β1 and miRNA-122. Conversely, the expression
of N-cadherin significantly decreased with an increase in miR-122
expression, resulting in the inhibition of EMT of HSCs. Thus, the
upregulation in miRNA-122 expression may result in the inhibition
of EMT of HSCs. This result was in line with reports from Omran
et al and Nakamura et al (14,15),
wherein the morphology and functional study demonstrated that the
overexpression of miRNA-122 may effectively promote the
differentiation and maturation of liver cells. The expression of
miRNA-122 at appropriate concentrations may adjust the balance
between liver cell proliferation and differentiation, as well as
that between EMT and mesenchymal epithelial transition. To clarify
the role of the TGF-β1 signaling pathway in this process, changes
in the expression of the key protein Smad4 in the TGF-β1 signaling
pathway were studied. Results indicated that HSCs stimulated by
TGF-β1 exhibited upregulated expression of Smad4; however,
miRNA-122 treatment significantly decreased the expression of
Smad4, which suggested the inhibitory effect of miRNA-122 on the
activation of HSCs induced by TGF-β1 and EMT.
Taken together, the present findings demonstrated
the effects of miRNA-122 on HSC epithelium. However, the regulation
of HSC activation is typically a complex signaling network. It was
suggested that miRNA-122 may regulate the activation of HSCs
mediated by TGF-β1 signaling pathways; whether the exact mechanism
involves direct interactions between the two is unknown. Therefore,
further studies must be performed to study the regulatory effects
of miRNA-122 in liver fibrosis in order to extend its application
as a potential agent against liver fibrosis.
Acknowledgements
Hepatic stellate cells HSC-T6 cells were kindly
provided by Professor Hong Shi of the Traditional Chinese and
Western Medicine Institute, Fujian University of Chinese Medicine
(Fuzhou, China).
Funding
The present study was funded by the Fuzhou Science
and Technology Project (grant nos. 2014-S-137-1 and 2014-S-137-5)
and the Fujian Science and Technology Guiding Project (grant no.
2015D002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
LW and BC performed the experiments, participated in
data collection and drafted the manuscript. QZ and WL performed
statistical analyses and designed the present study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomita K, Teratani T, Suzuki T, Shimizu M,
Sato H, Narimatsu K, Usui S, Furuhashi H, Kimura A, Nishiyama K, et
al: Acyl-CoA: Cholesterol acyltransferase 1 mediates liver fibrosis
by regulating free cholesterol accumulation in hepatic stellate
cells. J Hepatol. 61:98–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duval F, Moreno-Cuevas JE, González-Garza
MT, Rodríguez-Montalvo C and Cruz-Vega DE: Liver fibrosis and
protection mechanisms action of medicinal plants targeting
apoptosis of hepatocytes and hepatic stellate cells. Adv Pharmacol
Sci. 2014:3732952014.PubMed/NCBI
|
|
3
|
Yang AT, Hu DD, Wang P, Cong M, Liu TH,
Zhang D, Sun YM, Zhao WS, Jia JD and You H: TGF-β1 induces the dual
regulation of hepatic progenitor cells with both anti- and proliver
fibrosis. Stem Cells Int. 2016:14926942016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JH, Huang XR, Zhu HJ, Johnson R and Lan
HY: Role of TGF-beta signaling in extracellular matrix production
under high glucose conditions. Kidney Int. 63:2010–2019. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dropmann A, Dediulia T, Breitkopf-Heinlein
K, Korhonen H, Janicot M, Weber SN, Thomas M, Piiper A, Bertran E,
Fabregat I, et al: TGF-β1 and TGF-β2 abundance in liver diseases of
mice and men. Oncotarget. 7:19499–19518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan L, Ye L, Zhuang L, Zou X, Liu S,
Zhang Y, Zhang L, Jin C and Huang Y: VEGFC/VEGFR3 axis mediates
TGFβ1-induced epithelial-to-mesenchymal transition in non-small
cell lung cancer cells. PLoS One. 13:e02004522018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang QL, Tao YY, Yuan JL, Shen L and Liu
CH: Salvianolic acid B preventsepithelial-to-mesenchymal transition
through the TGF-beta1 signal transduction pathway in vivo and in
vitro. BMC Cell Biol. 11:312010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bi WR, Yang CQ and Shi Q: Transforming
growth factor-β1 induced epithelial-mesenchymal transition in
hepatic fibrosis. Hepatogastroenterology. 59:1960–1963.
2012.PubMed/NCBI
|
|
9
|
Girard M, Jacquemin E, Munnich A, Lyonnet
S and Henrion-Caude A: miR-122, a paradigm for the role of
microRNAs in the liver. J Hepatol. 48:648–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y,
Guo H, Fei M and Sun S: Plasma microRNA-122 as a biomarker for
viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem.
56:1830–1838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis Starkey PJ, Dear J, Platt V, Simpson
KJ, Craig DG, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM,
et al: Circulating microRNAs as potential markers of human
drug-induced liver injury. Hepatology. 54:1767–1776. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su TH, Chen M, Liu CJ, Chen CL, Ting TT,
Tseng TC, Chen PJ, Kao JH and Chen DS: Serum microRNA-122 level
correlates with Virologic responses to pegylated interferon therapy
in chronic hepatitis C. Proc Natl Acad Sci USA. 110:7844–7849.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding X, Ding J, Ning J, Yi F, Chen J, Zhao
D, Zheng J, Liang Z, Hu Z and Du Q: Circulating microRNA-122 as a
potential biomarker for liver injury. Mol Med Rep. 5:1428–1432.
2012.PubMed/NCBI
|
|
14
|
Omran AA, Osman KS, Kamel HM, Abdel-Naem
EA and Hasan DE: MicroRNA-122 as a novel non-invasive marker of
liver fibrosis in hepatitis C virus patients. Clin Lab.
62:1329–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura M, Kanda T, Jiang X, Haga Y,
Takahashi K, Wu S, Yasui S, Nakamoto S and Yokosuka O: Serum
microRNA-122 and Wisteria floribunda agglutinin-positive Mac-2
binding protein are useful tools for liquid biopsy of the patients
with hepatitis B virus and advanced liver fibrosis. PLoS One.
12:e01773022017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waidmann O, Köberle V, Brunner F, Zeuzem
S, Piiper A and Kronenberger B: Serum microRNA-122 predicts
survival in patients with liver cirrhosis. PLoS One. 7:e456522012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW,
Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al: MicroRNA-122, a
tumor suppressor microRNA that regulates intrahepatic metastasis of
hepatocellular carcinoma. Hepatology. 49:1571–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao DD, Yang J, Lei XF, Mi GL, Li SL, Li
K, Xu CQ and Yang HL: Expression of microRNA-122 and microRNA-22 in
HBV-related liver cancer and the correlation with clinical
features. Eur Rev Med Pharmacol Sci. 21:742–747. 2017.PubMed/NCBI
|
|
19
|
Wang N, Wang Q, Shen D, Sun X, Cao X and
Wu D: Downregulation of microRNA-122 promotes proliferation,
migration, and invasion of human hepatocellular carcinoma cells by
activating epithelial-mesenchymal transition. Onco Targets Ther.
9:2035–2047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Zhu X, Wu L, Yang R, Yang Z, Wang Q
and Wu F: MicroRNA-122 suppresses cell proliferation and induces
cell apoptosis in hepatocellular carcinoma by directly targeting
Wnt/β-catenin pathway. Liver Int. 32:752–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Y, Wang J, Han J, Luo D and Sun Z:
MiR-122 inhibits epithelial-mesenchymal transition in
hepatocellular carcinoma by targeting Snail1 and Snail2 and
suppressing WNT/β-cadherin signaling pathway. Exp Cell Res.
360:210–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma L, Yang X, Wei R, Ye T, Zhou JK, Wen M,
Wen RT, Li P, Dong B, Liu L, et al: MicroRNA-214 promotes hepatic
stellate cell activation and liver fibrosis by suppressing Sufu
expression. Cell Death Dis. 9:7182018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui X, Zhang X, Yin Q, Meng A, Su S, Jing
X, Li H, Guan X, Li X, Liu S and Cheng M: F-actin cytoskeleton
reorganization is associated with hepatic stellate cell activation.
Mol Med Rep. 9:1641–1647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu F, Ji S, Su L, Wan L, Zhang S, Dai C,
Wang Y, Fu J and Zhang Q: Adipose-derived mesenchymal stem cells
inhibit activation of hepatic stellate cells in vitro and
ameliorate rat liver fibrosis in vivo. J Formos Med Assoc.
114:130–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu H, Shen Y, Hong J, Xia Q, Zhou F and
Liu X: The contribution of TGF-β in Epithelial-Mesenchymal
Transition (EMT): Down-regulation of E-cadherin via snail.
Neoplasma. 62:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen T, Nie HY, Gao X, Yang J, Pu J, Chen
Z, Cui X, Wang Y, Wang H and Jia G: Epithelial-mesenchymal
transition involved in pulmonary fibrosis induced by multi-walled
carbon nanotubes via TGF-beta/Smad signaling pathway. Toxicol Lett.
226:150–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Molè-Bajer J, Bajer AS and Inoué S:
Three-dimensional localization and redistribution of F-actin in
higher plant mitosis and cell plate formation. Cell Motil
Cytoskeleton. 10:217–228. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong X, Fan Y, Zhang Y, Luo C, Duan X,
Yang L and Pan J: Inserted rest period resensitizes MC3T3-E1 cells
to fluid shear stress in a time-dependent manner via
F-actin-regulated mechanosensitive channel(s). Biosci Biotechnol
Biochem. 78:565–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boesch-Saadatmandi C, Wagner AE, Wolffram
S and Rimbach G: Effect of quercetin on inflammatory gene
expression in mice liver in vivo-role of redox factor 1, miRNA-122
and miRNA-125b. Pharmacol Res. 65:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bala S, Petrasek J, Ward J, Alao H, Levin
I and Szabo G: Serum microrna-122 and mir-155 as biomarkers of
liver injury and inflammation in models of acute and chronic liver
disease. Gastroenterology. 140:9062012. View Article : Google Scholar
|