Introduction
Perimenopause refers to the physiological process in
females form ovarian function begins to decline to 1 year after
menopause (1,2). Ovarian dysfunction and unstable
expression of sex hormones can cause women's menopause syndromes,
among which insomnia is one of the most common types (3,4).
Incidence of insomnia is ~30–60%, and with the growth of aging
population, the incidence of insomnia is predicted to be further
increased (5). Long-term insomnia
not only has a great impact on the patient's normal activities but
also reduces the patient's physiological function and causes a
series of complications (6,7). Therefore, it is necessary to pay
attention to the problem of insomnia in perimenopausal women.
At present, the mechanism of insomnia in
perimenopausal women is still unclear. Many studies have reported
that liver depression plays a pivotal role in insomnia during
perimenopausal period. Liver depression, also known as stagnation
of liver-qi syndrome, refers to liver failure and stagnation,
manifested as emotional depression, chest or abdominal distension,
pain and so on. Those studies believed that insomnia in
perimenopausal develops from liver (8,9).
Interleukin-1β (IL-1β) is an important immune factor, and it is
also a well-studied sleep-related cytokine in recent years
(10). Rethorst et al
(11) reported that IL-1β can
effectively improve post-exercise narcolepsy in patients with
depression. Zielinski et al (12) also found that the expression of IL-1β
was significantly increased in patients with chronic sleep
disorders. Therefore, a study of the relationship between IL-1β and
insomnia in perimenopausal women is important for understanding its
pathogenesis and clinical prevention and treatment.
In this study, the relationship between non-organic
insomnia and the patient's liver depression score and serum IL-1β
expression levels in perimenopausal women was studied. Our study
provided guidance for clinical prevention and treatment of the
perimenopausal non-organic sleep disorder (PNSD) in perimenopausal
women.
Materials and methods
Research subjects
A total of 268 cases of perimenopausal patients from
the Department of Traditional Chinese Medicine of Fujian Provincial
Hospital and the Department of Traditional Chinese Medicine of
Jinshan Branch of Fujian Provincial Hospital were selected from
March 2014 to June 2017. Among them, 182 patients developed
non-organic insomnia (observation group). The remaining 86 patients
were included in the control group. Diagnostic criteria for
perimenopausal women refer to ‘Obstetrics and Gynecology’. Age
range is 45–60 years. All patients had no menopause or menopause
for ≤1 year, and the follicle-stimulating hormone (FSH) was >10
IU/l. Diagnostic criteria for non-organic insomnia refer to the
‘International Classification of Diseases, 10th edition (ICD-10,
2003)’. Patients suffered from sleep disorders, including
difficulty falling asleep, poor quality of sleep, insomnia, for
>30 days. The number of sleep disturbances occurred ≥3 times a
week, and sleep disturbances obviously caused the patient's
distress or reduced social and professional functions. Patients
with ovarian disease, unexplained irregular vaginal bleeding, a
history of female and progesterone replacement therapy,
cardiovascular disease and other organ diseases, autoimmune
diseases, alcohol abuse, and smoking habit were excluded. The study
was approved by the Ethics Committee of Provincial Clinical Medical
College of Fujian Medical University (Fuzhou, China). Patients who
participated in this research had complete clinical data. Signed
written informed consents were obtained from the patients and/or
guardians.
Specimen collection
Fasting venous blood (5 ml) was extracted from those
non-menopausal women in the morning of the first week of the
menstrual cycle. Serum was separated within 1 h to detect
IL-1β.
IL-1β detection method
Expression of IL-1β in serum was detected by
enzyme-linked immunosorbent assay (ELISA). Coating liquid was used
to dilute IL-1β, and 200 µl of diluted IL-1β was added into each
well, followed by incubation at 4°C overnight. After washing with
ddH2O, blocking with blocking solution (200 µl/well) was
performed. Serum (50 µl/well) was added, and negative NC, positive
NC and blank control groups were set. Enzyme-labeled rabbit
anti-human IL-1β polyclonal antibody (1:300, cat. no. 16806-1-AP,
ProteinTech Group, Inc.; Wuhan Sanying Biotechnology, Wuhan, China)
was added and incubated at 37°C for 45 min. After washing for 5
times, 30 sec to 1 min for each time, 100 µl of substrate was added
into each well, and incubation was performed at 37°C for 15 min.
Finally, the reaction was terminated by adding 0.05 ml of sulfuric
acid at 2 mol/l per well. Color development was performed within 15
min. The kit for IL-1β was purchased from Roche Diagnostics (Basel,
Switzerland).
Observation indicators
Serum levels of IL-1β were measured by ELISA.
According to the ‘syndrome differentiation’, weighted threshold
method is used to determine the weight of disease location and
venereal factors, and 100 is used as universal threshold to judge
whether the diagnosis of each syndrome element is valid. If the
symptom is severe, its quantitative diagnosis value multiplies 1.5,
if the symptom is mild, multiply 0.7, and accumulate the
contribution degree of the related elements of liver depression as
the integral of liver depression syndrome element. Liver depression
scores: score <70 as grade 0; ≥70 and <100 as grade 1; ≥100
and <150 as grade 2; ≥150 as grade 3 (13). Pittsburgh Sleep Quality Index (PSQI)
was used to assess 30-day insomnia severity.
Statistical analysis
Statistical analysis was performed by using SPSS
22.0 software [Asia Analytics (formerly SPSS China), Shanghai,
China]. Count data are expressed as percentages and processed by
χ2 test. Normal measurement data are expressed as mean ±
standard deviation (SD). Follicle-stimulating hormone (FSH),
luteinizing hormone (LH), estradiol (E2) and testosterone (T) did
not meet the normal distribution and were expressed as median (M).
Independent sample t-test was used for comparison between groups,
and variance analysis was used for comparison among groups with LSD
test as a post hoc test. Spearman's correlation analysis was used
to analyze the relationship between IL-1β and PNSD and hepatic
depression. P<0.05 indicates that the difference was
statistically significant.
Results
General data
The average age of 182 PNSD patients in observation
group was 54.9±4.55 years. Eighty-six patients in control group
were females in perimenopausal period without non-organic insomnia,
and the average age was 53.5±4.15 years. There were no differences
in FSH, LH, E2, T levels, and menopausal status between the two
groups (p>0.05). However, there was a difference in depression
and anxiety scores between the two groups. Depression and anxiety
scores in the observation group were higher than those in control
group (p<0.05, Table I).
 | Table I.Clinical characteristics of the two
groups of patients. |
Table I.
Clinical characteristics of the two
groups of patients.
| Variables | Control group
(n=86) | Observation group
(n=182) | χ2/t | P-value |
|---|
| Age (years) | 53.5±4.15 | 54.9±4.55 | 0.691 | 0.490 |
| FSH (IU/l) | 14.5 | 15.3 | 1.636 | 0.085 |
| LH (IU/l) | 5.4 | 7.7 | 1.942 | 0.051 |
| E2 (pg/ml) | 32.4 | 33.9 | 0.067 | 0.965 |
| T (ng/ml) | 0.14 | 0.15 | 0.412 | 0.633 |
| Depression score | 45.69±10.47 | 51.95±9.57 | 4.849 | <0.001 |
| Anxiety score | 45.26±7.84 | 51.29±10.32 | 4.802 | <0.001 |
| Menopause status [n
(%)] |
|
| 0.050 | 0.895 |
| Not
menopause | 50 (58.14) | 112 (61.54) |
|
|
|
Menopause | 36 (41.86) | 70
(38.46) |
|
|
Liver depression score results in the
two groups
The liver depression scores in the observation group
were significantly higher than those in control group (p<0.05).
Liver depression grading showed a significant difference between
the two groups in proportion of patients with grade 0 and 3 liver
depression, and the proportion of patients with grade 0 liver
depression in control group was significantly higher than that in
the observation group (p<0.05), while proportion of patients
with grade 3 liver depression in the observation group was
significantly higher than that in control group (p<0.05). There
was no significant difference between the two groups in the
proportion of people with grade 1 and 2 liver depression
(p>0.05, Table II).
 | Table II.Results of liver depression
scoring. |
Table II.
Results of liver depression
scoring.
| Variables | Control group
(n=86) | Observation group
(n=182) | χ2/t | P-value |
|---|
| Average score | 108.5±57.6 | 158.4±64.5 | 6.113 | <0.001 |
| Depression
grades |
|
| 24.485 | <0.001 |
| 0 [n
(%)] | 32 (37.21) | 23 (12.64) | 20.139 | <0.001 |
| 1 [n
(%)] | 12 (13.95) | 18 (9.89) | 0.604 | 0.406 |
| 2 [n
(%)] | 16 (18.60) | 46 (25.27) | 1.110 | 0.278 |
| 3 [n
(%)] | 26 (30.23) | 95 (52.20) | 10.509 | 0.001 |
PSQI scores of the two groups of
patients
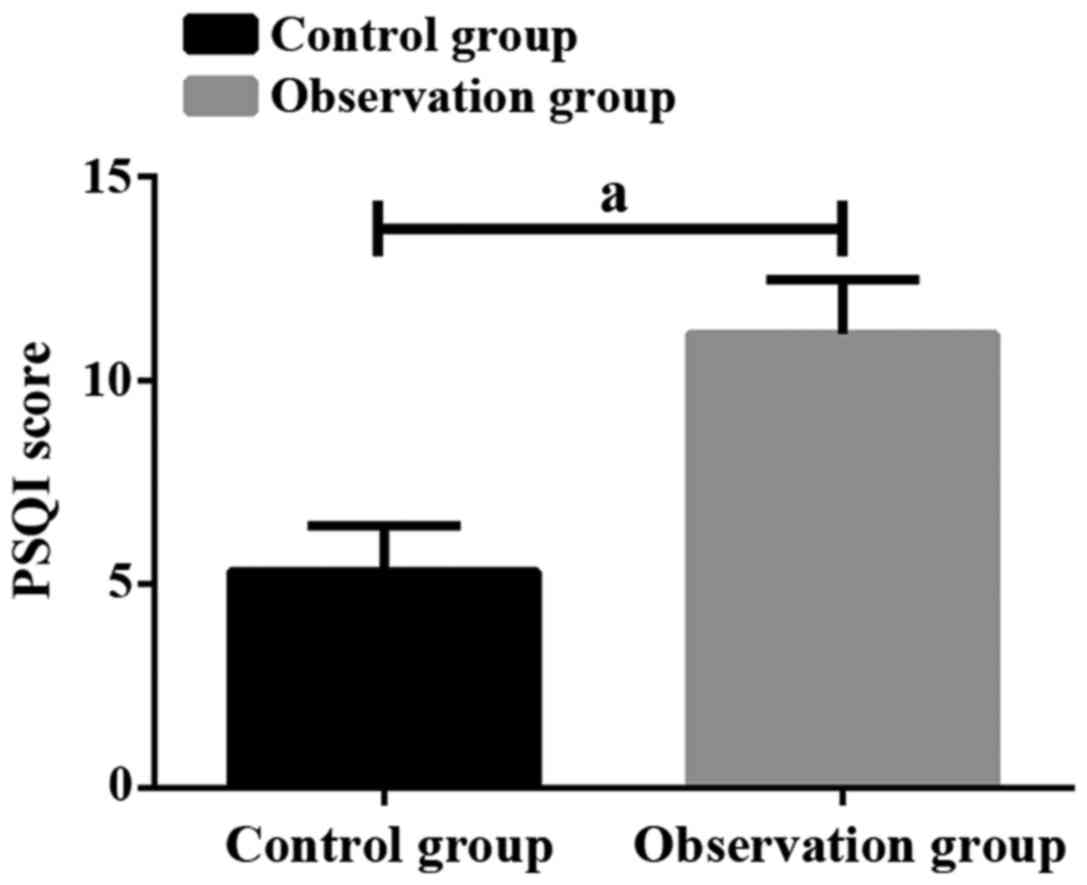
Average PSQI score in control group was 5.32±1.11,
and average PSQI score in observation group was 11.15±1.33. There
was a significant difference between the two groups (p<0.05,
Fig. 1). Higher liver depression in
observation group was accompanied by higher PSQI score. PSQI scores
were significantly different in patients with different liver
depression grades (p<0.05, Fig.
2).
IL-1β expression levels in the two
groups
The average expression level of IL-1β in the
observation group was significantly higher than that in control
group (p<0.05, Table III).
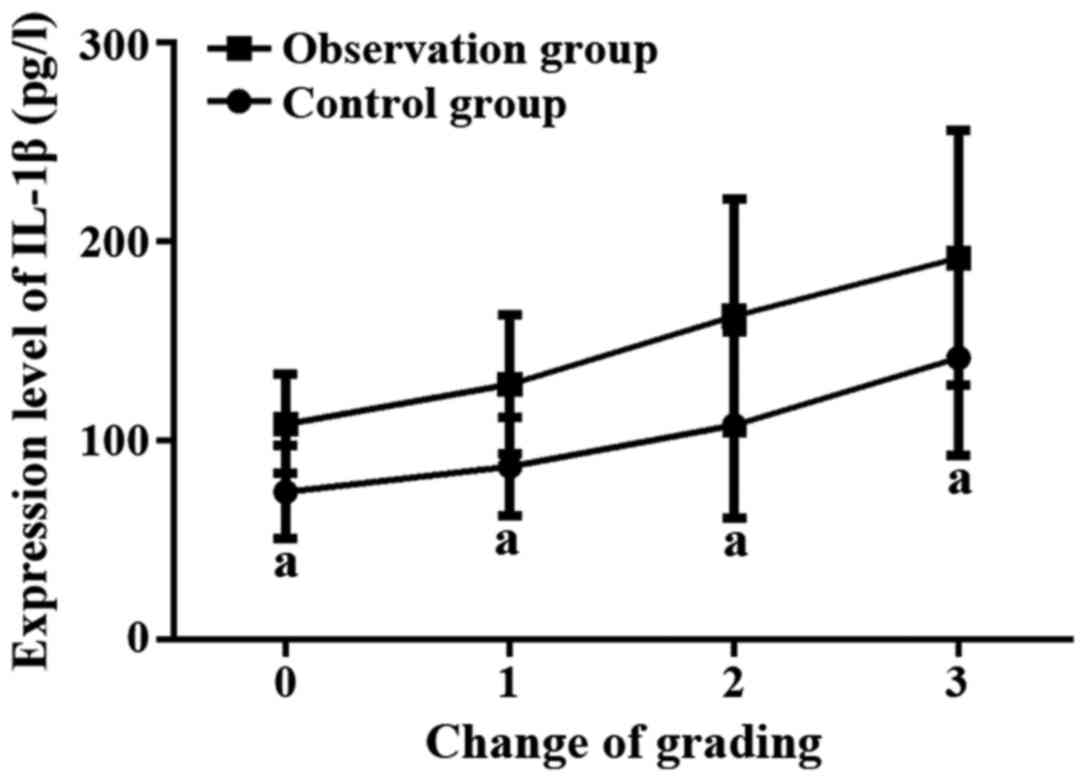
According to the liver depression grading results, the expression
level of IL-1β was significantly higher in the observation group
than in control group at each liver depression grade (p<0.05).
The expression level of IL-1β was different in patients with
different liver depression grades within the same group. Higher
liver depression grades are accompanied by higher expression level
of IL-1β (p<0.05, Fig. 3).
 | Table III.Expression levels of IL-1β in the two
groups of patients (pg/l). |
Table III.
Expression levels of IL-1β in the two
groups of patients (pg/l).
| Variables | Control group
(n=86) | Observation group
(n=182) | t | P-value |
|---|
| Average level | 102.5±57.4 | 147.6±65.9 | 5.444 | <0.001 |
| Grade 0 | 74.1±23.4 | 108.2±24.8 | 2.365 | 0.018 |
| Grade 1 |
86.9±24.7a |
128.1±34.8a | 2.775 | 0.006 |
| Grade 2 |
107.6±46.7a,b |
162.4±58.6a,b | 3.508 | 0.001 |
| Grade 3 |
141.4±48.9a–c |
191.7±64.2a–c | 2.469 | 0.014 |
Correlation analysis
Spearman's correlation analysis showed that PSQI was
positively correlated with liver depression score, and the level of
IL-1β was positively correlated with the liver depression grade in
patients with PNSD (r=0.724, p=0.012; r=0.765, p=0.008) (Table IV).
 | Table IV.Correlation analysis. |
Table IV.
Correlation analysis.
| Variables | IL-1β | PSQI | Liver depression
score |
|---|
| PSQI | r=0.812 |
| r=0.793 |
|
| P=0.002 |
| P=0.005 |
| Liver
depression | r=0.765 | r=0.724 |
|
| score | P=0.008 | P=0.012 |
|
Discussion
The most common type of perimenopausal sleep
disorder is non-organic insomnia (14). Liver depression is one of the most
important syndromes in patients with non-organic insomnia. Chinese
medicine believes that liver blood deficiency can cause insomnia
(15). Western medicine believes
there is a close relationship between sleep and the immune system
(16). IL-1β is an important immune
factor regulating sleep arousal behavior. IL-1β can be transported
through its IL-1β receptor to the site of action of NREM sleep in
the preoptic area of the hypothalamus, which in turn regulates
firing patterns of hypothalamus and brain stem neurons and sleep
arousal activity (17,18). In this study, the serum levels of
IL-1β in 182 patients with PNSD were measured, and correlations
with the degree of insomnia and the degree of liver depression were
investigated. Our findings provided guidance for the clinical
prevention and treatment of PNSD patients.
In this study, 182 PNSD patients were included in
the observation group, and 86 perimenopausal healthy women were
included in the control group. Results showed that PSQI scores of
the observation group were significantly higher than those of
control group. With high reliability and validity, PSQI can be used
to subjectively evaluate severity of insomnia in the past 1 month,
and it has become a commonly used clinical insomnia severity rating
scale worldwide (19–21). In this study, liver depression scores
were graded referred to ‘syndrome differentiation’ (13). Scores of liver depression in the
observation group were significantly higher than those in control
group, and most of PNSD patients had different degrees of liver
depression. Patients with PNSD mostly showed grade 2 and 3 liver
depression, and the proportion of grade 3 patients was
significantly higher in the observation group than in control
group, indicating that most patients with PNSD had
moderate-to-heavy liver depression. Most perimenopausal healthy
women had grade 0 liver depression, and the proportion of grade 0
patients was significantly higher in the control group than in
observation group, indicating that perimenopausal healthy women
usually do not suffer from liver depression. Results of this study
also showed that with the increase in liver depression grade, PSQI
scores and the severity of insomnia of patients in observation
group were also getting higher and higher. Correlation analysis
also showed that there was a positive correlation between the liver
depression and the PSQI scores. We also measured the expression
level of IL-1β in fasting blood. Test results showed that the
expression level of IL-1β in the observation group was
significantly higher than that in control group, and the expression
level of IL-1β was also increased with the increase in grade of
liver depression. Correlation analysis showed that the expression
level of IL-1β was positively correlated with the liver depression
grade and PSQI scores. Therefore, we can speculate that a higher
expression level of IL-1β may cause more severe insomnia and higher
degree of liver depression in PNSD patients. Animal experimental
studies have found that high levels of oxidative stress and
expression of IL-1β in the brain tissue of sleep-deprived animals
(22,23). IL-1β is a well-known sleep-regulating
cytokine (24). Therefore, we
speculate that PNSD patients have increased stress response, which
stimulates IL-1β expression, so as to protect neurological function
by regulating related neuroactive substances in patients with
insomnia. Due to limited time, this study failed to further study
the factors related to insomnia and sleep. In addition, the sample
size of this study is also small. Further studies with bigger
sample size are still needed to further confirm our conclusion.
In summary, the expression level of IL-1β in women
with PNSD was significantly upregulated, and different degrees of
liver depression also exist. Higher expression level of IL-1β is
accompanied by more serious liver depression and higher degree of
insomnia.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (General Program) (81473599), the
Innovative Medical Project of Fujian Province (2016-CX-8), and the
Youth Research Project of Fujian Province (2016-1-6).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and YC wrote the manuscript and collected the
specimens. HW and JH were responsible for ELISA. KM and JZ recorded
and analyzed the PSQI. SL contributed to the statistical analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Provincial Clinical Medical College of Fujian Medical University
(Fuzhou, China). Patients who participated in this research had
complete clinical data. Signed written informed consents were
obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martini J, Knappe S, Garthus-Niegel S and
Hoyer J: Mental disorders in women: Natural course during
premenstrual phases, peripartum period and perimenopause. Fortschr
Neurol Psychiatr. 84:432–449. 2016.(In German). PubMed/NCBI
|
|
2
|
Mosconi L, Berti V, Quinn C, McHugh P,
Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S,
Isaacson RS, et al: Correction: Perimenopause and emergence of an
Alzheimer's bioenergetic phenotype in brain and periphery. PLoS
One. 13:e01933142018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber MT, Maki PM and McDermott MP:
Cognition and mood in perimenopause: A systematic review and
meta-analysis. J Steroid Biochem Mol Biol. 142:90–98. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brinton RD, Yao J, Yin F, Mack WJ and
Cadenas E: Perimenopause as a neurological transition state. Nat
Rev Endocrinol. 11:393–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mosconi L, Berti V, Guyara-Quinn C, McHugh
P, Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S,
Isaacson RS, et al: Perimenopause and emergence of an Alzheimer's
bioenergetic phenotype in brain and periphery. PLoS One.
12:e01859262017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin VT, Pavlovic J, Fanning KM, Buse
DC, Reed ML and Lipton RB: Perimenopause and menopause are
associated with high frequency headache in women with migraine:
Results of the American Migraine Prevalence and Prevention Study.
Headache. 56:292–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brooks HL, Pollow DP and Hoyer PB: The VCD
mouse model of menopause and perimenopause for the study of sex
differences in cardiovascular disease and the metabolic syndrome.
Physiology (Bethesda). 31:250–257. 2016.PubMed/NCBI
|
|
8
|
Guo HM, Liu M, Xiang YT, Zhao J, Ungvari
GS, Correll CU, Ng CH, Chiu HF and Duan ZP: Insomnia in adults with
chronic hepatitis B, liver failure, and cirrhosis: A case-control
study. Perspect Psychiatr Care. 53:67–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeung WF, Chung KF, Zhang NL, Zhang SP,
Yung KP, Chen PX and Ho YY: Identification of Chinese medicine
syndromes in persistent insomnia associated with major depressive
disorder: A latent tree analysis. Chin Med. 11:42016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu JJ, McDonald VM, Baines KJ and Gibson
PG: Airway IL-1β and systemic inflammation as predictors of future
exacerbation risk in asthma and COPD. Chest. 148:618–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rethorst CD, Greer TL, Toups MS, Bernstein
I, Carmody TJ and Trivedi MH: IL-1β and BDNF are associated with
improvement in hypersomnia but not insomnia following exercise in
major depressive disorder. Transl Psychiatry. 5:e6112015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zielinski MR, Kim Y, Karpova SA, McCarley
RW, Strecker RE and Gerashchenko D: Chronic sleep restriction
elevates brain interleukin-1 beta and tumor necrosis factor-alpha
and attenuates brain-derived neurotrophic factor expression.
Neurosci Lett. 580:27–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang SF, Wang Q, Jiao LJ, Huang YL,
Garfield D, Zhang J and Xu L: Astragalus-containing Traditional
Chinese Medicine, with and without prescription based on syndrome
differentiation, combined with chemotherapy for advanced
non-small-cell lung cancer: A systemic review and meta-analysis.
Curr Oncol. 23:e188–e195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Słopień R, Wichniak A, Pawlak M, Słopień
A, Warenik-Szymankiewicz A and Sajdak S: Disturbances of sleep
continuity in women during the menopausal transition. Psychiatr
Pol. 49:615–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janjic V, Radmanovic B, Dejanovic SD,
Ravanic D and Borovcanin M: P.8.b.006: Side effects of zolpidem and
temazepam in treating primary insomnia. Eur Neuropsychopharmacol.
24:S737–S738. 2014. View Article : Google Scholar
|
|
16
|
Black DS and Slavich GM: Mindfulness
meditation and the immune system: A systematic review of randomized
controlled trials. Ann N Y Acad Sci. 1373:13–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talwar H, Bauerfeld C, Bouhamdan M, Farshi
P, Liu Y and Samavati L: Corrigendum to ‘MKP-1 negatively regulates
LPS-mediated IL-1β production through p38 activation and HIF-1α
expression’. (Cell Signal. 34 (1–10) (2017) Epub 2017 Feb 24). Cell
Signal. 38:2392017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee WS, Choi YJ, Cheon YH, Hong MJ, Lee
CH, Lee MS, Lee SI and Yoo WH: AB0146: Rebamipide inhibits
IL-1β-induced proliferation of rheumatoid arthritis synovial
fibroblasts through the phosphor-Jun N-terminal kinase (P-JNK)
pathway. Ann Rheum Dis. 74:9392015. View Article : Google Scholar
|
|
19
|
Reis C, Pilz LK, Keller L, Roenneberg T
and Paiva T: PSQI largely ignores sleep on work-free days both in
the general population and in clinical sleep medicine samples.
Sleep Med. 40:e2772017. View Article : Google Scholar
|
|
20
|
Xia T, Li S, Ma R, Guan S, Li J, Li H,
Zhang H, Lin Q, Zhao Z and Wang B: Effects of liver depression and
psychological stress on human uterine leiomyoma cells by an
AR-cAMP-PKA signal transduction pathway. Taiwan J Obstet Gynecol.
56:291–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meller W, Welle N, Sutley K and Thurber S:
Depression and liver transplant survival. Psychosomatics. 58:64–68.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niznikiewicz MM, Gerashchenko D, Mckenna
JT, Basheer R, Strecker RE, McCarley RW and Zielinski MR: 0021:
Sleep deprivation activates NLRP3 inflammasomes in neurons and
glia. Sleep. 40 suppl 1:A82017. View Article : Google Scholar
|
|
23
|
Zielinski MR, Gerashchenko D, Karpova SA,
Konanki V, McCarley RW, Sutterwala FS, Strecker RE and Basheer R:
The NLRP3 inflammasome modulates sleep and NREM sleep delta power
induced by spontaneous wakefulness, sleep deprivation and
lipopolysaccharide. Brain Behav Immun. 62:137–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chennaoui M, Gomez-Merino D, Drogou C,
Geoffroy H, Dispersyn G, Langrume C, Ciret S, Gallopin T and Sauvet
F: Effects of exercise on brain and peripheral inflammatory
biomarkers induced by total sleep deprivation in rats. J Inflamm
(Lond). 12:562015. View Article : Google Scholar : PubMed/NCBI
|