Introduction
Infantile hemangioma (IH), which is a unique
vascular tumor that recapitulates both the formation as well as the
spontaneous disappearance of blood vessels, is a type of benign
tumor with high incidence in infants (1,2). Most
IHs finally involute and resolve without intervention during
childhood. The lesions that result from the atrophy of IHs can form
permanent residual changes, including scarring and fibro-fatty
residuum (3). Currently, the leading
candidate for the cellular origin of IHs is hemangioma stem cells
(HemSC), which have been demonstrated to be highly proliferative in
culture and capable of differentiating into multiple cell types.
including endothelial cells, adipocytes, and osteocytes. Thus, it
is of considerable interest to investigate the mechanism of
adipogenesis in HemSCs.
IGF-2 is a small but powerful polypeptide that has
been shown to have several biological functions and play an
important role in regulating tumor growth (4). As an endogenous regulator, the
insulin-like growth factor-2 (IGF-2) could sustain the nature of
embryonic stem cells (5). Several
studies also have demonstrated that the expression of IGF-2 was
markedly upregulated during adipogenesis in cells (6–8).
Moreover, recent studies have shown that IGF-2 was highly expressed
during the proliferating phase of hemangiomas (9–11),
implicating the potential role of IGF-2 in HemSCs activity. IGF-2
binds to three kinds of receptors: type-1 IGF receptor (IGF-1R),
type-2 IGF receptor (IGF-2R), and the insulin receptor (IR). The
activation of each receptor by the ligand results in different
biological effects (4). The insulin
receptor is pivotal in the maintenance of glucose homeostasis, and
the IGF-1R regulates cellular proliferation, differentiation,
migration, and protection from apoptosis (12). Although IGFR-2R has no intrinsic
catalytic activity, it has been postulated that it may serve as a
membrane-bound IGF-binding protein that inactivates IGF-2 (13). Most biological outcomes of IGF-2 have
been attributed to its interaction with IGF-1R or IR (14). Several other studies have confirmed
that the phosphoinositide 3-kinase (PI3K)/AKT branch of the IGF-1R
signaling pathway is a significant influence on adipocyte
differentiation (15,16). These previous findings raise the
following questions: What role does IGF-2 play in the adipogenesis
of HemSCs? It is modulated by IGF-1R or PI3K/AKT signaling?
In the present study, we investigated the hypothesis
that IGF-2 affects the proliferation and adipogenic differentiation
of HemSCs through IGF-related pathways. In recent years,
propranolol has been used as a first-line therapy in many cases.
Despite the widespread use of this effective therapy, its mechanism
of action in IHs is not yet understood. However, previous studies
have shown that propranolol could accelerate adipogenesis in
HemSCs. Thus, we hypothesized that propranolol-induced promotion of
the dysregulated adipogenesis may be mediated by IGF-2 via
IGF-1R/PI3K/AKT pathway. The findings of the present study may
provide novel insights into the specific mechanism of propranolol's
effects on IH.
Materials and methods
Preparation of hemangioma
specimens
The Ethics Committee of the Second Affiliated
Hospital of Anhui Medical University (PJbb2017-004) approved the
collection of abscized human hemangiomas. The tissues were used
instantly for cell isolation and cultivation in in vitro
experiments.
Isolation and identification of
HemSCs
The proliferating IH tissues resected from the
patients were immediately immersed in a growth medium at 4°C
[Dulbecco's modified Eagle's medium, high glucose, 10% fetal bovine
serum (FBS; Biological Industries, Kibbutz Beit Haemek, Israel),
and 1% penicillin-streptomycin (PS; Beyotime Institute of
Biotechnology, Haimen, China)] and then quickly taken to our
laboratory. The fatty and skin tissue were resected, the samples
were rinsed three times in phosphate-buffered saline (PBS) and then
minced. A 0.2% compound of collagenase (cat. no. 17454; Serva
Electrophoresis GmbH, Heidelberg, Germany) was used to digest the
samples at 37°C for 2 h until they were chylous. They were then
filtered through a 100-micron cell strainer. From this single cell
suspension, cells expressing CD133 were selected using a magnetic
beads technology (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
and then cultured on fibronectin-coated plates (Corning
Incorporated, Corning, NY, USA) in EGM-2 media (cat. no. CC-4176;
Lonza Group, Ltd., Basel, Switzerland) that was supplemented by 20%
FBS and 1% PS.
Proliferation assay
Logarithmic growth phase HemSCs were seeded into
96-well tissue culture plates (Corning Incorporated) at an initial
density of 2×103. After serum starvation for 24 h, IGF-2
(cat. no. 100-12-50UG; PeproTech, Inc., Rocky Hill, NJ, USA) at 0,
10, 20, 100, and 200 ng/ml, respectively, was added to EBM-2
containing 5% FBS in the experimental groups. After 72 h in the
culture, CCK-8 reagents (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) were added to disparate wells under different
treatments. The absorbency was read at 490 nm using a microplate
reader (Biotek ELx 800; BioTek Instruments, Inc., Winooski, VT,
USA).
To examine the growth kinetics of IGF-2-treated
HemSCs, HemSCs were seeded into 96-well plates (Corning
Incorporated) at a density of 1.5×103 cells/well. After
serum starvation, the cells were treated with 100 ng/ml IGF-2
containing 5% FBS. CCK-8 was added at 0, 1, 3, 5, and 7 days after
culture and incubated at 37°C for 4 h. The results of the OD values
at 490 nm were tested by a microplate reader (Biotek Elx 800;
BioTek Instruments, Inc.).
Immunohistochemistry
When the cells had increased by 70% in 24 well
plates, they were washed in PBS followed by fixing in 4%
poly-formaldehyde for 15 min, drying in air for 5 min, and
permeabilizing in 0.5% Triton X-100 for 10 min. Endogenous
peroxidase activity was inactivated using 3% hydrogen peroxide for
5–10 min at room temperature. The slides were incubated in blocking
solution (reagent A) for 15–20 min, and then the blocking solution
was out-welled without washing. Between each step, the cells were
washed in PBS, the slides were incubated overnight at 4°C, and the
primary antibodies were diluted by PBS to achieve the required
concentration. The primary antibodies were replaced by PBS in the
negative control group. The secondary antibodies were incubated for
10–15 min at 37°C. The cells were observed under a bright-field
microscope for 3–10 min immediately after the DAB solution (cat.
no. K166724B; 50 µl DAB: 1 ml DAB substrate, kept away from light)
was added to the slides, which underwent hematoxylin staining for
0.5–1 min and gum seal after drying at room temperature. The cells
were photographed using bright-field microscope illumination
(Olympus IX71; Olympus Corporation, Tokyo, Japan).
Oil red O-staining
To evaluate the adipogenic differentiation of
IGF-2-treated HemSC, 5.0×104 cells were seeded on 6-well
plates in EGM-2/10% FBS. When the cells were oversaturated, the
original medium was changed to adipogenic differentiation media
(cat. no. HUXMF-90031; Cyagen Biosciences, Santa Clara, CA, USA)
with IGF-2 (100 ng/ml), IGF-2 (100 ng/ml) plus OSI-906 (cat. no.
S1091; 1 µM; Selleck Chemicals, Houston, TX, USA), or no treatment
for 10 days. The oil red o-stained cells were observed using an
inverted microscope. Photos were taken using the Eclipse E800
microscope (Nikon Corporation, Tokyo, Japan).
Western blot analysis
HemSCs were grown on 10-cm tissue culture plates
(Corning Incorporated). The cells were cultured in EGM-2/FBS-10%
containing IGF-2 (100 ng/ml), IGF-2 (100 ng/ml) plus OSI-906 (1
µM), IGF-2 (100 ng/ml) plus LY294002 (cat. no. HY-10108; 10 µM;
MedChem Express Co., Ltd., Shanghai, China), or no treatment.
Protein extracts were subjected to SDS-PAGE on 8–15% polyacrylamide
gels. The samples were then transferred to PVDF membranes. The
membranes were blocked by 5% non-fat milk for 2 h and then
incubated first with primary antibodies overnight at 4°C and then
with secondary antibodies. The following primary antibodies were
used: Anti-PPARγ (cat. no. bs-0530P), anti-C/EBPα (cat. no.
bs-1630R), anti-C/EBPβ (cat. no. bs-1396R), anti-adiponectin (cat.
no. 0471R; all from BIOSS, Beijing, China), and anti-pAKT (cat. no.
9271), anti-AKT (cat. no. 9272), anti-IGF-2 (cat. no. 25690), and
anti-β-actin (cat. no. 3700; Cell Signaling Technology, Inc.,
Danvers, MA, USA).
Cell culture and treatment with
propranolol
HemSCs were maintained on fibronectin-coated 10-cm
plates in EGM-2/FBS-20%. The medium was changed every two days.
HemSCs at passage numbers 5 to 15 were used in all experiments.
During the media changes, propranolol hydrochloride (cat. no.
P8688; Sigma-Aldrich, St. Louis, MO, USA) was added to the culture
media at the indicated concentrations.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using the TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
cDNA was synthesized by the reverse transcription of 2 µg of total
RNA using a PrimeScript™ RT reagent (Takara, Dalian, China). A
semi-quantitative real-time polymerase chain reaction was conducted
using the Applied Biosystems 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the SYBR Premix Ex
TaqTM (Takara). The results were normalized to β-actin expression
levels, and the samples were analyzed in triplicate. The gene
expression levels of C/EBPα, C/EBPβ and PPARγ were then determined
(Table I).
 | Table I.Primers. |
Table I.
Primers.
| Gene | Primers (5′-3′) |
|---|
| PPARγ (F) |
TCTCCAGCATTCTACTCCACA |
| PPARγ (R) |
CAGGCTCCACTTTGATTGC |
| C/EBPα
(F) |
TGGACAAGAACAGCAACGAG |
| C/EBPα
(R) |
TTGTCACTGGTCAGCTCCAG |
| C/EBPβ
(F) |
TTTCGAAGTTGATGCAATCG |
| C/EBPβ
(R) |
CAACAAGCCCGTAGGAACAT |
| β-actin
(F) |
CTGGAACGGTGAAGGTGACA |
| β-actin
(R) |
AAGGGACTTCCTGTAACAATGCA |
Statistical analyses
The results of two independent experiments were
expressed as the mean ± SD (n=3 for each experiment). In
comparing the two groups, an unpaired Student's t-test was used to
assess the differences, and analysis of variance was applied for
the analysis of the mean values among multiple groups. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
IGF-2 stimulated cell proliferation in
HemSCs
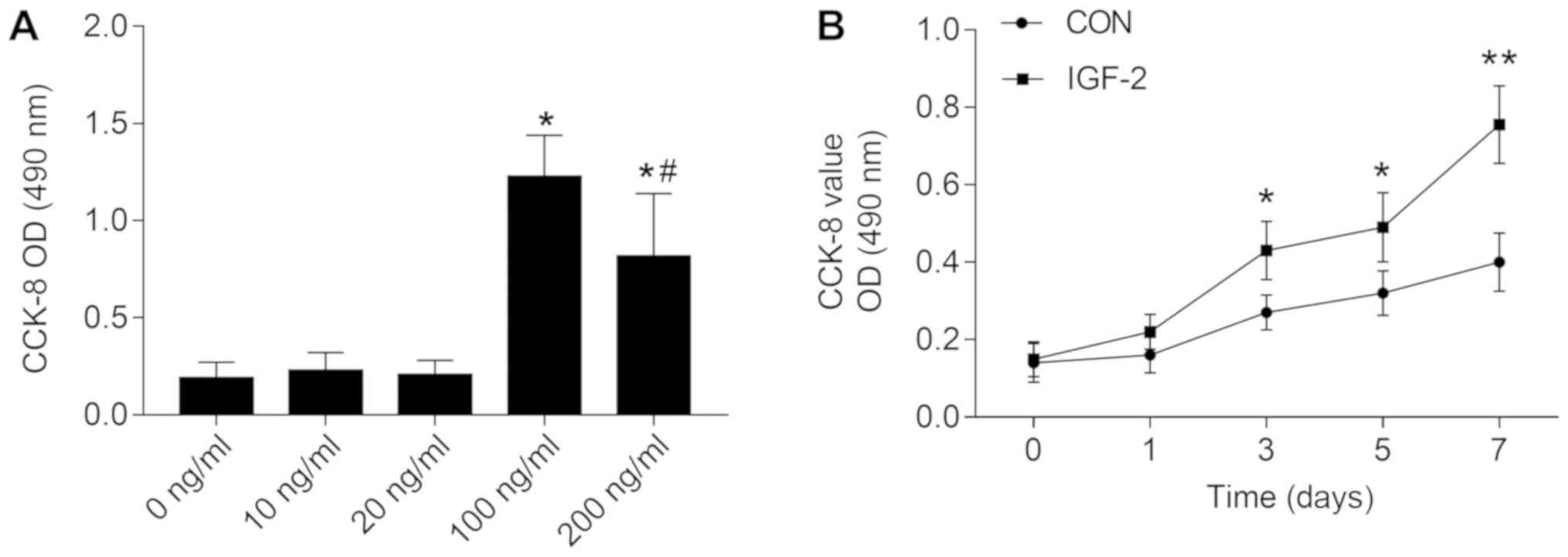
A CCK-8 assay was performed to determine whether
IGF-2 influenced the proliferation of HemSCs in vitro. The
results showed that 10–200 ng/ml of IGF-2 promoted the
proliferation of HemSCs. The concentration at 100 ng/ml was the
most appropriate among all the groups (Fig. 1A). When 100 ng/ml IGF-2 was used for
the cell growth curve, the IGF-2-treated cells promoted increased
cell proliferation compared to the non-treatment group from day 1
to day 7 (Fig. 1B).
The expression of IGF-2 and its
different receptors in HemSCs
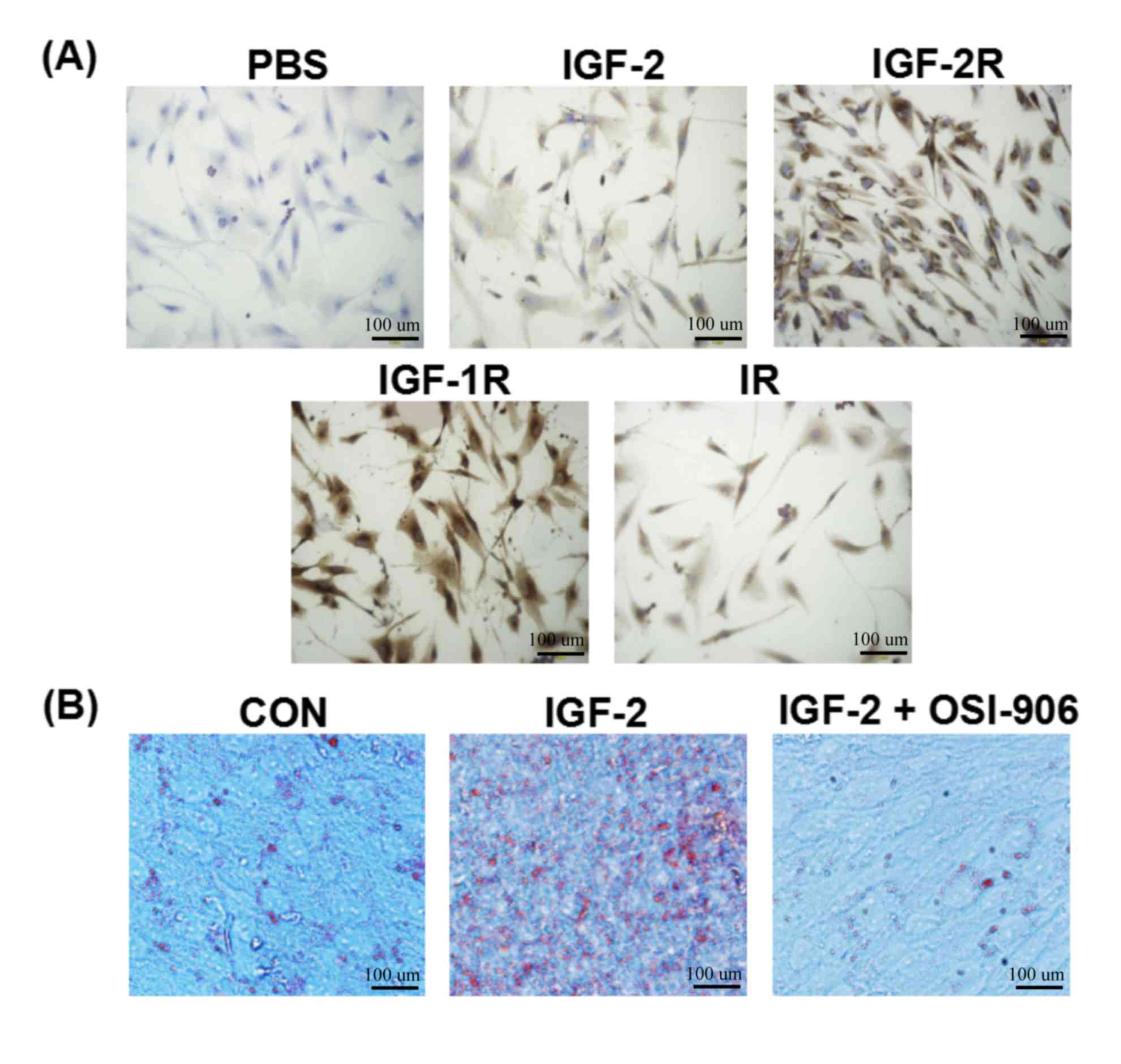
As shown in Fig. 2A,
the expression of IGF-2, IGF-2R, IGF-1R, and IR was positive in the
HemSCs. In cell localization, the dyeing of IGF-2 was primarily in
the cytoplasm. IGF-2R, IGF-1R, and IR were in both the membrane and
the cytoplasm.
IGF-2 enhanced lipogenesis during
differentiation of HemSCs into adipocytes
In order to determine whether the induction of IGF-2
accelerated the differentiation of adipocytes, HemSCs was exposed
to IGF-2 (100 ng/ml), IGF-2 (100 ng/ml) plus OSI-906 (1 µM), or no
treatment for 10 days in adipogenic differentiation media (Cyagen
Biosciences). Phase-contrast microscopy was used after oil red
o-staining. The results indicated that IGF-2 treatment stimulated
the accumulation of lipogenesis in the HemSCs, which was shown by
the increase in cell numbers and the density of lipid droplets in
the HemSCs treated with adipogenic differentiation media containing
IGF-2 compared to the adipogenic differentiation media alone or the
adipogenic differentiation media containing IGF-2 plus OSI-906 (1
µM) group (Fig. 2B). The results
further confirmed the adipogenic effects of IGF-2 on HemSCs. The
results also showed that the IGF-1R inhibitor (OSI-906, 1 µM)
suppressed the IGF-2 inducing lipid accumulation.
IGF-2 enhanced adipogenic
differentiation and AKT protein phosphorylation inhibited by
OSI-906 or LY294002
We also aimed to determine whether IGF-2 could
regulate the adipogenic differentiation of HemSCs into adipocytes
via IGF receptors. The results of the western blot analysis showed
that the expressions of disparate transcription factors and
adipocyte specific proteins were increased in the IGF-2 treated
group compared to the control group. C/EBPα, C/EBPβ, PPARγ, and
adiponectin were increased, which indicated that IGF-2 enhanced
adipogenesis in HemSCs. Interestingly, the IGF-2-treated medium in
the presence of OSI-906 or LY294002 or LY294002 alone could repress
the IGF-2-induced adipogenesis and inhibit the protein expressions
of C/EBPα, C/EBPβ, PPARγ, and adiponectin (Fig. 3A). In addition, the p-AKT level was
attenuated compared with that in the IGF-2 treated cells (Fig. 3B). These findings suggested that the
IGF-2 mediated the induction of adipocyte differentiation by
upregulating the phosphorylation of AKT via the IGF-1R-PI3K
signaling pathway in the HemSCs.
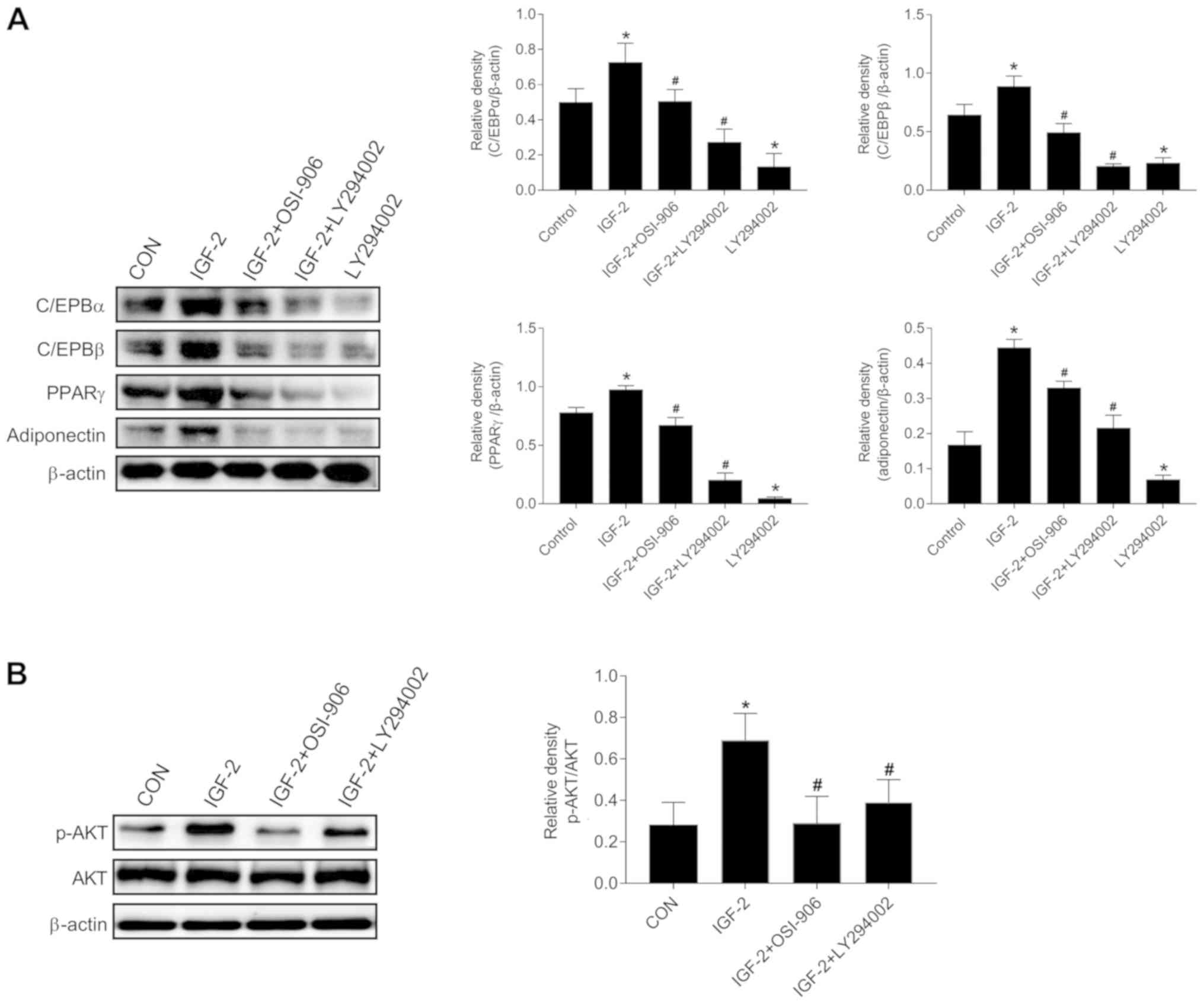 | Figure 3.Effects of IGF-2 on the expression of
adipogenic markers in HemSCs. The C/EBPα, C/EBPβ, PPAR, and
adiponectin protein levels were analyzed by western blot analysis.
(A) The results showing protein levels in untreated and treated
HemSCs for C/EBPα, C/EBPβ, PPARγ, and adiponectin in IGF-2 (100
ng/ml), IGF-2 (100 ng/ml) with OSI-906 (1 µM), IGF-2 (100 ng/ml)
with LY294002 (10 µM), and LY294002 (10 µM). β-actin was used as a
loading control. (B) Western blot analysis showed p-AKT and total
AKT as well as β-actin protein bands in confluent HemSCs cultures
exposed to the indicated treatments for 1 h. Groups: No treatment;
IGF-2 (100 ng/ml); IGF-2 (100 ng/ml) plus OSI-906 (1 µM); IGF-2
(100 ng/ml) plus LY294002 (10 µM). Quantification of the p-AKT
protein levels showed an obvious increase in the IGF-2 group
(P<0.05). No changes were detected in the total AKT protein
levels among the groups (P>0.05). Values are mean ± SD.
n=3, *P<0.05 vs. Control group. #P<0.05 vs.
IGF-2. IGF-2, insulin-like growth factor-2; HemSC, hemangioma stem
cell; p-AKT, phosphorylated AKT. |
Effects of propranolol on HemSCs
HemSCs were grown in EGM-2/20% FBS at vrious
nanomolar concentrations of propranolol (0, 50, and 100 µM). During
24 h of adipogenic differentiation, the gene expression levels of
C/EBPα, C/EBPβ (CCAAT/enhancer-binding protein) and PPAR
(peroxisome proliferator-activated receptor) were observed. In the
50-µM propranolol solution, the C/EBPα RNA level was no changed
compared with the control group, whereas it was increased after
intervention in 100 µM propranolol solution compared to the control
group. The RNA levels of C/EBPβ and PPARγ were significantly
elevated compared with the control group after intervention with 50
and 100 µM of propranolol solution (Fig.
4B). The changes in the levels of protein expression of C/EBPα,
C/EBPβ, and PPARγ were similar to those in RT-qPCR in the detection
of the adipogenic differentiation index in HemSCs. However, no
significant change in C/EBPα was found (Fig. 4A). Interestingly, several
concentrations of propranolol-treated HemSCs showed marked
increases in IGF-2 compared to the control group (Fig. 4C). Furthermore, IGF-2 may have been
involved in this effect, which was shown by its increased levels
following the treatment by pronanolol.
Discussion
Traditionally, IH has been regarded as a benign
tumor of the microvasculature. The present findings showed that the
cellular origin of IHs was HemSCs, which has the subject of
extensive research in the biology of IH (1). In the present study, the spontaneous
disappearance of IHs provided a new angle to explore the mechanisms
controlling the phases of the proliferation and the involution of
hemangiomas. Several previous studies attempted to confirm the
molecules that stimulated the adipogenic differentiation of HemSCs
to aid the development of drugs for the treatment of IH. However,
whether IGF-2 promotes or inhibits the adipogenic differentiation
of HemSCs in vitro remains unclear. In this study, we
observed that IGF-2 significantly improved the proliferation of
HemSCs in a dose-dependent manner. In vitro, the highly
enhanced differentiation of HemSCs was derived from the
proliferating IH tissues. Furthermore, the IGF-2-enhanced
differentiation appeared to be mediated by IGF-1R through the
activation of the PI3K-AKT signaling pathway in HemSCs.
IGF-2 is key in regulating tumor growth and in
several biological functions (4).
IGF-2 stimulated both the proliferation and the adipogenesis of
cell populations derived from adult rat bone (6). We presupposed that IGF-2 might
influence the proliferation and differentiation of HemSCs. Our
research showed that IGF-2 significantly facilitated the
proliferation of HemSCs in a dose-dependent manner, and it
increased the lipid accumulation of HemSCs in vitro. The
inhibition of IGF-1R by OSI-906 blocked the IGF-2-induced
adipogenic differentiation of HemSCs. Adipogenesis is closely
regulated by transcription factors including members of the PPARs
and CCAAT/enhancer-binding protein (C/EBP) families (17). C/EBPs have diverse roles in the
regulation of pre-adipocyte differentiation. Furthermore, in
pre-adipocytes, C/EBPβ accelerates the induction rate of C/EBPα,
which is an integral part of the genetic cascade that causes
adipogenesis (18). C/EBPα is
another transcription factor that is induced during adipocyte
differentiation, which cooperates with PPARγ to stimulate the
adipocyte process and, adiponectin promotes preadipocyte
differentiation via the PPARγ pathway (19). The results of the present research
demonstrated that IGF-2 played an important role not only in the
early-stage of adipogenesis but also in the later stage of lipid
accumulation, which was indicated by the upregulated C/EBPα,
C/EBPβ, PPARγ, and adiponectin protein levels of the IGF-2-HemSCs,
which was confirmed by the results of oil red o-staining. IGF-2R
functions as a type of decoy receptor via the binding and
inactivation of IGF-2, which appeared to increase proliferation and
stimulate differentiation in granulosa cells via the IGF-1R
(20,21). Several studies confirmed that the
PI3K/AKT branch of the IGF-1R signaling pathway influences
adipocyte differentiation (15,16). In
3T3-L1 pre-adipocytes, the inhibition of PI3K by LY294002
restrained adipocyte differentiation (22). In this research, we found that
treatment with the IGF-1R inhibitor-OSI-906 and PI3K
inhibitor-LY294002 in combination with IGF-2 reduced the expression
of C/EBPα, C/EBPβ, PPARγ, and adiponectin with compared with
treatment with IGF-2 alone. The same changes were also observed in
the LY294002 single-treated group compared to the group without
IGF-2. Furthermore, the phosphorylation of AKT was promoted by the
treatment with IGF-2, whereas it was inhibited by the treatment
with OSI-906 or LY294002. These findings suggest that the effects
of IGF-2 on adipocyte differentiation in HemSCs are through the
IGF-1R/PI3K/AKT pathway.
Propranolol has proved to be efficacious in
problematic IHs. Previous studies have found that propranolol
changed HemSCs proliferation and differentiation into adipocytes
(23). In basal media, we
demonstrated that treatment with propranolol caused an obvious
increase in transcripts in multiple pro-adipogenic genes in the
absence of adipogenic stimulation. The RNA levels of PPARγ and
C/EBPβ were significantly increased compared with the control group
after intervention with 50 and 100 µM of propranolol solution. In
contrast, C/EBPα was not changed by 50 µM of propranolol. These
results were in accordance with Wong, Li, and England (23–25). The
protein expression level of C/EBPα, C/EBPβ and PPARγ were obviously
enhanced in 100 µM of propranolol, but C/EBPα was not significantly
altered in 50 µM group. The changes in the protein expression level
of PPARγ, C/EBPα and C/EBPβ were similar to those of RT-qPCR in
detection of adipogenic differentiation index in HemSCs. We
speculated that the pro-adipogenic gene expression was perturbed
during early adipogenesis (23,25).
Elevated expressions of gene and protein levels of PPARγ were seen
in later stages of adipogenesis during terminal differentiation
(18). The gene level was increased
in the propranolol treatment group, which explained the increase in
lipogenesis observed in propranolol-treated HemSCs. This phenomenon
was consistent with the results of Wong, A. (23).
The present study has the following limitations. The
population of patients in this study was small. Although we found
that propranolol enhanced adipogenesis in HemSCs, we did not
explore this effect in an animal study to confirm that propranolol
regulates IGF-2, thereby affecting HemSCs in adipogenesis.
Based on the results of this study, we conclude that
IGF-2 could promote proliferation and adipogenic differentiation in
HemSCs. Furthermore, IGF-2 could enhance the adipogenesis of HemSCs
through the IGF-1R and PI3K pathways. Propranolol and IGF-2 could
play an important role during adipogenic differentiation and may
have clinical implications for the treatment of IH.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Integrated
Project of Public Welfare Technology Application of Anhui Province,
Science, and Technology Agency of Anhui (grant no.
1501ld04048).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KZ, FW and JH were involved in drafting the
manuscript and data analysis. KZ assisted with acquisition of data.
KZ and YL performed and analyzed western blot analyses, RT-qPCR and
IHC. JX, HL, DC and XH provided excellent technical assistance. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the second Hospital of Anhui Medical University. Written informed
consents were signed by the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kilcline C and Frieden IJ: Infantile
hemangiomas: How common are they? A systematic review of the
medical literature. Pediatr Dermatol. 25:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleiman A, Keats EC, Chan NG and Khan ZA:
Evolution of hemangioma endothelium. Exp Mol Pathol. 93:264–272.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng CE and Friedlander SF: Infantile
hemangiomas, complications and treatments. Semin Cutan Med Surg.
35:108–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halje M, Nordin M, Bergman D and Engström
W: Review: The effect of insulin-like growth factor II in the
regulation of tumour cell growth in vitro and tumourigenesis in
vivo. In Vivo. 26:519–526. 2012.PubMed/NCBI
|
|
5
|
Bendall SC, Stewart MH, Menendez P, George
D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau
A, Yang J, Bossé M, et al: IGF and FGF cooperatively establish the
regulatory stem cell niche of pluripotent human cells in vitro.
Nature. 448:1015–1021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia D and Heersche JN: Insulin-like growth
factor-1 and −2 stimulate osteoprogenitor proliferation and
differentiation and adipocyte formation in cell populations derived
from adult rat bone. Bone. 27:785–794. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cha MH, Kim IC, Lee BH and Yoon Y:
Baicalein inhibits adipocyte differentiation by enhancing COX-2
expression. J Med Food. 9:145–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellows CG, Jia D, Jia Y, Hassanloo A and
Heersche JN: Different effects of insulin and insulin-like growth
factors I and II on osteoprogenitors and adipocyte progenitors in
fetal rat bone cell populations. Calcif Tissue Int. 79:57–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Picard A, Boscolo E, Khan ZA, Bartch TC,
Mulliken JB, Vazquez MP and Bischoff J: IGF-2 and FLT-1/VEGF-R1
mRNA levels reveal distinctions and similarities between congenital
and common infantile hemangioma. Pediatr Res. 63:263–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ritter MR, Dorrell MI, Edmonds J,
Friedlander SF and Friedlander M: Insulin-like growth factor 2 and
potential regulators of hemangioma growth and involution identified
by large-scale expression analysis. Proc Natl Acad Sci USA.
99:7455–7460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Wylie-Sears J, Boscolo E, Mulliken
JB and Bischoff J: Genomic imprinting of IGF2 is maintained in
infantile hemangioma despite its high level of expression. Mol Med.
10:117–123. 2004.PubMed/NCBI
|
|
12
|
Hopkins A, Crowe PJ and Yang JL: Effect of
type 1 insulin-like growth factor receptor targeted therapy on
chemotherapy in human cancer and the mechanisms involved. J Cancer
Res Clin Oncol. 136:639–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spicer LJ, Voge JL and Allen DT:
Insulin-like growth factor-II stimulates steroidogenesis in
cultured bovine thecal cells. Mol Cell Endocrinol. 227:1–7. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Byrd JC and MacDonald RG: Mechanisms for
high affinity mannose 6-phosphate ligand binding to the
insulin-like growth factor II/mannose 6-phosphate receptor. J Biol
Chem. 275:18638–18646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SP, Ha JM, Yun SJ, Kim EK, Chung SW,
Hong KW, Kim CD and Bae SS: Transcriptional activation of
peroxisome proliferator-activated receptor-gamma requires
activation of both protein kinase A and Akt during adipocyte
differentiation. Biochem Biophys Res Commun. 399:55–59. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen
A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman
KG and Hay N: Dwarfism, impaired skin development, skeletal muscle
atrophy, delayed bone development, and impeded adipogenesis in mice
lacking Akt1 and Akt2. Genes Dev. 17:1352–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Madsen MS, Siersbaek R, Boergesen M,
Nielsen R and Mandrup S: Peroxisome proliferator-activated receptor
γ and C/EBPα synergistically activate key metabolic adipocyte genes
by assisted loading. Mol Cell Biol. 34:939–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musri MM and Parrizas M: Epigenetic
regulation of adipogenesis. Curr Opin Clin Nutr Metab Care.
15:342–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi SK, Park S, Jang S, Cho HH, Lee S,
You S, Kim SH and Moon HS: Cascade regulation of PPARγ(2) and
C/EBPα signaling pathways by celastrol impairs adipocyte
differentiation and stimulates lipolysis in 3T3-L1 adipocytes.
Metabolism. 65:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adashi EY, Resnick CE and Rosenfeld RG:
Insulin-like growth factor-I (IGF-I) and IGF-II hormonal action in
cultured rat granulosa cells: Mediation via type I but not type II
IGF receptors. Endocrinology. 126:216–222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spicer LJ and Aad PY: Insulin-like growth
factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine
granulosa cells through the IGF1 receptor: Role of
follicle-stimulating hormone and IGF2 receptor. Biol Reprod.
77:18–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J and Liao K: Protein kinase B/AKT 1
plays a pivotal role in insulin-like growth factor-1 receptor
signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem.
279:35914–35922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong A, Hardy KL, Kitajewski AM, Shawber
CJ, Kitajewski JK and Wu JK: Propranolol accelerates adipogenesis
in hemangioma stem cells and causes apoptosis of hemangioma
endothelial cells. Plast Reconstr Surg. 130:1012–1021. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li HX, Xiao L, Wang C, Gao JL and Zhai YG:
Review: Epigenetic regulation of adipocyte differentiation and
adipogenesis. J Zhejiang Univ Sci B. 11:784–791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
England RW, Hardy KL, Kitajewski AM, Wong
A, Kitajewski JK, Shawber CJ and Wu JK: Propranolol promotes
accelerated and dysregulated adipogenesis in hemangioma stem cells.
Ann Plast Surg. 73 Suppl 1:S119–S124. 2014. View Article : Google Scholar : PubMed/NCBI
|