Introduction
Ulcerative colitis (UC), an inflammatory bowel
disease (IBD) affecting the colon, is characterized by a chronic,
relapsing, organ-specific inflammatory state of the mucosa
(1). The incidence and prevalence of
UC are higher in countries with greater economic development,
particularly in the northern countries of Western Europe, Canada,
USA, Australia and New Zealand (2).
The incidence of UC has recently increased (3). The pathogenesis of UC is associated
with various factors, including genetic predisposition (4), environmental risk factors (5) and the state of the immune system
(6). Furthermore, the disorder of
the immune system is considered an important factor (7), and psychological stress is considered
one of the risk factors for UC (8).
The recent increase in the pace of human life,
competition, pollution and the number of stress stimuli contributed
to greater susceptibility to disease. Research into the influence
of stress on disease status has received increasing attention from
clinicians. Stress causes alterations in psychological and
physiological functions due to recognition and evaluation of the
stimuli from a stress source (9).
The mechanism by which psychological factors influence the body has
not been extensively studied. It is challenging to design
experiments to quantitatively measure psychological factors and
evaluate psychosomatic associations. However, animal models of
stress have been previously established and used to study
physiological responses (10).
Therefore, the present study aimed to observe the effects of stress
on the general condition, characteristics of the colonic mucosa and
immunity-associated indicators of normal control mice and mouse
models of UC mice. In addition, the present study investigated the
effect of stress on the occurrence, development, prognosis and
possible pathogenesis of UC.
Materials and methods
Materials
Mice were weighed 1 h before the initiation of the
model, and a calculation was made to obtain a 5%
2,4,6-trinitrobenzenesulfonic acid solution (TNBS; 150 mg/kg;
Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The final solution
was mixed to achieve a solution of 5% TNBS and 380 g/l ethanol
using a 4:1 volume ratio of ethanol to TNBS.
The laboratory equipment used in the present study
included a LabSystems Multiskan MS plate reader (Thermo Labsystems,
Helsinki, Finland), anesthesia machine (Beijing Yiren Hengye
Technology Co. Ltd, Beijing, China), optical microscope (Olympus
Corporation, Tokyo, Japan), water jacketed incubator (Shanghai
Yiheng Scientific Instruments, Co., Ltd., Shanghai, China) and
gavage needles (Changzhou Jinliyuan Medical Instrument Co. Ltd,
Changzhou, China). Enzyme-linked immunosorbent assay (ELISA) kits
for secretory (s) immunoglobulin (Ig)A (SBJ-R0054), interleukin
(IL)-6 (SBJ-H0465), IL-8 (SBJ-R0033), tumor necrosis factor (TNF)-α
(SBJ-R0040), complement component (C)3 (SBJ-H0199) and C4
(SBJ-R0368) were obtained from SBJBio (Nanjing, China), and the
ELISA kit for IgA (FU-D1033) was obtained from Beijing Xin
Fangcheng Biotechnology Co., Ltd. (Beijing, China).
Animals
A total of 80 experimental specific pathogen-free
BALB/c mice (age, 6–8 weeks; weight, 18–22 g) were purchased from
the Institute of Laboratory Animal Science, Chinese Academy of
Medical Sciences (Beijing, China). Mice were maintained at a
specific pathogen-free facility with 40–70% humidity and 24–26°C
temperature, with a 12-h light/dark cycle and free access to food
and water. The research program was approved by the Ethics
Committee of Tianjin Nankai Hospital. The mice were allowed to
acclimate to laboratory conditions for 7 days and then fasted for
36 h with ad libitum access to water. The mice were
subsequently deprived of water 1 h before the experiment and
randomly divided into 4 groups (n=20/group): i) Control group (A);
ii) stress group (B), iii) UC + stress group (C); and UC model
alone (D). Mice in groups C and D were treated with TNBS-ethanol
solution to induce UC. After 5 days, the mice in groups B and C
were restrained in water, as described below.
Establishment of the animal model
Establishment of the animal model of
UC
The present study used a modified TNBS protocol
combined with an addition of an alcohol complex to establish the
animal model of UC (11). Mice were
fasted for 36 h with free access to water. Animals were
subsequently weighed 1 h before the experiment in order to
calculate the drug dose to be administered and were anaesthetized
by isoflurane inhalation, as previously described (anesthesia
machine oxygen flow rate, 0.6 nl/min; isoflurane concentration,
4–6%; maintenance anesthesia, isoflurane 1–2% for 1 min using an
anesthesia tube and plastic funnel to cover the nose and mouth of
the mice) (12). Anesthesia was
considered successful when the mice were calm, supine and breathing
smoothly. For administration of the TNBS-ethanol solution, a gavage
needle was connected to a 1-ml syringe. The needle was lubricated
with liquid paraffin and slowly inserted into the anus of each
mouse to a depth of ~1–2 cm. The solution was slowly injected with
the gavage needle continuously pushed. The solution was completely
injected at a depth of 4–5 cm into the anus. The tail of the mouse
was slowly lifted to clench the anus to prevent the outflow of
liquid and in order to ensure that the TNBS solution was dispersed
in the colon. In addition, the anesthesia table and the mice were
maintained at angle of 45–60°C and the position of the mice was
rotated for 5 min to ensure that the TNBS solution was in contact
with the wall of the colon. The mice naturally woke and became
alert, and were subsequently released into the cages and provided
free access to food and water. Measurements were taken 5 days
following induction of the model of UC.
Establishment of the animal model of
stress
Groups B and C were subjected to stress via the
water immersion restraint method. Mice were fasted for 24 h with
ad libitum access to water. Mice were anaesthetized in a
cage and immersed in water continuously for 4 h (water temperature,
25±1°C; water level, cartilago ensiformis) as previously described
(13).
General condition of the mice
The following characteristics of mice were observed
and recorded: Mental state (via duration of sleep, time of waking
and response to external stimuli), physical activity (14), food consumption, body weight, fur
gloss (15), stool type, fecal
occult blood and mortality rate. The disease activity index (DAI)
was calculated as previously described by Murthy et al
(16) (Table I), using the following formula:
DAI=[weight loss (%) + characterization of feces +
hematochezia)/3.
 | Table I.Disease activity index scores. |
Table I.
Disease activity index scores.
| Score | Weight loss
(%) | Characterization of
feces | Hematochezia |
|---|
| 0 | 0 | Normal | Negative |
| 1 | 1–5 | – | – |
| 2 | 6–10 | Semi-loose | Occult blood
(+) |
| 3 | 11–15 | – | – |
| 4 | >15 | Loose | Bloody visible by
naked eye |
Macroscopic scores
Mice were restrained and blood samples were
collected via orbital puncture following anesthesia as described
above. Mice were euthanized by cervical dislocation and dissected
to open the abdominal cavity and access the colon. Colonic lavage
was performed. Following removal of the colon lavage fluid and
brushing of intestinal tissue, the colon was cut into sections
along the mesenteric side and fixed with a pin. The colon was
subsequently observed by the naked eye and scored according to the
criteria described in Table II
(17,18).
 | Table II.Macroscopic scores of the colonic
mucosa. |
Table II.
Macroscopic scores of the colonic
mucosa.
| Score | Alterations in
colonic mucosa |
|---|
| 0 | No damage |
| 1 | Mucosal hyperemia,
edema, no ulcer |
| 2 | Mucosal hyperemia,
edema, mild erosion, no ulcer |
| 3 | Mucosal hyperemia,
edema, moderate erosion, single ulcer |
| 4 | Mucosal hyperemia,
edema, severe erosion, multiple ulcers |
| 5 | Mucosal hyperemia,
edema, severe erosion, ulcer >1 cm |
Microscopic scores
Distal colon sections (0.5 cm from the anus;
dimensions, lx0.5 cm) were obtained for analysis, fixed with 4%
formalin at room temperature for 24 h, and embedded in paraffin.
Subsequently, 4-µm-thick sections were prepared and
hematoxylin-eosin staining was performed at room temperature. For
each slice, 3 fields of view were randomly selected for analysis
and scored according to the parameters previously described by
Gaudio et al (19) (Table III).
 | Table III.Ulcerative colitis pathological
scoring standard. |
Table III.
Ulcerative colitis pathological
scoring standard.
| Pathological
morphology | Score |
|---|
| Destruction of
epithelium and glands |
|
|
Morphologically normal | 0 |
| Focal
destruction of the epithelial surface and/or focal crypt
dropout | 1 |
| Zonal
destruction of the epithelial surface and/or zonal crypt loss | 2 |
| Diffuse
and/or mucosal ulceration involving submucosa and/or diffuse crypt
losses | 3 |
| Dilatation of
glandular crypts |
|
Normal | 0 |
| Focal
dilatation | 1 |
| Zonal
dilatation | 2 |
| Dilated
crypts | 3 |
| Depletion and loss
of goblet cells |
|
|
Normal | 0 |
|
Slightly depleted goblet
cells | 1 |
| Zonal
or moderately depleted goblet cells | 2 |
|
Diffusely or completely
depleted goblet cells | 3 |
| Inflammatory cell
infiltration |
| Absence
of infiltrate | 0 |
|
Infiltrate at the
subepithelial and lamina propria level or crypt bases | 1 |
|
Infiltrate reaching muscularis
mucosae | 2 |
| Severe
and extensive infiltrate reaching submucosa and/or involving
muscularis propria | 3 |
| Edema |
|
Absent | 0 |
|
Focal | 1 |
| Zonal
and/or moderately diffuse | 2 |
|
Extensive or severe | 3 |
| Vascular
congestion |
|
Absent | 0 |
|
Focal | 1 |
|
Zonal | 2 |
|
Diffuse | 3 |
| Crypt
abscesses |
|
Absent | 0 |
|
Focal | 1 |
|
Zonal | 2 |
|
Diffuse | 3 |
| Atrophia |
|
Absent | 0 |
|
Focal | 1 |
|
Zonal | 2 |
|
Diffuse | 3 |
Detection of immune factors
Following the induction of the animal model of
stress, mice were dried off to prevent water from contaminating the
microcentrifuge tubes and causing hemolysis. Following blood
collection for detection of immunoglobulin (Ig)A, interleukin
(IL)-6, IL-8, TNF-α, C3 and C4 by orbital puncture, mice were
sacrificed, and the abdominal cavity was dissected to isolate the
colon, as detailed above, which was subsequently washed thrice with
2 ml physiological saline. The saline was removed, and the solution
was centrifuged for 20 min at 4°C (1,369.5 × g). The supernatant
was removed to detect soluble (s)IgA. All samples were processed
using ELISA kits, according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between any 2 groups were determined by one-way
analysis of variance with the Student-Newman-Keuls post hoc test.
SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used
for all calculations. P<0.05 was considered to indicate a
statistically significant difference.
Results
General condition of the mice
Following 36 h of fasting with ad libitum
access to water, the maximum body weight loss of mice was 1.06 g.
The rage of weight loss was 0.85±0.21 g. The following observations
were made regarding the general condition of group A mice: Glossy
fur, responsive to stimuli, physically active and flexible, healthy
weight and granular stool. In comparison with group A, mice in
groups C and D exhibited slow activity, increased duration of
sleeping, loose and dull skin, decreased appetite, bloody stool and
weight loss (Fig. 1). Mice in group
B exhibited excitability, and increased breathing and heart rate in
the early stage of the stress experiment. These mice gradually
became subdued with smooth breathing and reduced responses to
external stimuli. Group C mice exhibited similar characteristics to
group B mice but their excitement (physical activity, propensity to
cry and struggle when being handled) intensity was weaker and more
rapidly suppressed. Preoperative DAI values for the mice in each
group exhibited no significant differences (P>0.05). The DAI
values of each group at 5 days post-administration of TNBS-ethanol
solution were in the following order from high to low:
C>D>B>A. There was a statistically significant difference
between group A and both groups C and D (P<0.01). Furthermore,
the DAI index in group B was different compared with both groups C
and D (P<0.01). DAI scores were not significantly different
between groups C and D (P>0.05; Fig.
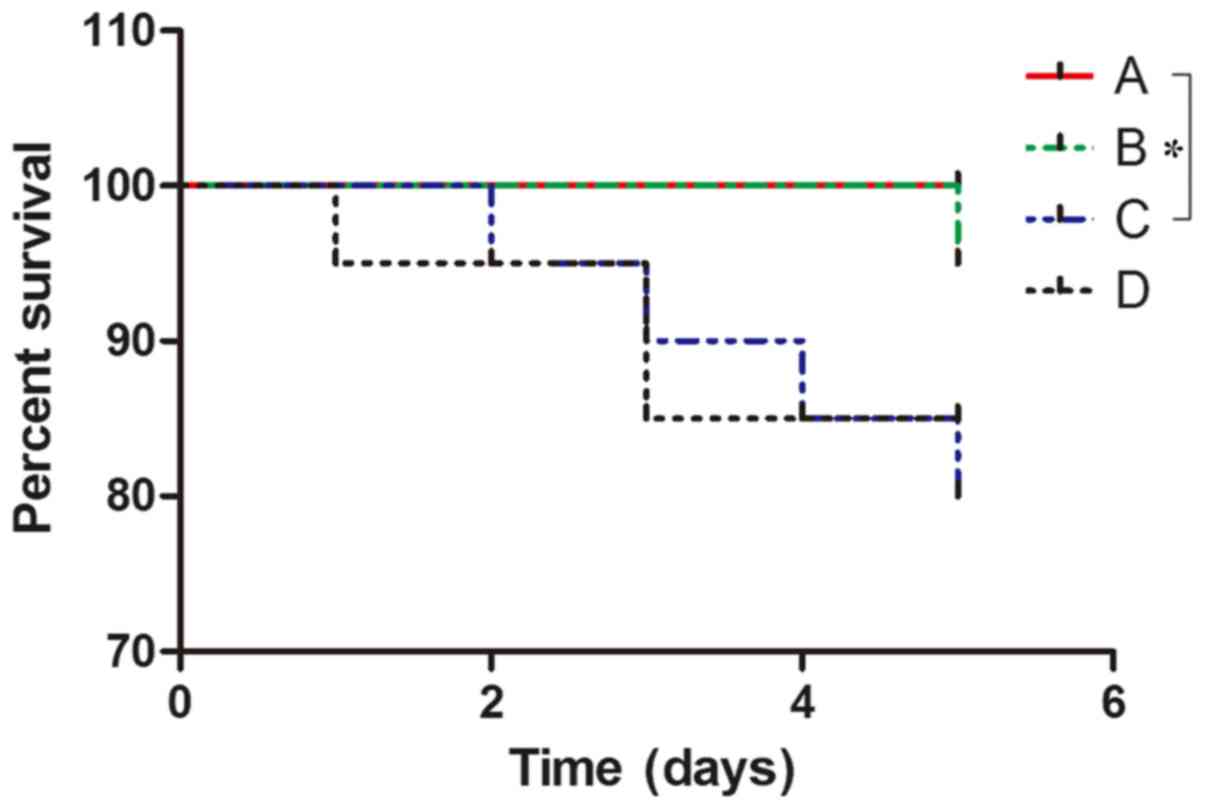
1). No mortality was observed in group A. Mortality was
observed in one mouse in group B due to asphyxia; 4 in group C,
including 2 from intestinal necrosis, 1 from mechanical intestinal
obstruction and 1 from asphyxia; and 3 in group D, including 1 case
of mortality due to intestinal necrosis and 2 due to mechanical
intestinal obstruction (Fig. 2). The
survival of mice in group A was better than that ingroup C, and the
difference was statistical significant (P=0.0455). However, there
was no significant difference in survival among groups B, C and D
at day 5.
Macroscopic and microscopic
characteristics of the colonic mucosa
Macroscopic characteristics were evaluated by the
naked eye. Normal colonic mucosa should appear red and smooth with
no injury, as in group A. However, following induction of the UC
model, colonic mucosa became extensively congested and edema and
ulcers were observed along with the development of rough mucus
membrane with mucosal erosion, which was obvious in group C. Group
B had a less marked manifestation than group C (Fig. 3).
Pathological analysis using microscopy indicated
inflammatory cell infiltration, diffuse mucosal hyperaemia,
extensive edema, epithelial and glandular damage, decreased number
of goblet cells, and crypt abscesses (Fig. 4). Groups B, C and D had different
degrees of injury. Among them, group C was the most severe. The
above results indicated that stress can aggravate injury of the
colonic mucosa (Figs. 1, 3 and 4).
Serum levels of IgA and secretory sIgA
in colonic lavage fluid
The levels of serum IgA and sIgA in the colonic
lavage fluid of each group 5 days post-administration were in the
following order from the highest to the lowest: A>B>D>C.
Statistically significant differences were identified between
groups A and C (P<0.01), and groups B and C (P<0.01). In
comparison with group D, the level of group C was also declined
(P<0.05; Fig. 5).
 | Figure 5.The serum expression levels of IL-6,
IL-8, TNF-α, C3, C4, IgA and sIgA in different groups. A, control
group; B, stress group; C, UC and stress group; D, UC model alone.
*P,0.05, **P<0.01. IL, interleukin; TNF, tumor necrosis factor;
Ig, immunoglobulin; s, soluble; C, complement component; ns, not
significant. |
Serum levels of IL6, IL8, TNF-α, C3
and C4
The serum levels of IL6, IL8, TNF-α, C3 and C4 in
each group 5 days post-administration were in the following order
from the highest to the lowest: C>D>B>A. Significant
differences were observed in IL-6, IL-8 and TNF-α expression
between groups A and C, and between groups B and C (P<0.01).
Group C exhibited higher levels than group D (P<0.05; Fig. 5). However, there was no significant
difference in the levels of C3 and C4 between groups.
Discussion
UC is an immune-mediated chronic inflammatory
disease with symptoms including abdominal pain, bloody diarrhea and
fatigue. UC is associated with numerous complications involving all
systems of the body and its chronic nature follows an unpredictable
course with instances of exacerbation and remission (20). In recent years, it has become an
increasingly common disease of the digestive system. With
increasing incidence, UC can severely affect the physical and
mental health, and the quality of life of patients (21). Previous studies indicated that UC may
be associated with a complex interaction of genetic, environmental,
immune and psychological factors in susceptible individuals. The
interaction between these factors results in severe intestinal
inflammation (22). Psychological
effects from UC and UC medications are experienced by many patients
with UC (23).
Adaptation to environmental stimuli is necessary to
maintain homeostasis. Stress can be defined as a threat to an
organism's homeostasis (8).
Psychological stress is defined as a psychological alteration
resulting from patients' feelings of unease, worry, and/or fear.
Psychological stress induces gastrointestinal symptoms, including
dyspepsia and abdominal pain (24,25), and
increased colonic motility (26).
Stress is a risk factor, trigger and perpetuating factor in UC
(27). Mechanisms by which stress
affects the nervous system to alter the immune function at both the
systemic and gut mucosal level are currently under investigation
(28,29).
The results of the present study indicated that
stress can aggravate the injury of the colonic mucosa and immune
system. The effects of stress are complex and depend on the
duration and intensity of the stimulus. A small amount of stress
has been hypothesized to enhance immune function; however,
increased intensity of stress can be detrimental due to excessive
production of neuroendocrine-derived mediators that weaken immune
responses to invasive pathogens (30). By acting on the catecholamine
receptors of immune cells, stress may reduce the capacity of the
sympathetic nervous system to release large amounts of
catecholamines, thereby weakening the immune response (31). In addition, stress can cause
intestinal motility dysfunction and intestinal flora imbalance, and
induce spasms of intestinal smooth muscles and vessels, thus
leading to a lack of blood and oxygen supply and an increase in
mucosal barrier dysfunction (32).
Stress may be classified into 4 types, including
physical, social, cultural and psychological (33). Among these, psychological stress is
one of the most ubiquitous and notable (34). A number of experimental methods have
been previously used to induce animal models of stress, including
restraint, water immersion, thermal stress and fatigue (35,36).
Among these methods, the former two lead to pronounced stress and
exhibited numerous advantages, including simplicity and low
variability among different groups (10,37).
Furthermore a combination of restraint and water immersion can
successfully induce experimental anxiety to study the underlying
psychological or emotional factors (38).
The mouse models of UC established in the present
study consumed less food, exhibited decreased activity, weight
loss, diarrhea, bloody stool, a decrease in fur glossiness, edema
in colonic mucosa, congestion and ulcer formation, as observed by
the naked eye. Furthermore, these mice exhibited infiltration of
inflammatory cells, loss of goblet cells and ulcer formation, as
exhibited by pathological analysis. All of the above parameters
suggested that the model of UC was successfully induced. In
addition, stress induced a decline in the general condition (mental
state, activity, food consumption, body weight and fur gloss), an
increase in DAI scores of mice with UC and aggravated macroscopic
and microscopic damage of the colonic mucosa. These results
indicated that stress may be a pathogenic factor for patients with
UC and can result in disease exacerbation. Stress treatment, such
as anti-anxiety therapy, may be a part of a comprehensive treatment
strategy for patients with UC.
Healthy intestinal mucosa is protected against
external stimuli by a complex mucosal defense barrier (39). In mouse models of UC, the colon
mucosa was thin and extensively congested with edema and ulcers,
which may be due to the defects of the intestinal barrier function
and immune mechanisms (40).
Compared with the control group, the level of serum
IgA and colonic lavage fluid sIgA in the mouse models of UC with
stress decreased significantly, demonstrating that stress produced
the downward trend. Previous studies have reported a reduction in
sIgA levels in patients with IBD, which is supported by the results
of the present study (41). IgA and
sIgA are the most abundant Igs in humans and the first line of
specific immunological defense against environmental antigens
(42). Possible reasons for the
decreased levels of IgA in mouse models of UC with stress are that
the stress acted on the neuroendocrine immune regulation network,
which decreased the release of large amounts of catecholamines by
the sympathetic nerves, whereas catecholamines acted on
catecholamine receptors of immune cells to weaken the body's
immunity (43). Alternatively,
stress may act through stimulating the release of glucocorticoids
by the pituitary adrenal cortex system of the brain to induce
immune suppression (44). Immune
suppression in addition to the weakened colonic mucosal barrier
function, may cause reduced resistance to external stimulation and
aggravated severity of UC (45).
This abnormal intestinal immune response may be involved in the
pathogenesis of UC.
TNF-α is an important proinflammatory cytokine
associated with cell apoptosis, inflammation, metabolism and
thrombosis. TNF-α stimulates the secretion of IL-6 and −8 to
promote the activation of nuclear factor (NF)-κB, which induces
proinflammatory and immunomodulatory gene expression (46). Active NF-κB can further stimulate the
expression of TNF-α and increase the intensity of inflammation
(47). The development of
neuroendocrine immunology introduces a new perspective for
understanding the mechanisms underlying stress (48). Stress can activate the peripheral and
central immune system responses and induce the release of
inflammatory mediators, including TNF-α, IL-6 and IL-8 (49). Activated immune system mediates the
process of psychological disorder through the interaction with
neuronal and neuroendocrine systems (50). An animal model of UC used in the
present study exhibited significantly increased serum IL-6, IL-8
and TNF-α expression levels. It has been previously demonstrated
that stress may lead to neuroendocrine immune system abnormalities
that increase the secretion of IL-6, IL-8 and TNF-α. Therefore, it
may be hypothesized that in the present study, stress exacerbated
UC due to increased secretion of IL-6, IL-8 and TNF-α via the
neuroendocrine-immune system.
Serum C3 and C4 serve important roles in immune
defense, immune regulation, and immune pathology. Decreased
expression levels of these factors may cause a decreased ability to
resist external pathogens and increased susceptibility to illness
(51). In the present study,
induction of the UC model and stress exhibited no significant
effects on serum levels of C3 and C4, and the reason may be
associated with insufficient sample size or the type of stress and
the duration of stimulation. Alternatively, stress may have no
effect on C3 and C4; however, further studies are required to test
this hypothesis.
Using the water immersion with restraint method of
stress induction, the present study concluded that stress may
increase colonic mucosa damage in mice with UC and interfere with
the immune regulation involving TNF-α, IgA, sIgA, C3 and C4,
thereby increasing the severity of UC in mice. The above data
suggested that, at least in some UC mice, stress may reflect
primary disease activity via immune damage, and that it is possible
that stress treatment rather than etiological and medical treatment
would be more effective. Psychological stress is common among
patients with UC and can lead to reduced quality of life (52). Given the association between UC and
stress, clinicians may consider the treatment of stress is an
integral part of therapies for patients with UC.
The present study has certain limitations. First, an
animal model was used for exposure to a stress-inducing
environment. The experiment should be continued following the
elimination of the stress environment to evaluate the change of
colonic mucosa and serum levels of IgA, IL-6, IL-8, TNF-α, C3 and
C4. Furthermore, the underlying mechanism of action of stress in
the development of UC remains to be elucidated. The mechanism of
stress in the progression of UC requires further investigation.
Future studies should investigate the association between stress
and the nervous, endocrine, and immune systems in the context of
the pathogenesis of UC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Tianjin
Foundation of Science and Technology (grant no. 2013KY25).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and YT were responsible for the conception and
design of the study. WN, XW, QZ and YX assisted in animal
experiments. YG, SL and XL assisted in the ELISA and
immunohistochemistry assays. YG, YT and QZ wrote, reviewed and
revised the manuscript. All authors participated in final approval
of the manuscript.
Ethics approval and consent to
participate
The research program was approved by the Ethics
Committee of Tianjin Nankai Hospital (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeRoche TC, Xiao SY and Liu X:
Histological evaluation in ulcerative colitis. Gastroenterol Rep.
2:178–192. 2014. View Article : Google Scholar
|
|
2
|
Parente JM, Coy CS, Campelo V, Parente MP,
Costa LA, da Silva RM, Stephan C and Zeitune JM: Inflammatory bowel
disease in an underdeveloped region of Northeastern Brazil. World J
Gastroenterol. 21:1197–1206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shivashankar R, Tremaine WJ, Harmsen WS
and Loftus EV Jr: Incidence and prevalence of crohn's disease and
ulcerative colitis in olmsted county, minnesota from 1970 through
2010. Clin Gastroenterol Hepatol. 15:857–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kabi A, Nickerson KP, Homer CR and
McDonald C: Digesting the genetics of inflammatory bowel disease:
Insights from studies of autophagy risk genes. Inflamm Bowel Dis.
18:782–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo AY, Stevens BW, Wilson RG, Russell CN,
Cohen MA, Sturgeon HC, Thornton A, Giallourakis C, Khalili H,
Nguyen DD, et al: Early life environment and natural history of
inflammatory bowel diseases. BMC Gastroenterol. 14:2162014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vadasz Z, Rainis T, Nakhleh A, Haj T,
Bejar J, Halasz K and Toubi E: The involvement of immune
semaphorins in the pathogenesis of inflammatory bowel diseases
(IBDs). PLoS One. 10:e01258602015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cătană CS, Berindan Neagoe I, Cozma V,
Magdaş C, Tăbarăn F and Dumitraşcu DL: Contribution of the
IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease.
World J Gastroenterol. 21:5823–5830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mawdsley JE and Rampton DS: Psychological
stress in IBD: New insights into pathogenic and therapeutic
implications. Gut. 54:1481–1491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo J, Mrug S and Knight DC: Emotion
socialization as a predictor of physiological and psychological
responses to stress. Physiol Behav. 175:119–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pohl CS, Medland JE and Moeser AJ:
Early-life stress origins of gastrointestinal disease: Animal
models, intestinal pathophysiology, and translational implication.
Am J Physiol Gastrointest Liver Physiol. 309:G927–G941. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majewska-Szczepanik M, Góralska M,
Marcińska K, Zemelka-Wiącek M, Strzępa A, Dorożyńska I and
Szczepanik M: Epicutaneous immunization with protein antigen TNP-Ig
alleviates TNBS-induced colitis in mice. Pharmacol Rep.
64:1497–1504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hohlbaum K, Bert B, Dietze S, Palme R,
Fink H and Thöne-Reineke C: Severity classification of repeated
isoflurane anesthesia in C57BL/6JRj mice - Assessing the degree of
distress. PLoS One. 12:e01795882017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YM, Lu GM, Zou XP, Li ZS, Peng GY and
Fang DC: Dynamic functional and ultrastructural changes of gastric
parietal cells induced by water immersion-restraint stress in rats.
World J Gastroenterol. 12:3368–3372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horvath G, Kekesi G, Petrovszki Z and
Benedek G: Abnormal motor activity and thermoregulation in a
schizophrenia rat model for translational science. PLoS One.
10:e01437512015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharpley CF, McFarlane JR and Slominski A:
Stress-linked cortisol concentrations in hair: What we know and
what we need to know. Rev Neurosci. 23:111–121. 2011.PubMed/NCBI
|
|
16
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Souza MM, Aguilar-Nascimento JE,
Gomes-da-Silva MH and Carlos Junior R: Effects of budesonide and
probiotics enemas on the colonic mucosa of rats with experimental
colitis. Acta Cir Bras. 22:34–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Araki Y, Andoh A, Fujiyama Y and Bamba T:
Development of dextran sulphate sodium-induced experimental colitis
is suppressed in genetically mast cell-deficient Ws/Ws rats.
Clin Exp Immunol. 119:264–269. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaudio E, Taddei G, Vetuschi A, Sferra R,
Frieri G, Ricciardi G and Caprilli R: Dextran sulfate sodium (DSS)
colitis in rats: Clinical, structural, and ultrastructural aspects.
Dig Dis Sci. 44:1458–1475. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bürger M, Schmidt C, Teich N and Stallmach
A: Medical therapy of active ulcerative colitis. Viszeralmedizin.
31:236–245. 2015.PubMed/NCBI
|
|
21
|
Cawthorpe D and Davidson M: Temporal
comorbidity of mental disorder and ulcerative colitis. Perm J.
19:52–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wehkamp J, Götz M, Herrlinger K, Steurer W
and Stange EF: Inflammatory bowel disease. Dtsch Arztebl Int.
113:72–82. 2016.PubMed/NCBI
|
|
23
|
Bannaga AS and Selinger CP: Inflammatory
bowel disease and anxiety: Links, risks, and challenges faced. Clin
Exp Gastroenterol. 8:111–117. 2015.PubMed/NCBI
|
|
24
|
de Sousa Rodrigues ME, Bekhbat M, Houser
MC, Chang J, Walker DI, Jones DP, Oller do Nascimento CM, Barnum CJ
and Tansey MG: Chronic psychological stress and high-fat
high-fructose diet disrupt metabolic and inflammatory gene networks
in the brain, liver, and gut and promote behavioral deficits in
mice. Brain Behav Immun. 59:158–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moloney RD, O'Mahony SM, Dinan TG and
Cryan JF: Stress-induced visceral pain: Toward animal models of
irritable-bowel syndrome and associated comorbidities. Front
Psychiatry. 6:152015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang SX and Wu WC: Effects of
psychological stress on small intestinal motility and bacteria and
mucosa in mice. World J Gastroenterol. 11:2016–2021. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keefer L, Keshavarzian A and Mutlu E:
Reconsidering the methodology of ‘stress’ research in inflammatory
bowel disease. J Crohns Colitis. 2:193–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Golbidi S, Frisbee JC and Laher I: Chronic
stress impacts the cardiovascular system: Animal models and
clinical outcomes. Am J Physiol Heart Circ Physiol.
308:H1476–H1498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shah E, Rezaie A, Riddle M and Pimentel M:
Psychological disorders in gastrointestinal disease: Epiphenomenon,
cause or consequence? Ann Gastroenterol. 27:224–230.
2014.PubMed/NCBI
|
|
30
|
Radek KA: Antimicrobial anxiety: The
impact of stress on antimicrobial immunity. J Leukoc Biol.
88:263–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Priyadarshini S and Aich P: Effects of
psychological stress on innate immunity and metabolism in humans: A
systematic analysis. PLoS One. 7:e432322012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rho SG, Kim YS, Choi SC and Lee MY: Sweet
food improves chronic stress-induced irritable bowel syndrome-like
symptoms in rats. World J Gastroenterol. 20:2365–2373. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karl JP, Hatch AM, Arcidiacono SM, Pearce
SC, Pantoja-Feliciano IG, Doherty LA and Soares JW: Effects of
psychological, environmental and physical stressors on the gut
microbiota. Front Microbiol. 9:20132018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stults-Kolehmainen MA and Sinha R: The
effects of stress on physical activity and exercise. Sports Medl.
44:81–121. 2014. View Article : Google Scholar
|
|
35
|
Nirmal J, Babu CS, Harisudhan T and
Ramanathan M: Evaluation of behavioural and antioxidant activity of
Cytisus scoparius Link in rats exposed to chronic unpredictable
mild stress. BMC Complement Altern Med. 8:152008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim TK, Park JY and Han PL: Physiological
parameters in the blood of a murine stress-induced depression model
before and after repeated passive exercise. Endocrinol Metab.
30:371–380. 2015. View Article : Google Scholar
|
|
37
|
Guo S, Gao Q, Jiao Q, Hao W, Gao X and Cao
JM: Gastric mucosal damage in water immersion stress: Mechanism and
prevention with GHRP-6. World J Gastroenterol. 18:3145–3155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Padgett DA, Marucha PT and Sheridan JF:
Restraint stress slows cutaneous wound healing in mice. Brain Behav
Immun. 12:64–73. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lechuga S and Ivanov AI: Disruption of the
epithelial barrier during intestinal inflammation: Quest for new
molecules and mechanisms. Biochim Biophys Acta Mol Cell Res.
1864:1183–1194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Antoni L, Nuding S, Wehkamp J and Stange
EF: Intestinal barrier in inflammatory bowel disease. World J
Gastroenterol. 20:1165–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marteau P, Colombel JF, Nemeth J, Vaerman
JP, Dive JC and Rambaud JC: Immunological study of histologically
non-involved jejunum during Crohns disease: Evidence for reduced in
vivo secretion of secretory IgA. Clin Exp Immunol. 80:196–201.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reyna-Garfias H, Miliar A, Jarillo-Luna A,
Rivera-Aguilar V, Pacheco-Yepez J, Baeza I and Campos-Rodríguez R:
Repeated restraint stress increases IgA concentration in rat small
intestine. Brain Behav Immun. 24:110–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heffner KL: Neuroendocrine effects of
stress on immunity in the elderly: Implications for inflammatory
disease. Immunol Allergy Clin North Am. 31:95–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meyer JS and Hamel AF: Models of stress in
nonhuman primates and their relevance for human psychopathology and
endocrine dysfunction. ILAR J. 55:347–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gersemann M, Wehkamp J and Stange EF:
Innate immune dysfunction in inflammatory bowel disease. J Intern
Med. 271:421–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aggarwal BB, Gupta SC and Sung B:
Curcumin: An orally bioavailable blocker of TNF and other
pro-inflammatory biomarkers. Br J Pharmacol. 169:1672–1692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luo SY, Le Z, Lv XH and Zhong ZG: Study on
effect of total flavonoids of Oldenlendia difflusa on ulcerative
colitis and its immunological mechanism Zhongguo Zhong Yao Za Zhi.
39:896–900. 2014.(In Chinese). PubMed/NCBI
|
|
48
|
Sheridan JF, Dobbs C, Jung J, Chu X,
Konstantinos A, Padgett D and Glaser R: Stress-induced
neuroendocrine modulation of viral pathogenesis and immunity. Ann N
Y Acad Sci. 840:803–808. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Slavich GM and Irwin MR: From stress to
inflammation and major depressive disorder: A social signal
transduction theory of depression. Psychol Bull. 140:774–815. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peng YL, Wang WY, Jiang CL and Wang YX:
Roles of cytokines in stress-induced depression. Sheng Li Xue Bao.
65:229–236. 2013.(In Chinese). PubMed/NCBI
|
|
51
|
Ricklin D, Reis ES, Mastellos DC, Gros P
and Lambris JD: Complement component C3-The ‘Swiss Army Knife’ of
innate immunity and host defense. Immunol Rev. 274:33–38. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sajadinejad MS, Asgari K, Molavi H,
Kalantari M and Adibi P: Psychological issues in inflammatory bowel
disease: An overview. Gastroenterol Res Pract. 2012:1065022012.
View Article : Google Scholar : PubMed/NCBI
|