Introduction
Statins are HMG-CoA reductase inhibitors. There are
many statins in the market, such as simvastatin, lovastatin,
pravastatin and simvastatin (1). It
has been confirmed that statins can make HMG-CoA unable to convert
to mevalonic acid which is a necessary substance for the synthesis
of cholesterol, which eventually leads to the inhibition of
cholesterol synthesis in the body (2). Furthermore, it has been found that
after preconditioning with pravastatin, it can effectively inhibit
apoptosis of renal ischemia reperfusion injury, and has a better
effect on renal protection (3).
Statins are also becoming increasingly prominent in the prevention
and treatment of heart disease. Necrosis in the ischemic central
region is the main manifestation of myocardial ischemia and
reperfusion, and there will be obvious apoptosis in the semi-dark
zone (4). In this study, an animal
model of simvastatin preconditioning myocardial ischemia
reperfusion injury is used to observe the effect of the expression
of apoptosis inhibitor caspase-3.
Materials and methods
Animals and groups
A total of 48 healthy male SD rats aged 8 weeks and
weighing 160–240 g were purchased from Laboratory Animal Center of
Sichuan University (Chengdu, China), randomly numbered and divided
into 4 groups, namely, the blank group, the sham operation group,
the ischemia-reperfusion group and simvastatin group with 12 rats
in each group. The feeding environment of the rats was of SPF
grade. The ambient temperature was 22±1°C and the ambient humidity
was 45±3%. The rats in each group could eat and drink freely
according to the circadian rhythm.
The study was approved by the Ethics Committee of
Yancheng TCM Hospital Affiliated to Nanjing University of Chinese
Medicine (Yancheng, China). All the experimental operations
involved in this experiment were carried out in accordance with the
relevant provisions of the NIH guidelines for the use of laboratory
animals.
Reagent consumables
Simvastatin was purchased from Shanghai Yanjing
Biological Technology Co., Ltd. (Shanghai, China), nitro
tetrazolium chloride (NBT) was purchased from Shanghai Haoran Bio
Technologies Co., Ltd. (Shanghai, China), caspase-3 rabbit
polyclonal antibody was purchased from Cell Signaling Technology,
Inc. (Danvers, USA; cat. no. 9662), and SABC immunohistochemical
staining kits were purchased from Shanghai S&S Bio & Tech
Co., Ltd. (Shanghai, China; http://www.ssmotor-sh.com/), upgraded packing of one
step TUNEL apoptosis in situ assay kits were purchased from
Jiangsu Keygen Biotech Co., Ltd. (Jiangsu, China). ECL luminescent
liquids was purchased from Cell Signaling Technology Inc.).
Administration
SD rats in the blank group were reared normally.
Simvastatin was compounded into suspension state by using medical
saline. The amount of 20 mg/kg was administered to SD rats at ten
days before operation, once a day at the same time, the same volume
of isosmotic saline was used for ten days in the sham operation and
ischemia reperfusion groups, once a day at the same time.
Animal model of ischemia-reperfusion
injury
According to the method provided by Hadi et
al (5), ischemia reperfusion
model was established. The 4 groups of SD rats were fasted for 12 h
before the model was built. In addition to the blank group, all the
SD rats in all the other groups were anesthetized with 10%
concentration of chloral hydrate. The limbs of the SD rats were
fixed on the operating table. The rat chests were disinfected by
medical alcohol after hair removal, the trachea was cut by the
surgical blade, using mechanical auxiliary ventilation and the
chest was opened along the left edge of the sternum to expose the
heart position of the rat. The ligation of the left anterior
descending branch of the left coronary artery was covered with the
gauze after the wetting of the saline. After half an hour, the
ligature was cut through with surgical scissors to restore the
blood flow to the reperfusion of the coronary artery. SD rats were
sacrificed after 3 h making the model, and the damaged parts of the
heart were selected. Formalin solution was used to fixing and
paraffin-embedded section was carried out. Then the immunochemistry
and TUNEL were performed. Only those with thread without ligation
were the sham operation group, and no animals died during the
ligation and reperfusion.
Detection of arrhythmia
Referring to the previous scoring methods for
ventricular arrhythmias (VA) (6),
the score of VE with no VA or <5 times is 0 points, and the
score of VE with ≥5 times is 1 point. T at a time <60 sec is 2
points, VT at a time ≥60 sec or multiple accumulation <60 sec is
3 points, and multiple accumulation >60 sec is 4 points. VT
occurrence is 5 points, VF occurrence continuously over 5 min or
death during the observation period is 6 points.
Detection of myocardial infarction
area
The left ventricle was sliced to a thickness of ~2
mm, 5 pieces in total, and put in a 0.25% NBT dyeing solution to
stain at 37°C for 15 min. At the time of necrosis of the
myocardium, gray white could be seen, while the non-necrotic
myocardium was blue. The water was absorbed with clean absorbent
paper, the necrotic area of the myocardium was slowly cut down and
weighed. The weight of the necrotic region of the myocardium was
recorded, and the proportion of the myocardial weight in the
myocardial infarction area accounted for the weight of the left
ventricle was calculated.
Immunohistochemical staining
Immunohistochemical examination was performed
according to the manufacturers instructions. Paraffin-embedded
treatment was carried out on myocardial tissue block (fixed with 4%
polyformaldehyde at 4°C overnight), paraffin section dewaxing,
hydration, PBS washing. After blocking for 15 min, 10% serum at
room temperature was blocked in non-specific background for 15 min,
anti-caspase-3 monoclonal antibody was added (dilution, 1:200; cat.
no. 9662; Cell Signaling Technology, Inc.), and stored in a
refrigerator at 4°C overnight. Rinsing by PBS after taking out the
horseradish peroxidase (HRP) labeled secondary antibody (dilution,
1:5,000; cat. no. 3875; Cell Signaling Technology, Inc.) the biotin
labeled secondary antibody was added, then incubated at room
temperature for 30 min before rinsing. Streptomyces antibiotic
protein peroxidase solution was incubated at room temperature for
30 min and then washed again. After DAB staining, rinsing, redying,
sealing, and observing under an optical microscope (LSM880; Carl
Zeiss AG with Airyscan, Oberkochen, Germany), the cytoplasm of
caspase-3 positive cells were brownish or brown color.
Positive cell rate % = positive cells
count/(positive cells count + negative cells count) ×100%.
Detection of apoptosis by TUNEL
Strictly referring to the operation procedure of one
step TUNEL apoptosis in situ assay kits, the tissue block
was rinsed with dimethylbenzene and gradient alcohol, and then
rinsed and dried after incubating proteinase K at room temperature,
and added 50 µl TUNEL liquid, rinsed after incubating in a dark wet
box at room temperature for 1 hour, and the 50 µl converted-POD was
added, and incubated for another 30 min. After washing, 100 µl DAB
substrate was added and incubated for 10 min at room temperature
and then rinsed, redyed and dehydrated. After rinsing with xylene,
it was sealed and observed under the microscope. Under the optical
microscope, it was found that there were brown granules in
cytoplasm and nucleus, which was a positive expression. In the
positive cell distribution area, 5 different visual fields were
randomly selected. The average number was used as the quantitative
standard of apoptosis, and the percentage of positive cells was
calculated.
Detection of expression of caspase-3
by western blotting
The protein was extracted according to the steps in
the tissue protein extraction instructions. The supernatant was
taken as total protein after centrifugation at 12,000 × g for 10
min at 4°C. The protein was quantified by BCA protein quantitative
kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Sampling buffer system with equal concentration was prepared and
boiled at 95°C for 15 min. A total of 10% SDS-PAGE gel was placed
and each pore was 15 µl. Then, 100V was used for electrophoresis.
After the end of electrophoresis, the protein was transferred to a
PVDF membrane by wet method. The membrane was transferred for 2 h
(90V), and then it was immersed in the closed liquid, and placed on
the rocking bed for 2 h. The caspase-3 (dilution, 1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.) and β-actin primary antibody
(dilution, 1:1,000; cat. no. 3700; Cell Signaling Technology, Inc.)
was added, then stored in a refrigerator at 4°C overnight, and
β-actin was used as the internal reference. TBST was washed for 10
min at room temperature 3 times. Horseradish enzyme labeled sheep
against rabbit IgG antibody (dilution, 1:5,000; cat. no. 3875; Cell
Signaling Technology, Inc.) was added and incubated for 1 h at room
temperature and then, TBST was washed for 10 min at room
temperature 5 times. Fresh ECL luminescent liquid (Cell Signaling
Technology Inc.; mixed with liquids A and B) was added to a PVDF
film to develop in the dark. After chromogenic photography, the
strips obtained were processed and analyzed by ImageJ software
(ImageJ2×; Rawak Software, Inc., Dresden, Germany).
Statistical analysis
The SPSS 19.0 software (SPSS Inc., Chicago, IL, USA)
was used to analyze the data, and the measurement data were
expressed as mean ± standard deviation (mean ± SD). Analysis of
variance (ANOVA) was used for comparison among groups, and the
Bonferroni method was used for comparison between two groups if the
variance was homogeneous, while the Welchs method was used for
analysis if the variance was not homogeneous. P<0.05 was
considered to be statistically different.
Results
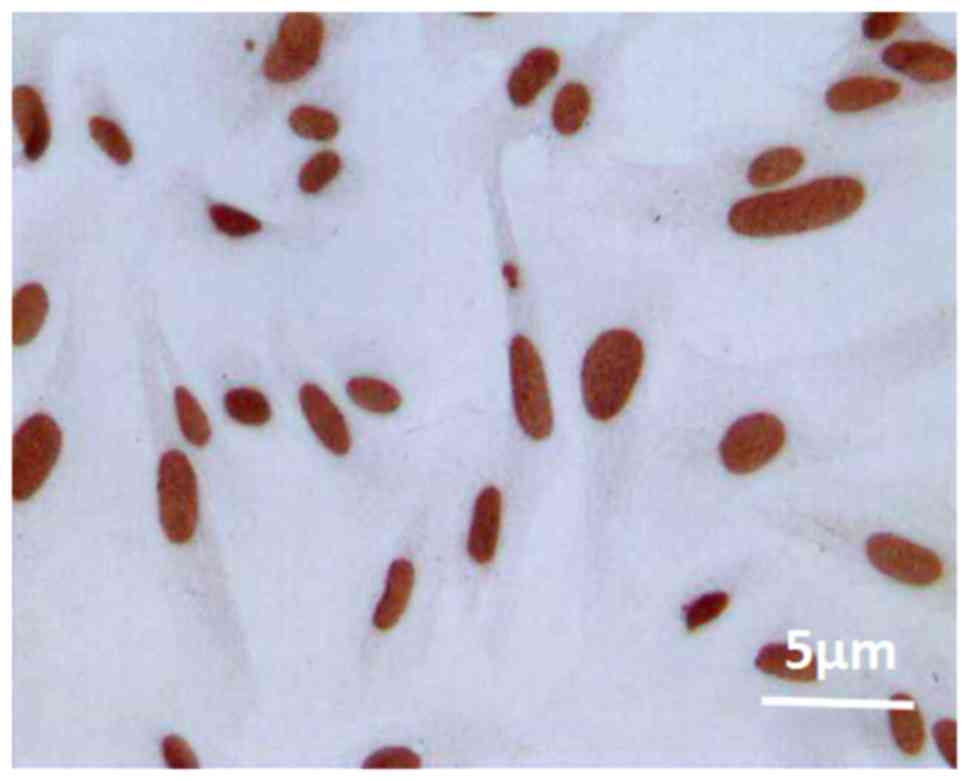
Staining diagram of positive
apoptosis
Under the optical microscope, it was found that
there were brown granules in cytoplasm and nucleus, indicating that
the cells in the myocardium were apoptotic (Fig. 1).
Effect of ischemia-reperfusion on
infarct area in SD rats
NBT staining showed that the myocardium of SD rats
in the blank and sham operation groups were all stained blue, and
no gray white was seen. The myocardium of SD rats in the ischemia
reperfusion and simvastatin groups showed a more obvious gray white
infarct area which in the simvastatin group was significantly
smaller than the ischemia reperfusion group. The difference between
the two groups was statistically significant (P=0.007) (Table I).
 | Table I.The myocardial infarction area of SD
rats after ischemia reperfusion (mean ± standard deviation). |
Table I.
The myocardial infarction area of SD
rats after ischemia reperfusion (mean ± standard deviation).
| Groups | No. of cases | Myocardial infarction
range (%) |
|---|
| Blank | 12 | 0 |
| Sham operation | 12 | 0 |
| Ischemia
reperfusion | 12 |
30.16±2.53a |
| Simvastatin | 12 |
23.95±1.89a,b |
Effect of simvastatin on expression of
caspase-3 in SD rats
The expression of caspase-3 in the
ischemia-reperfusion group was significantly higher than that in
the sham operation group (P<0.001). The expression of caspase-3
in the simvastatin group SD rats was significantly lower than the
expression of caspase-3 in the SD rats of the ischemia reperfusion
group, and the difference between the two groups was significant
(P=0.027) (Fig. 2).
Effect of simvastatin on apoptotic
cells in SD rats
Apoptosis was detected in a very small number of
cells both in the blank group and the sham operation group. There
was a large number of apoptotic cells in the ischemia reperfusion
and simvastatin groups, and the number of apoptotic cells in the
simvastatin group SD rats was significantly lower than the number
of apoptotic cells in the SD rats of the ischemia reperfusion
group. The difference between the two groups was statistically
significant (P=0.018) (Fig. 3).
Reperfusion arrhythmia monitoring
Arrhythmia in SD rats was not found in the blank and
sham operation groups, and the arrhythmia score was 0, while
arrhythmia in SD rats was observed in the ischemia reperfusion and
simvastatin groups, and the arrhythmia score of the simvastatin
group was significantly lower than the arrhythmia score of the
ischemia reperfusion group, and the two groups were evaluated. The
difference was statistically significant (P=0.033) (Table II).
 | Table II.The score of arrhythmia (mean ±
standard deviation). |
Table II.
The score of arrhythmia (mean ±
standard deviation).
| Groups | No. of cases | Arrhythmia score |
|---|
| Blank | 12 | 0 |
| Sham operation | 12 | 0 |
| Ischemia
reperfusion | 12 | 4.2±1.5a |
| Simvastatin | 12 | 3.1±2.3b |
Detection of expression of caspase-3
protein by western blotting
Compared with the blank and sham operation groups,
the expression of caspase-3 protein in the ischemia-reperfusion and
simvastatin groups was significantly higher than the simvastatin
group, and the expression of caspase-3 protein in the simvastatin
group was significantly lower than the expression of caspase-3
protein in the ischemia-reperfusion group. The difference was
statistically significant (P=0.038) (Fig. 4).
Discussion
Four types of cardiac dysfunction occurred after
myocardial ischemia reperfusion injury, including reflux,
myocardial stunning, fatal reperfusion injury and reperfusion
arrhythmia (7,8). At present, there are different
explanations for the mechanism of myocardial ischemia-reperfusion
injury, such as oxygen free radical theory (9), calcium overload theory (10) and inflammatory response theory
(11). Kocak et al (12) suggested that apoptosis would occur
during myocardial ischemia-reperfusion injury, and apoptosis plays
a decisive role in the final infarct area of myocardium. When a
specific drug is used to intervene in the process of apoptosis, the
effective inhibition of apoptosis can make the area of the
myocardial infarction area up to 70%, significantly improving the
state of heart function (13).
Therefore, the use of anti-apoptotic drugs in clinical treatment of
myocardial ischemia reperfusion injury have a great
significance.
Apoptosis is also known as programmed cell death
(PCD), and caspase-3 is a very important starting factor (14), which mediates PCD process. It has
been shown that caspase-3 can be activated by its own cleavage by
oligomerization, and can activate a variety of downstream
proteases, such as cysteine protease, and participate in the
process of inhibiting cell proliferation and inducing cell
apoptosis (15). Zheng et al
(16) found that caspase-3
inhibitors can effectively inhibit the apoptosis of rabbit
cardiopulmonary bypass cardiomyocytes. It is presumed that it may
be associated with the inhibition of the activity of caspase-3.
Wang et al (17) found that
the use of different doses of atorvastatin preconditioning acute
myocardial ischemia animal model, can significantly reduce
apoptosis of myocardial cells and inhibit the expression of
caspase-3 gene. Huang et al (18) found that atorvastatin can inhibit
apoptosis of myocardial cells in rats with heart failure after
myocardial infarction, and then reduce the structural
reconstruction of the heart and improve the cardiac function.
In this study, the expression of caspase-3 was
significantly increased in the ischemia-reperfusion and simvastatin
groups, which was mainly distributed in the gray white region,
indicating that the cardiomyocytes of the SD rats were obviously
apoptotic after ischemia-reperfusion, and apoptosis of the
simvastatin group was significantly lower than that of the ischemia
reperfusion group (P<0.05); the positive rate of caspase-3 was
decreased (P<0.05), the area of myocardial infarction area was
decreased (P<0.05) and the rate of arrhythmia was significantly
decreased (P<0.05). The effect of myocardial apoptosis in the
simvastatin group was significantly inhibited, and the incidence of
cardiac infarction and arrhythmia was reduced, so as to exert a
better protective effect on myocardium, which is in accordance with
the results of Ma et al (19). At present, it is not known how
simvastatin can protect the myocardium by reducing the expression
of caspase-3 through anti-apoptosis. However, Liu et al
(20) indicated that the expression
of p53 and caspase-3 in simvastatin decreased significantly in the
the simvastatin group with simvastatin preconditioning renal
ischemia reperfusion, and the level of oxygen free radicals were
also decreased (P<0.05). It is presumed that simvastatin may
scavenge the excess oxygen free radicals induced by renal
ischemia-reperfusion injury, and then decrease the oxidative damage
effect of myocardial cells, reduce the expression of p53 and
caspase-3, and ultimately inhibit cell apoptosis. However, for the
role of myocardial ischemia reperfusion injury, we need to further
design experiments for verification.
In conclusion, simvastatin has a protective effect
on myocardial ischemia reperfusion injury, and its mechanism may be
associated with reducing the expression of caspase-3 and inhibiting
apoptosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS conceived and designed the study. RP and JS were
responsible for the construction of the ischemia-reperfusion injury
model. WS and HS detected arrhythmia and myocardial infarction
area. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yancheng TCM Hospital Affiliated to Nanjing University of Chinese
Medicine (Yancheng, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sopková J, Vidomanová E, Strnádel J,
Škovierová H and Halašová E: The role of statins as therapeutic
agents in cancer. Gen Physiol Biophys. 36:501–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nykänen AI, Tuuminen R and Lemström KB:
Donor simvastatin treatment and cardiac allograft
ischemia/reperfusion injury. Trends Cardiovasc Med. 23:85–90. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akarsu M, Saygun O, Aydinuraz K, Aydin O,
Daphan CE, Tanrıkulu FB, Kisa U and Comu FM: The effects of
simvastatin on ischemia reperfusion injury in an experimental colon
anastomosis model. Indian J Surg. 79:390–395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Zhang Z and Yan H: Simvastatin
inhibits ischemia/reperfusion injury-induced apoptosis of retinal
cells via downregulation of the tumor necrosis factor-α/nuclear
factor-κB pathway. Int J Mol Med. 36:399–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hadi NR, Al-Amran F, Yousif M and Zamil
ST: Antiapoptotic effect of simvastatin ameliorates myocardial
ischemia/reperfusion injury. ISRN Pharmacol. 2013:8150942013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang LH, Li J, Gu JP, Qu MX, Yu J and
Wang ZY: Butorphanol attenuates myocardial ischemia reperfusion
injury through inhibiting mitochondria-mediated apoptosis in mice.
Eur Rev Med Pharmacol Sci. 22:1819–1824. 2018.PubMed/NCBI
|
|
7
|
Zhou T, Guo S, Wang S, Li Q and Zhang M:
Protective effect of sevoflurane on myocardial ischemia-reperfusion
injury in rat hearts and its impact on HIF-1α and caspase-3
expression. Exp Ther Med. 14:4307–4311. 2017.PubMed/NCBI
|
|
8
|
Zhao Y, Feng Q, Huang Z, Li W, Chen B,
Jiang L, Wu B, Ding W, Xu G, Pan H, et al: Simvastatin inhibits
inflammation in ischemia-reperfusion injury. Inflammation.
37:1865–1875. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Herz W and Babiker F: Acute intravenous
infusion of immunoglobulins protects against myocardial
ischemia-reperfusion injury through inhibition of caspase-3. Cell
Physiol Biochem. 42:2295–2306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang J, Han JI and Han S: Effect of
pretreatment with simvastatin on spinal cord ischemia-reperfusion
injury in rats. J Cardiothorac Vasc Anesth. 27:79–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuuminen R, Holmström E, Raissadati A,
Saharinen P, Rouvinen E, Krebs R and Lemström KB: Simvastatin
pretreatment reduces caspase-9 and RIPK1 protein activity in rat
cardiac allograft ischemia-reperfusion. Transpl Immunol. 37:40–45.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kocak FE, Kucuk A, Ozyigit F, Tosun M,
Kocak C, Kocak A, Ekici MF, Yaylak F and Genc O: Protective effects
of simvastatin administered in the experimental hepatic
ischemia-reperfusion injury rat model. J Surg Res. 199:393–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song L, Gao LN, Wang J, Thapa S, Li Y,
Zhong XB, Zhao HW, Xiang XR, Zhang FG and Ji P: Stromal
cell-derived factor-1α alleviates calcium-sensing receptor
activation-mediated ischemia/reperfusion injury by inhibiting
caspase-3/caspase-9-induced cell apoptosis in rat free flaps.
BioMed Res Int. 8945850:20182018.
|
|
14
|
Han QF, Wu L, Zhou YH, Wang LH, Zhang DY,
Liu T and Yao HC: Simvastatin protects the heart against ischemia
reperfusion injury via inhibiting HMGB1 expression through PI3K/Akt
signal pathways. Int J Cardiol. 201:568–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi X, Cui X, Wu P, Wang S, Wang G, Yang X,
Yang F, Zheng S and Li Z: Effects of N-acetylcysteine on apoptosis
induced by myocardial ischemia reperfusion injury in rats' heart
transplantation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
27:1234–1239. 2013.(In Chinese). PubMed/NCBI
|
|
16
|
Zheng C, Wu Z, Tian L, Li D, Wang X, He Y,
He Y, Jin W, Li M, Zhu Q, et al: Long noncoding RNA AK12348 is
involved in the regulation of myocardial ischaemia-reperfusion
injury by targeting PARP and caspase-3. Heart Lung Circ.
27:e51–e58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang SY, Cui XL, Xue FS, Duan R, Li RP,
Liu GP, Yang GZ and Sun C: Combined morphine and limb remote
ischemic perconditioning provides an enhanced protection against
myocardial ischemia/reperfusion injury by antiapoptosis. J Surg
Res. 202:13–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang CH, Lai CC, Yang AH and Chiang SC:
Myocardial preconditioning reduces kidney injury and apoptosis
induced by myocardial ischaemia and reperfusion. Eur J Cardiothorac
Surg. 48:382–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma J, Qiao Z and Xu B: Effects of ischemic
preconditioning on myocardium Caspase-3, SOCS-1, SOCS-3, TNF-α and
IL-6 mRNA expression levels in myocardium IR rats. Mol Biol Rep.
40:5741–5748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu K, Chen H, You QS, Ye Q, Wang F, Wang
S, Zhang SL, Yu KJ and Lu Q: Curcumin attenuates myocardial
ischemia-reperfusion injury. Oncotarget. 8:112051–112059. 2017.
View Article : Google Scholar : PubMed/NCBI
|