Introduction
Renal podocytes serve a crucial role in glomerular
filtration and constitute the major component of the filtration
barrier (1). In a study of patients
with Alzheimer's disease, podocyte exfoliation from the glomerulus
was accelerated with increasing age and the number of podocytes
decreased with age (2). The
reduction of podocyte number and density induced diseases,
including proteinuria, glomerulosclerosis and renal dysfunction
(3). Oestrogen affects a variety of
physiological and pathological functions of the kidney, including
the regulation of haemodynamics, mesangial cells, the mesangial
matrix, collagen metabolism, cytokines, the release of inflammatory
mediators and glomerular filtration (4,5). Studies
have revealed that oestrogen inhibits glomerular podocyte apoptosis
through oestrogen-associated receptors (6,7).
The protein tyrosine phosphatase receptor type O
(PTPRO) is a type of phosphotyrosine protein phosphatase (PTP)
receptor that was first identified and cloned in the human
glomerulus (8). PTPRO has six
protein subtypes, of which the full-length subtype is expressed in
several organs, including the kidneys, brain, lungs, liver and
mammary glands, while one of the truncated subtypes is mainly
expressed in macrophages and lymphocytes (9). PTPRO is a transmembrane protein, and
its intracellular domain contains the PTP domain that catalyses the
dephosphorylation of tyrosine residues (8). PTP dephosphorylation is indispensable
in cell signal transduction, which greatly influences and regulates
the biological behaviour of cells, including cell proliferation,
differentiation and apoptosis (10,11). One
study demonstrated that the increased expression of PTPRO in
oestrogen-induced tumorigenesis could facilitate endocrine therapy
in breast cancer (12). Mutations in
PTPRO are a cause of autosomal-recessive nephrotic syndrome
(13). Additionally, antibodies
directed against PTPRO caused increased glomerular protein
permeability (14). In particular,
antibodies to phosphatases of the extracellular domain resulted in
impairment of the permeability barrier (14). These studies indicate that oestrogen
mediates glomerular dysfunction, which may be associated with the
regulation of PTPRO, however the mechanism remains unclear. The
current study mainly investigated the effect of oestrogen on the
apoptosis of renal podocytes, but also explored its possible signal
transduction mechanism, which may provide a novel target for the
treatment of childhood nephrotic syndrome.
Materials and methods
Cell culture and treatment
A mouse podocyte cell line derived from kidneys
(MPC5) was obtained from the Biotechnology Co., Ltd. Shanghai
Enzyme Research (Shanghai, China). Human primary renal podocytes
derived from kidneys (HUM-iCELL-u004) were obtained from iCell
Bioscience Inc. (Shanghai, China). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
foetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml
streptomycin (all Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and maintained at 37°C in a humidified incubator
with 5% CO2.
Cells were washed once with PBS prior to stimulation
with 17β-oestradiol (E2) at dosages of 5, 10, 50 or 100 nM, or the
E2 antagonist, tamoxifen, (both Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at dosages of 0.1 or 5 µM for 7 days at 37°C.
During stimulation, cell viability and proliferation were analysed
by the MTT assay, and apoptosis were analysed by flow
cytometry.
Construction of the PTPRO
overexpression vector
Cells were transfected with PTPRO or ERβ
overexpression vectors 5 days after the stimulation. The
pcDNA3.1(+)/PTPRO expression vector was constructed by cloning a
PTPRO fragment from normal mouse cDNA (Sangon Biotech Co., Ltd.,
Shanghai, China) into pcDNA3.1(+; Invitrogen; Thermo Fisher
Scientific, Inc.) between the BamH I and EcoR I sites
to express PTPRO in abundance in E. coli DH5α cells (Takara
Biotechnology Co., Ltd., Dalian, China). The primers for PTPRO were
as follows: Forward: 5′-GGAACCACTGACCTGTCCCACTC-3′, reverse:
5′-CTCGGTGTTGCTCCCTCTCTCAG-3′. Then, the 1 µg pcDNA3.1(+)/PTPRO
plasmid was transfected into MPC5 cells using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The stably
transfected clones were screened for G418 resistance using 50 mg/ml
Geneticin™ Selective Antibiotic (G418 Sulfate; Invitrogen; Thermo
Fisher Scientific, Inc.) at 24 h after transfection. Briefly, cells
were cultured after 24 h of transfection in DMEM with 1,200 µg/ml
G418 sulfate at 37°C for 14 days, and then the positive clones were
observed with a light microscope at a magnification of ×200. The
target gene was then detected by western blot analysis. The
pcDNA3.1(+)/ERβ expression vector was constructed by cloning a ERβ
fragment from normal mouse cDNA (Sangon Biotech Co., Ltd.) into the
pcDNA3.1(+) plasmid (Invitrogen; Thermo Fisher Scientific, Inc.).
Then 1 µg pcDNA3.1(+)/ERβ plasmid or pcDNA3.1(+) plasmid (control)
were transfected into MPC5 cells with or without E2 treatment using
Lipofectamine 2000 at 37°C for 24 h. The primers for ERβ were as
follows: Forward: 5′-CCTCGTTCTGGACAGGGATG-3′, reverse:
5′-AGAAGCATCAGGAGGTTGGC-3′.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
MPC-5 cells or human primary renal podocytes were
treated with 5, 10, 50 or 100 nM E2 or 0.1 or 5 µM tamoxifen. Total
RNA was extracted from cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Isolated RNA was electrophoresed
in a 1% agarose gel to detect the purity of total RNA. The
first-strand cDNA was synthesized in a 10 µl reaction system using
1 µg total RNA and SuperScript® III Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.), and
placed in a PCR thermocycler at 37°C for 60 min. PCR amplification
was performed using the PCR amplification kit (Taq; Takara
Biotechnology Co., Ltd., Dalian, China). The thermocycling
conditions were as follows: 94°C for 5 min, then 30 cycles of 94°C
for 45 sec, 56°C for 45 sec and 72°C for 45 sec. The number of
cycles used was determined by comparing the results of RT-PCR
analyses with different numbers of cycles (Fig. 1). With the gradual accumulation of
PCR products, the amplified DNA fragments no longer increase
exponentially, and enter the linear growth phase or stationary
phase, that is the ‘stagnation effect’, it named as plateau phase
(15). When cells were in the
plateau phase, the relative mRNA expression identified in the
30-cycle analysis was similar to that of the 35-cycle analysis.
However, the relative mRNA expression identified in the 30-cycle
analysis was significantly higher compared with that of 28-cycle
analysis and significantly lower compared with that of 35-cycle
analysis (both P<0.05). Therefore, 30 cycles were in the
exponential phase. The specific primers were designed using Primer
Premier 6.0 software (Premier Biosoft International, Inc., Palo
Alto, CA, USA) and synthesized by Sangon Biotech Co., Ltd. The
primers for PTPRO (mouse) were as follows: Forward:
5′-ACCACTGACCTGTCCCACTC-3′; reverse: 5′-AGGTGTTGCTCCCTCTCTCA-3′.
The primers for PTPRO (human) were as follows: Forward:
5′-TCTGCAGATGGCTAGGGAGT-3′; reverse: 5′-AGACATGAGGGTAGCAGGGT-3′.
The primers for β-actin (human) were as follows: Forward:
5′-CCGTTCCGAAAGTTGCCTTTT-3′; reverse: 5′-GAGGCGTACAGGGATAGCAC-3′.
The PCR product was electrophoresed in a 1% agarose gel and the
bands were visualized by ethidium-bromide staining. Bio-Rad Gel
Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to observe the bands. Each band was analysed by Quantity One
4.62 software (Bio-Rad Laboratories, Inc.). The intensity of the
target band were normalised to the β-actin band.
Western blotting
MPC-5 cells were treated by 100 nM E2 or PBS
(control) for 5 days, and then were incubated with
pcDNA3.1(+)/PTPRO expression vector or pcDNA3.1(+) scramble
plasmids. Subsequently, total proteins were extracted from cells
using Cell Total Protein Extraction kit (Amresco, LLC, Solon, OH,
USA) and quantified with Bicinchoninic Acid Protein Concentration
Determination kit (Beyotime Institute of Biotechnology, Shanghai,
China). All antibodies were purchased from Abcam (Cambridge, UK).
The proteins (20 µg/lane) were separated by SDS-PAGE in a 10% gel
followed by electrotransfer to nitrocellulose membranes. The
membranes were blocked by 5% FBS at room temperature for 45 min,
and probed using primary antibodies against PTPRO (1:500; cat. no.
ab231560), ERβ (1:1,000; cat. no. ab3577), signal transducer and
activator of transcription (STAT3; 1:1,000; cat. no. ab68153),
phosphorylated (p-)STAT3 (Y705; 1:1,000; cat. no. ab76315), p-STAT3
(S727; 1:2,000; cat. no. ab30647), tyrosine-protein kinase JAK1
(JAK1; 1:1,000; cat. no. ab47435), p-JAK1 (Y1022+Y1023; 1:1,000;
cat. no. ab138005), JAK2 (1:5,000; cat. no. ab39636), p-JAK2
(Y1007; 1:1,000; cat. no. ab195055) and β-actin (1:10,000; cat. no.
ab8227) overnight at 4°C. They were also probed using primary
antibodies against ERα (1:600; cat. no. ab75635) for 4 h at room
temperature. The membranes were then incubated horseradish
peroxidase-conjugated secondary antibodies (1:20,000; cat. no.
ab7090) at room temperature for 1 h. β-actin was used as an
internal reference. Bands were revealed with an
Electro-Chemi-Luminescence (ECL) reagent (EMD Millipore, Billerica,
MA, USA) and recorded on X-ray films (Kodak, Rochester, NY, USA).
The densitometry of each band was quantified using a Gel imaging
system and Quantity One 4.62 software.
Cell viability and proliferation
assays
After cells were incubated with E2 or tamoxifen for
5 days, cell viability and proliferation was analysed using MTT
Cell Proliferation and Cytotoxicity Assay kit (Beyotime Institute
of Biotechnology) every day for 7 days. The dimethyl sulfoxide was
used to dissolve the purple formazan. The optical density value was
recorded at a wavelength of 450 nm. Then, time was plotted on the
abscissa and absorbance on the ordinate to plot a cell growth
curve. The assay was repeated four times for each sample.
Cell apoptosis assay
After cells were incubated with E2 or tamoxifen for
5 days, apoptotic cells were measured by flow cytometry using an
Annexin V-fluorescein isothiocyanate/propidium iodide apoptosis
detection kit (Abcam). The fluorescence intensity was detected at
488 nm using flow cytometry. Cells were sorted by the FACSCalibur
flow cytometer and analysed using CellQuest software (version 5.1;
both BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Data are presented as mean ± standard deviation of
at least four replicates per group. One-way analysis of variance
and Fisher's Least Significant Difference were used to compare
multiple groups. Data were analysed by SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). Statistical differences were calculated
using t-tests in Fig. 3D-G.
Results
Oestrogen promotes cell viability and
proliferation, and prevents apoptosis in podocytes
Cell viability increased with increasing E2 dosages,
but decreased with increasing dosages of tamoxifen in MPC-5 cells
(Fig. 2A); these changes were
significant compared with the control cells. After 4 days, cell
proliferation significantly increased with the duration of 100 nM
E2 stimulation, whereas, after 3 days, it significantly decreased
with the duration of 5 µM tamoxifen stimulation in MPC-5 cells
compared with control cells (Fig.
2B). E2 (100 nM) stimulation significantly inhibited apoptosis,
whereas tamoxifen (5 µM) stimulation significantly accelerated
apoptosis in MPC-5 cells (Fig. 2C).
Cell proliferation increased with increasing E2 dosages, but
decreased with increasing dosages of tamoxifen in human primary
renal podocytes (Fig. 2D); these
changes were significant compared with the control cells. After 5
days, cell proliferation significantly increased with the duration
of 100 nM E2 stimulation, whereas, after 4 days, it significantly
decreased with the duration of 5 µM tamoxifen stimulation in MPC-5
cells compared with control cells (Fig.
2B). E2 (100 nM) stimulation significantly inhibited apoptosis,
whereas tamoxifen (5 µM) stimulation significantly accelerated
apoptosis human primary renal podocytes (Fig. 2F). These results indicate that E2 can
promote cell viability and proliferation, and prevent the apoptosis
of podocytes. Additionally, it was also determined that the effect
of oestrogen on viability, proliferation and apoptosis were the
same in mouse cell lines (MPC-5) as in human cell lines (human
primary renal podocytes), suggesting that the effect was not
species-specific.
Oestrogen and its receptors inhibit
PTPRO expression in podocytes
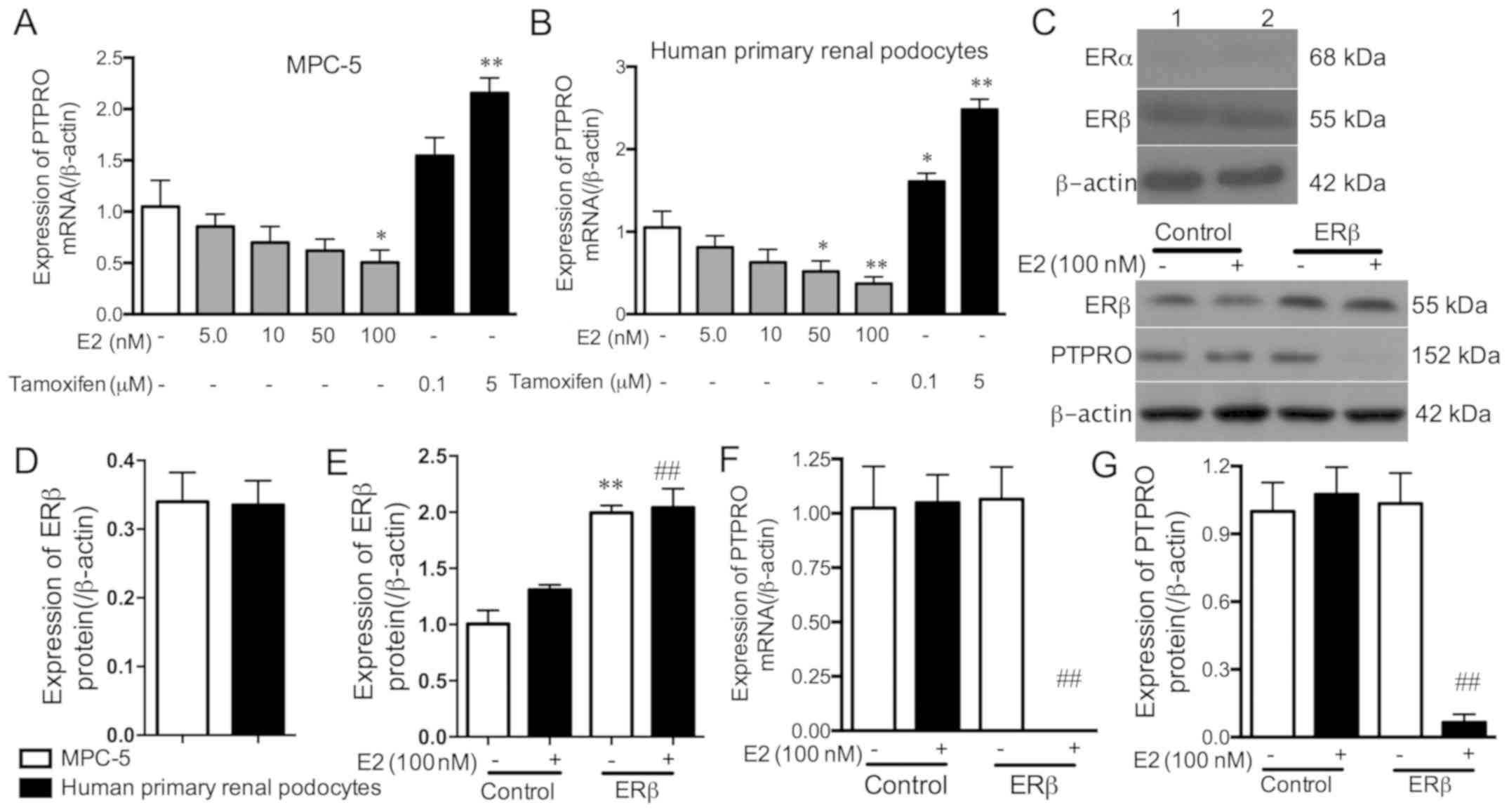
The mRNA expression levels of PTPRO decreased with
increasing E2 dosages, but this was only significant with 100 nM E2
in MPC-5 cells, and 50 and 100 nM E2 in human primary renal
podocytes compared with the controls (Fig. 3A and B). PTPRO mRNA expression
increased with 5 µM tamoxifen in MPC-5 cells, and with 0.1 and 5 µM
tamoxifen in human primary renal podocytes compared with the
controls. These results indicate that E2 can inhibit the expression
of PTPRO in podocytes.
To explore the role of oestrogen receptors in
regulating PTPRO expression, the expression levels of ERα and ERβ
proteins were detected in MPC-5 cells and human primary renal
podocytes. The results demonstrated that the ERα protein was not
expressed, while the ERβ protein was expressed in MPC-5 cells and
human primary renal podocytes (Fig. 3C
and D). Subsequently, the ERβ overexpression vectors were
transfected into E2 (100 nM)-stimulated and non-stimulated MPC5
cells, and the transfection effect was detected by western blot
analysis (Fig. 3C and E). Compared
with control cells, ERβ expression was significantly increased by
ERβ overexpression vectors in E2-stimulated and non-stimulated
MPC-5 cells. The binding of E2 to increased levels of ERβ
significantly eliminated PTPRO mRNA and protein expression compared
with E2 (100 nM)-stimulated cells (Fig.
3C, F and G). These results suggest that E2 combines with ERβ
rather than ERα, to inhibit PTPRO expression in podocytes.
Oestrogen activates JAK2 by inhibiting
PTPRO in mouse podocytes
To further explore the molecular mechanism by which
E2 regulates PTPRO expression in podocytes, protein expression
levels of PTPRO, p-JAK1 and p-JAK2 were analysed. Firstly, PTPRO
overexpression vectors were transfected into E2-stimulated or
tamoxifen-stimulated MPC-5 cells, then PTPRO expression was
detected by western blot analysis. PTPRO overexpression vectors
significantly promoted E2-inhibited and tamoxifen-inhibited PTPRO
expression (Fig. 4A and B).
Phosphorylation of the Y1022 and Y1023 sites of JAK1 was not
affected by E2 or tamoxifen stimulation with or without PTPRO
overexpression (Fig. 4A and C). E2
significantly increased the expression of JAK2 phosphorylated at
the Y1007 compared with the control, while PTPRO overexpression
significantly dephosphorylated the Y1007 site of JAK2 in
E2-stimulated MPC-5 cells (Fig. 4A and
D). Additionally, tamoxifen significantly dephosphorylated the
Y1007 site of JAK2 compared with the control. PTPRO overexpression
significantly dephosphorylated the Y1007 site of JAK2 in
tamoxifen-stimulated MPC-5 cells. These results suggest that PTPRO
is involved in E2-induced JAK2 activation in podocytes, rather than
JAK1 activation.
Oestrogen activates STAT3 by
inhibiting PTPRO in podocytes
Previous studies have revealed that the suppressive
role of PTPRO in hepatocellular carcinoma or breast cancer could be
ascribed to the regulation of STAT3 expression (16). Therefore, in the current study, to
elucidate the underlying mechanism by which PTPRO is involved in
the inhibition of podocyte viability by oestrogen, the regulation
of STAT3 activity by PTPRO was analysed.
E2 significantly phosphorylated the Y705 and S727
sites of STAT3 compared with the control, and PTPRO overexpression
significantly dephosphorylated the Y705 and S727 sites of STAT3 in
E2-stimulated MPC-5 cells (Fig.
5A-C). p-STAT3(Y705) expression was significantly increased in
E2-stimulated and PTPRO overexpressed MPC-5 cells compared with the
control, whereas p-STAT3(S727) expression was only markedly
increased. Additionally, tamoxifen significantly dephosphorylated
Y705 and S727 sites of STAT3 compared with the control, and PTPRO
overexpression significantly dephosphorylated the Y705 and S727
sites of STAT3 in tamoxifen-stimulated MPC-5 cells. These results
indicate that PTPRO is involved in E2-induced STAT3
phosphorylation.
PTPRO is involved in the regulation of
the viability and apoptosis of podocytes through E2 binding
E2 significantly promoted cell viability and
prevented apoptosis compared with the controls, while PTPRO
overexpression significantly reversed these E2-induced effects
(Fig. 5D and E). The E2 antagonist,
tamoxifen, significantly suppressed cell viability and promoted
apoptosis compared with the control, while PTPRO overexpression
significantly amplified these E2-induced effects. These results
indicate that PTPRO is involved involved in the regulation of
podocyte viability and apoptosis through E2.
Discussion
Childhood nephrotic syndrome has multiple
aetiologies, which induce an increase of glomerular basement
membrane permeability and thus a large amount of protein is lost by
excretion into the urine (17,18).
Podocytes attach to the outer side of the glomerular basement
membrane to form the glomerular hemofiltration barrier between
vascular endothelial cells and the glomerular basement membrane
(19–21). Therefore, podocyte apoptosis will
increase glomerular basement membrane permeability, inducing
proteinuria (20,22,23),
which is one of the risk factors for the pathogenesis of childhood
nephrotic syndrome. However, little is known about the mechanism of
podocyte apoptosis.
In the current study, it was demonstrated that
oestrogen promotes podocyte proliferation and inhibits podocyte
apoptosis, which are associated with the binding of oestrogen to
its receptor, ERβ, rather than ERα, to eliminate PTPRO expression.
The mechanism may be associated with the activation of the
JAK2/STAT3 signalling pathway by oestrogen.
Oestrogen can inhibit or attenuate the progression
of chronic kidney disease caused by multiple issues, such as
urinary tract obstruction (24,25). A
study using a kidney-wrapped hypertension model in rats revealed
that the castration of male rats reduced proteinuria, and
glomerular and tubular damage, whereas the addition of
dihydrotestosterone inhibited this protective effect (26). The sterilization of female rats
increased glomerular and tubular damage, the addition of E2 reduced
castration-induced kidney damage, and the addition of
dihydrotestosterone inhibited the protective effect of E2 (26). Therefore, androgens were demonstrated
to aggravate renal injury caused by kidney-wrap-induced
hypertension, while oestrogen had a protective effect on the
condition. The current study indicated that oestrogen promoted the
proliferation of podocytes and inhibited the apoptosis of
podocytes.
PTPRO expression has been determined to be highest
in the kidneys and brain, but was also high in other tissues,
including the liver and mammary glands (27). Studies have revealed that the
oestrogen-oestrogen receptor complex (E2-ER) can regulate the
transcription of PTPRO, and ERα and ERβ serve different functions
in the regulatory process (12,16). In
the current study, although ERα promoted the transcriptional
activation of PTPRO, the transcriptional repression of PTPRO by ERβ
was demonstrated to be the more pronounced process. Additionally,
ERα was not expressed in MPC-5 and human primary renal podocytes.
E2 bound to ERβ to further induce the separation of c-jun and c-fos
from the activator protein 1 site in the promoter region of PTPRO,
thereby inhibiting the transcription of PTPRO (12). The aforementioned findings are
consistent with the current study, which demonstrated that the
expression of PTPRO was decreased with the increase of E2 dosages
in MPC-5 and human primary renal podocytes. In essence, E2
inhibited the expression of PTPRO by binding to ERβ, rather than
ERα.
Studies have revealed that PTPRO downregulation
prevents paediatric nephrotic syndrome. Notably, Ozaltin et
al (13) reported that mutations
in the PTPRO gene caused diffuse podocyte effacement and the
extensive microvillus transformation of podocytes, which
contributed to the occurrence of childhood nephrotic syndrome.
However, another study reported that, although PTPRO−/−
mice have shortened podocytes and a reduced total slit diaphragm
length, they did not appear to develop major proteinuria or present
with a reduction of glomerular filtration rate (28). The results of immunofluorescent and
western blot analyses revealed that PTPRO−/− increased
the fluorescence intensity and expression of vimentin in glomeruli
(28). Vimentin is a major component
of intermediate filaments, which are present in the major processes
of podocytes (29). Therefore, these
results appeared to suggest that the inhibition of PTPRO promoted
the flexibility of podocytes and prevented nephrotic syndrome. The
authors of the current study hypothesise that these contradictory
results may be associated with organism-specific differences in
functional redundancy of some phosphatases.
The activation of STAT3 by tyrosine phosphorylation
serves an essential role in the overall process of intracellular
signal transduction (30). Studies
demonstrated that, when cells undergo sustained stimulation from a
variety of cytokines and growth factors, including IL-6 and EGF,
their homologous receptors are recruited and activate JAK2 in a
tyrosine-phosphorylation-dependent manner, which may also lead to
the activation of STAT3 (31,32).
Additionally, another well-known tyrosine kinase, c-Src, was
revealed to be activated and contributed to STAT3 activation by
phosphorylating S727 and T705 in STAT3 (33,34).
Some molecular agents or proteins that attenuate STAT3 activity or
block upstream phosphorylation cascades may suppress cell growth,
such as that of PTPs (35,36). Other proteins or polypeptides,
including TROP2 and P4HB, exhibit the opposite effect, contributing
to the activation of STAT3 and its upstream signal molecules
(11,12). The current study demonstrated that
oestrogen activates Y705 and S727 phosphorylation sites of STAT3
and the Y1007 phosphorylation site of JAK2, indicating that the
JAK2/STAT3 signalling pathway is involved in oestrogen
regulation.
In the current study, oestrogen bound with ERβ,
rather than ERα, promoted podocyte proliferation and inhibited
podocyte apoptosis by inhibiting the expression of PTPRO and
activating the JAK2/STAT3 signalling pathway. The authors suggest
that the current study may provide novel ideas for the prevention
of childhood nephrotic syndrome. Additionally, it was also
determined that PTPRO can activate c-Src, p38 mitogen-activated
protein kinase (MAPK) and MAPK signals during the experiment,
inferring that these signals may be involved in the regulation of
oestrogen in podocyte proliferation (data not shown). Therefore,
this inference will be the focus of a future study by our
group.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
XG and WR conceived of the study. WR, HY, YB and YL
performed the experiments, and collected and analysed all data. WR
and HY prepared the manuscript, while XG, YB and YL revised the
manuscript. All authors edited the manuscript, contributed to the
writing of the manuscript, and read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Welsh GI and Saleem MA: Nephrin-signature
molecule of the glomerular podocyte? J Pathol. 220:328–237.
2010.PubMed/NCBI
|
|
2
|
Niranjan T, Bielesz B, Gruenwald A, Ponda
M P, Kopp JB, Thomas DB and Susztak K: The Notch pathway in
podocytes plays a role in the development of glomerular disease.
Nat Med. 14:290–298. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding F, Wickman L, Wang SQ, Zhang Y, Wang
F, Afshinnia F, Hodgin J, Ding J and Wiggins RC: Accelerated
podocyte detachment and progressive podocyte loss from glomeruli
with age in Alport Syndrome. Kidney Int. 92:1515–1525. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mauvais-Jarvis F, Clegg DJ and Hevener AL:
The role of estrogens in control of energy balance and glucose
homeostasis. Endocr Rev. 34:309–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercantepe T, Unal D, Selli J, Mercantepe
F, Unal B and Karabiyik TN: Protective effects of estrogen and
bortezomib in kidney tissue of post-menopausal rats: An
ultrastructural study. Ren Fail. 38:1129–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong W, Yu J, Wang Q, Li S, Song J, Jia Z,
Huang S and Zhang A: Estrogen-related receptor (ERR) gamma protects
against puromycin aminonucleoside-induced podocyte apoptosis by
targeting PI3K/Akt signaling. Int J Biochem Cell Biol. 78:75–86.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doublier S, Lupia E, Catanuto P,
Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ and Karl M:
Testosterone and 17β-estradiol have opposite effects on podocyte
apoptosis that precedes glomerulosclerosis in female estrogen
receptor knockout mice. Kidney Int. 79:404–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas PE, Wharram BL, Goyal M, Wiggins
JE, Holzman LB and Wiggins RC: GLEPP1, a renal glomerular
epithelial cell (podocyte) membrane protein-tyrosine phosphatase.
Identification, molecular cloning, and characterization in rabbit.
J Biol Chem. 269:19953–19962. 1994.PubMed/NCBI
|
|
9
|
Aguiar RC, Yakushijin Y, Kharbanda S,
Tiwari S, Freeman GJ and Shipp MA: PTPROt: An alternatively spliced
and developmentally regulated B-lymphoid phosphatase that promotes
G0/G1 arrest. Blood. 94:2403–2413. 1999.PubMed/NCBI
|
|
10
|
Alonso A, Sasin J, Bottini N, Friedberg I,
Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J and Mustelin
T: Protein tyrosine phosphatases in the human genome. Cell.
117:699–711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacob ST and Motiwala T: Epigenetic
regulation of protein tyrosine phosphatases: Potential molecular
targets for cancer therapy. Cancer Gene Ther. 12:665–672. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramaswamy B, Majumder S, Roy S, Ghoshal K,
Kutay H, Datta J, Younes M, Shapiro CL, Motiwala T and Jacob ST:
Estrogen-mediated suppression of the gene encoding protein tyrosine
phosphatase PTPRO in human breast cancer: Mechanism and role in
tamoxifen sensitivity. Mol Endocrinol. 23:176–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozaltin F, Ibsirlioglu T, Taskiran EZ,
Baydar DE, Kaymaz F, Buyukcelik M, Kilic BD, Balat A, Iatropoulos
P, Asan E, et al: Disruption of PTPRO causes childhood-onset
nephrotic syndrome. Am J Hum Genet. 89:139–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Charba DS, Wiggins RC, Goyal M, Wharram
BL, Wiggins JE, McCarthy ET, Sharma R, Sharma M and Savin VJ:
Antibodies to protein tyrosine phosphatase receptor type O (PTPro)
increase glomerular albumin permeability [P(alb)]. Am J Physiol
Renal Physiol. 297:F138–F144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Staněk L: Polymerase chain reaction: Basic
principles and applications in molecular pathology. Cesk Patol.
49:119–121. 2013.(In Czech). PubMed/NCBI
|
|
16
|
Hou J, Xu J, Jiang R, Wang Y, Chen C, Deng
L, Huang X, Wang X and Sun B: Estrogen-sensitive PTPRO expression
represses hepatocellular carcinoma progression by control of STAT3.
Hepatology. 57:678–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rheault MN: Nephrotic and nephritic
syndrome in the newborn. Clin Perinatol. 41:605–618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Su B G, Xiao HJ, Zhang HW, Liu XY,
Wang F and Ding J: Clinical characteristics of
glucocorticoid-induced eye adverse reactions in children with
primary nephrotic syndrome. Beijing Da Xue Xue Bao Yi Xue Ban.
49:794–797. 2017.(In Chinese). PubMed/NCBI
|
|
19
|
Bettaieb A, Koike S, Hsu MF, Ito Y, Chahed
S, Bachaalany S, Gruzdev A, Calvo-Rubio M, Lee KSS, Inceoglu B, et
al: Soluble epoxide hydrolase in podocytes is a significant
contributor to renal function under hyperglycemia. Biochim Biophys
Acta. 1861:2758–2765. 2017. View Article : Google Scholar
|
|
20
|
Ito Y, Hsu MF, Bettaieb A, Koike S, Mello
A, Calvo-Rubio M, Villalba JM and Haj FG: Protein tyrosine
phosphatase 1B deficiency in podocytes mitigates
hyperglycemia-induced renal injury. Metabolism. 76:56–69. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim NH: Podocyte hypertrophy in diabetic
nephropathy. Nephrology (Carlton). 10 Suppl:S14–S16. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delezay O, He Z, Hodin S, Saleem MA,
Mismetti P, Perek N and Delavenne X: Glomerular filtration drug
injury: In vitro evaluation of functional and morphological
podocyte perturbations. Exp Cell Res. 361:300–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abraham VC, Miller LN, Pratt SD, Putman B,
Kim L, Gopalakrishnan SM and King A: Implementation of a human
podocyte injury model of chronic kidney disease for profiling of
renoprotective compounds. Eur J Pharmacol. 815:219–232. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Guo Y, Zou H, Sun N, Zhao D, Liu
W, Dong Y, Cheng G and Yuan Q: Effect of estrogen deficiency on the
fixation of titanium implants in chronic kidney disease mice.
Osteoporos Int. 26:1073–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gluhovschi G, Gluhovschi A, Anastasiu D,
Petrica L, Gluhovschi C and Velciov S: Chronic kidney disease and
the involvement of estrogen hormones in its pathogenesis and
progression. Rom J Intern Med. 50:135–144. 2012.PubMed/NCBI
|
|
26
|
Ji H, Menini S, Mok K, Zheng W, Pesce C,
Kim J, Mulroney S and Sandberg K: Gonadal steroid regulation of
renal injury in renal wrap hypertension. Am J Physiol Renal
Physiol. 288:F513–F520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ming F and Sun Q: Epigenetically silenced
PTPRO functions as a prognostic marker and tumor suppressor in
human lung squamous cell carcinoma. Mol Med Rep. 16:746–754. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wharram BL, Goyal M, Gillespie PJ, Wiggins
JE, Kershaw DB, Holzman LB, Dysko RC, Saunders TL, Samuelson LC and
Wiggins RC: Altered podocyte structure in GLEPP1 (Ptpro)-deficient
mice associated with hypertension and low glomerular filtration
rate. J Clin Invest. 106:1281–1290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou J, Yaoita E, Watanabe Y, Yoshida Y,
Nameta M, Li H, Qu Z and Yamamoto T: Upregulation of nestin,
vimentin, and desmin in rat podocytes in response to injury.
Virchows Arch. 448:485–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barre B, Avril S and Coqueret O: Opposite
regulation of myc and p21waf1 transcription by STAT3 proteins. J
Biol Chem. 278:2990–2996. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alvarez JV, Greulich H, Sellers WR,
Meyerson M and Frank DA: Signal transducer and activator of
transcription 3 is required for the oncogenic effects of
non-small-cell lung cancer-associated mutations of the epidermal
growth factor receptor. Cancer Res. 66:3162–3168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dudka AA, Sweet SM and Heath JK: Signal
transducers and activators of transcription-3 binding to the
fibroblast growth factor receptor is activated by receptor
amplification. Cancer Res. 70:3391–3401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boccaccio C, Ando M, Tamagnone L, Bardelli
A, Michieli P, Battistini C and Comoglio PM: Induction of
epithelial tubules by growth factor HGF depends on the STAT
pathway. Nature. 391:285–288. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of Stat3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu F, Dube N, Kim JW, Cheng A,
Ibarra-Sanchez Mde J, Tremblay ML and Boisclair YR: Protein
tyrosine phosphatase 1B attenuates growth hormone-mediated
JAK2-STAT signaling. Mol Cell Biol. 23:3753–3762. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kleppe M, Soulier J, Asnafi V, Mentens N,
Hornakova T, Knoops L, Constantinescu S, Sigaux F, Meijerink JP,
Vandenberghe P, et al: PTPN2 negatively regulates oncogenic JAK1 in
T-cell acute lymphoblastic leukemia. Blood. 117:7090–7098. 2011.
View Article : Google Scholar : PubMed/NCBI
|