Introduction
Niederhuber et al (1) reported on the first use of the totally
implantable central venous port system (TICVPS) in 1982.
Subsequently, the use of the TICVPS in patients undergoing
chemotherapy, parenteral nutrition, intravenous injection,
transfusion or repetitive laboratory analysis has increased
(2,3). The TICVPS may reduce infection rates
and thrombosis arising from recurrent puncture of the veins in
patients with cancer (4,5). In addition, they also have no or only a
minor impact on patients' daily activities, and cosmetic results
after implantation are usually satisfactory (6–10). The
TICVPS provides safe access to the central vein and long-term
comfort and aesthetic satisfaction for patients who require
long-term venous access.
The TICVPS is being implemented in >15 million
patients per annum in the US, with associated complication rates
ranging from 5–19% (11,12). Various types of TICVPS, which all
provide venous access, are inserted by vascular surgeons,
interventional radiologist and oncologists using a number of
methods (13–19). Reported complications are mechanical
complications during or directly after the insertion, including
arterial puncture, nerve injury, hematoma and pneumothorax, and
long-term complications, including infection and thrombosis
(11). As the insertion techniques,
management of the central venous port and catheter material are
improved, the associated complications reduced, however when
complications occur, the length of hospitalization and cost of
medical care increase, and the rates of patient morbidity and
mortality increase (20,21). Thus, it is important to reduce
complications during or after TICVPS insertion.
The purpose of the present study was to describe the
procedures for TICVPS insertion and to assess various postoperative
complications associated with the TICVPS at our center. The present
study also sought to determine how to reduce TICVPS-associated
complications.
Patients and methods
Patients and data
The present study retrospectively reviewed data from
patients who underwent TICVPS insertion between January 2013 and
July 2015 at Pusan National University Yangsan Hospital (Yangsan,
Republic of Korea). Exclusion criteria were as follows: Patients
without fluoroscopy image results or follow-up not completed at
Pusan National University Yangsan Hospital after TICVPS insertion.
A single-type TICVPS (Districath; Districlass Medical SA,
Chaponnay, France) was used. All procedures were performed by 2
vascular surgeons at a single center. Patient data and surgical
radiographic imaging were collected from electronic medical records
and a picture archiving and communication system tool (Marosis
5.4.10.71 PACS viewer; Marotech, Inc., Seoul, Korea), including
operative and progress notes, as well as nursing records, in order
to identify and record complications.
TICVP insertion
The TICVP insertion procedures were performed in the
operating room, and the surgeons constantly monitored the
electrocardiogram (ECG), oxygen saturation and blood pressure of
patients during the procedure. Antibiotics (2 g flomoxef sodium)
were intravenously administered as a prophylaxis prior to the
procedure. The patient was placed in the supine position, and the
neck was slightly turned to the side opposite to that of the
procedure. Betadine was applied around the procedure site, which
was aseptically draped. The right internal jugular vein was
primarily selected as the access vein. The left internal jugular
vein was considered the second access vein if the right internal
jugular vein had an anatomical abnormality or in cases of right
breast cancer. The subclavian vein was used if the two internal
jugular veins could not be accessed. Once the access vein was
determined, venous puncture was performed under ultrasonography and
the guide wire was placed in the needle. Although the needle was
removed and the wire fixed into the vein with mosquito forceps, a
skin incision of ~2 cm was created for the pocket of the chamber at
the deltopectoral region, below the clavicle. After making the
pocket wide enough to insert the chamber (semicircle, ~2 cm in
diameter), a subcutaneous tunnel was created between the puncture
and pocket sites with a tunneler, which was connected at the
catheter end. The chamber was then placed at the pocket site, and
the catheter was cut to place the tip of the catheter at the
cavoatrial junction and into the puncture site. Finally, the
function of the TICVPS was confirmed by aspirating a small amount
of blood from the chamber with a non-coring needle. The blood flow
through the catheter, the catheter angle and the catheter tip
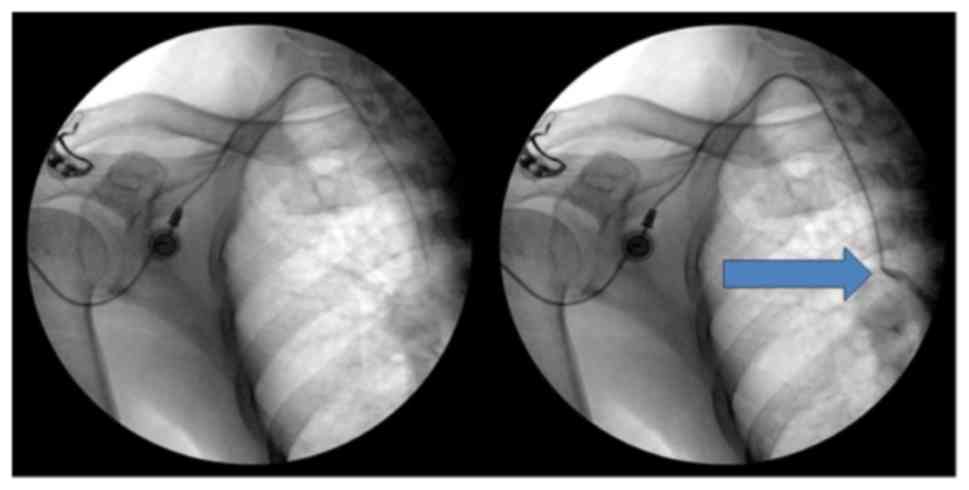
position were checked by injecting a small amount of contrast media
under fluoroscopy with a mobile C-arm (OEC9900 Elite; GE
Healthcare, Little Chalfont, UK; Figs.
1 and 2). The chamber was
sutured on the fascia of the pectoralis major muscle for fixation
of the chamber in the pocket after each checkpoint was confirmed.
The skin incision was sutured subcutaneously with monocryl 5-0 and
reinforced with Steri strips, and dressing was applied. The success
of the procedure was due to the following features showcasing good
clinical practice: i) All procedures were performed in a clean
operating room with monitoring, ii) ultrasonography and fluoroscopy
were used for guidance, iii) the procedure was performed by
experienced surgeons, iv) selection of a wide catheter angle
(>60°) and v) subcutaneous suture with Steri Strips.
Two surgeons had extensive experience with central
venous catheterization (CVC) under ultrasonography (>100 cases
annually during 4 years) and also performed endovascular surgery
(>200 cases annually) under ultrasonography and fluoroscopy, and
a similar operative technique was used for TICVPS placement. A
vascular surgeon experienced in TICVPS was defined as having
performed >400 CVCs and 200 TICVPs.
Post-insertion exam and follow-up
After the insertion, the patient had a 1-day post
insertion check-up by the Surgeon who had performed the TICVPS
insertion and the wound site was examined for any immediate
complications. The team of surgeons initiated the use of the TICVPS
to check its patency. Subsequently, the patients were followed up
over 30 days post-insertion and any observations were added to the
patients' medical records.
Analyses of the present study were performed by
reviewing the electronic medical records of the patients. The
definition of periprocedural complications was classified into
immediate, early and late complications. Immediate complications
are intra-procedural. Early complications were defined as
complications that arise within 24 h, which are mostly
procedure-associated, and also complications that occur within 30
days after the procedure. Late complications are those that are
detected beyond 30 days of insertion. The complication rates
published in previous studies vary in their type. The present study
focused on various important periprocedural complications.
Results
A total of 843 TICVPS were inserted in 827 patients
between January 2013 and July 2015. The procedure was successful in
all cases. TICVPS insertion was performed twice in 16 patients in
this study period. The demographic and clinical characteristics of
the patients are listed in Table I.
A total of 351 patients (42.4%) were males. The mean age of the
patients was 58.2 years (range, 18–86 years), and 448 (54.2%) were
below the age of 60 years. The average time the indwelling catheter
was worn was 275.41 days (range, 1–782 days), and 325 patients
(38.6%) had the indwelling catheter for >300 days. TICVPS
insertion was performed under local and general anesthesia in 715
(84.8%) and 128 cases (15.2%), respectively. Patients under general
anesthesia were simultaneously subjected to cancer surgery and
TICVPS insertion. The most common TICVPS insertion sites were the
right internal jugular, left internal jugular, right subclavian and
left subclavian veins in 724 (85.9%), 113 (13.4%), 4 (0.5%) and 2
cases (0.2%), respectively. The mean catheter angle was 72.5°
(range, 48.7–99.8°) and the mean body mass index of the patients
was 23.0 kg/m2 (range, 13.2–44.4).
 | Table I.Demographics and clinical
characteristics of the patients (n=827). |
Table I.
Demographics and clinical
characteristics of the patients (n=827).
| Variable | Value |
|---|
| Sex |
|
| Male | 351 (42.4) |
|
Female | 476 (57.6) |
| Age (years) | 58.2±11.6
(18–86) |
|
<60 | 448 (54.2) |
| ≥60 | 379 (45.8) |
| Port implantation
period (days) | 275.4±146.5
(1–782) |
|
<300 | 518 (61.4) |
| ≥300 | 325 (38.6) |
| Anesthesia |
|
|
Local | 715 (84.8) |
|
General | 128 (15.2) |
| Port implantation
site |
|
| Right
internal jugular vein | 724 (85.9) |
| Left
internal jugular vein | 113 (13.4) |
| Right
subclavian vein | 4 (0.5) |
| Left
subclavian vein | 2 (0.2) |
| Catheter angle
(°) | 72.5±20.0
(48.7–99.8) |
| Body mass index
(kg/m2) | 23.0±3.6
(13.2–44.4) |
|
<17 | 24 (2.9) |
| ≥17,
<25 | 619 (74.4) |
|
≥25 | 200 (23.7) |
| Underlying
disease |
|
|
Malignant solid tumor | 766 (90.9) |
|
Breast cancer | 182 (21.6) |
|
Gastrointestinal
cancer | 470 (55.8) |
|
Gynecological
cancer | 44 (5.2) |
|
Lung cancer | 32 (3.8) |
|
Other | 38 (4.5) |
| Hematologic
malignancy | 71 (8.4) |
| Benign disease | 6 (0.7) |
| Metastatic
cancer | 343 (40.7) |
Solid tumors, hematologic cancers and benign tumors
were present in 766 (90.9%), 71 (8.4%), and 6 patients (0.7%),
respectively, wherein TICVPS was inserted for fluid resuscitation,
parental nutrition or transfusion.
TICVPS-associated complications are described in
Table II. A total of 34 (4.0%)
complications were recorded. Catheter-associated complications were
the most common type of complication, occurring in 19 cases (2.3%).
Among these patients, 7 patients with catheter-associated infection
underwent TICVPS removal and received antibiotics based on the
results of the catheter tip culture. Catheter migration occurred in
8 patients, which was confirmed by chest radiography. Catheter tip
malposition occurred in the neck vein in 6 cases, and extravenous
catheter migration in 2 cases. Of these patients, 3 underwent
removal and re-insertion of the catheter and 5 were subjected to
re-positioning, including the 2 patients with extravenous catheter
migration. The other 4 patients with catheter thrombosis underwent
catheter removal (n=3) and anticoagulation therapy (n=1), including
1 patient whose catheter function was preserved.
 | Table II.Complications of totally implantable
central venous port system. |
Table II.
Complications of totally implantable
central venous port system.
| Type of
complication | n (%) |
|---|
| Chamber
site-associated | 11 (1.3) |
|
Infection | 5 (0.6) |
|
Erosion | 6 (0.7) |
|
Catheter-associated | 19 (2.3) |
|
Infection | 7 (0.8) |
|
Migration | 8 (1.0) |
|
Thrombosis | 4 (0.5) |
| Other | 4 (0.5) |
| Chamber
malposition | 2 (0.3) |
|
Discomfort | 1 (0.1) |
|
Malfunction | 1 (0.1) |
| Total | 34 (4.0) |
Complications of the chamber insertion site occurred
in 11 cases (1.3%; 5 infections and 6 erosions). All infections
were treated by administration of antibiotics and dressing. Of the
6 erosion cases, 2 underwent TICVPS removal and re-insertion, and 4
were treated with debridement, irrigation and restoration.
Chamber malposition occurred in 2 patients and they
underwent chamber repositioning. One patient had discomfort at the
insertion site and requested TICVPS removal. TICVPS malfunction
occurred in 1 case. Repositioning was performed first, but
malfunction was not resolved; hence, the TICVPS was removed and
another one was inserted.
Discussion
Insertion of the TICVPS is predominantly carried out
by surgeons, who perform venous cut down or use anatomic landmarks
to identify a suitable entry site. However, certain interventional
radiologists perform image-assisted percutaneous TICVPS insertion
using ultrasound guidance with the Seldinger technique at the
access site and fluoroscopy to check the catheter placement with
good success rates comparable to those of the surgeons (11). No significant differences in the rate
of infection between the angiographic suite and the operation
theater, were reported, with P<0.743. When intervention
radiologists performed TICVPS, the infection rate was relatively
high (>5%) (11). In the present
study, the overall complication rate was 4.0%, with no mortality
recorded in 843 cases of TICVPS insertion. In terms of the
complication rate, the present results are superior to those
reported in other studies (5–19%). A comparison of complications
reported in various studies is presented in Table III (12,17,20,22). All
patients of the present study underwent the insertion procedure
performed according to good clinical practice, as mentioned above.
Proper positioning of the catheter tip is important, as
shortcomings thereof are associated with TICVPS malfunction and
catheter-associated complications. In cases of catheter migration
or catheter tip malposition, catheter malfunction, pain or swelling
may occur. Son et al (23)
reported that the risk for catheter thrombosis was high when the
catheter tip was above the superior vena cava.
 | Table III.Comparison of the frequency of
complications (%) between different studies. |
Table III.
Comparison of the frequency of
complications (%) between different studies.
| Complication | Teichgräber et
al (17) (n=3,160) | Babu et al
(22) (n=180) | Kim et al
(20) (n=179) | McGee and Gould
(12) (review) | Present study
(n=827) |
|---|
| Pneumothorax | 0 | 3.7 |
| <0.1–0.2 | 0 |
| Bleeding
Hematoma | 0.2 | 7.4 |
| <0.1–2.2 | 0.2 |
| Malposition | 0 | 5.6 |
|
| 0.1 |
| Deep vein
thrombosis | 0.5 | 4.6 | 4.5 | 4.3 | 0.4 |
| Pain | 0.2 |
|
|
| 0.1 |
| Allergic
reaction | 0.02 |
|
|
| 0 |
| Catheter associated
bloodstream infection | 5.1 | 18.8 | 12.8 | 13.6 | 0.8 |
| Pocket
infection | 0.3 |
|
|
| 0.6 |
| Migration | 0.6 | 10 | 10 |
| 1 |
| Skin erosion | 0.2 | 0 | 0 | 9.3 | 0.7 |
| Accidental
dislodgement | 0.4 | 10.2 | 4.5 | 7.5 | 0.2 |
| Malfunction | 0.1 |
|
| 4.6 | 0.01 |
Schenck et al (24) recommended using intra-atrial ECG
techniques to determine the catheter tip position. In this study,
the electrical current transducer was connected to the catheter and
the cable attached to lead II of a standard ECG monitor. The
catheter was slowly advanced by monitoring the morphological
changes of the P wave until the tip reached the desired position.
The catheter tip is close to the sinoatrial node (i.e., in the
upper part of the right atrium) when the P wave reaches its maximum
height. One centimeter above this point, when the P wave is at half
of its maximal height, the tip is close to the atriocaval junction.
It may be justified to perform delayed postoperative chest
radiography to confirm central venous catheter line tip
placement.
The acceptable complication rate observed in the
present study (4.0%) may be due to several factors. The position of
the catheter tip was determined using fluoroscopy with contrast
media during the procedure. The blood flow in the catheter, as well
as the catheter angle and catheter tip position were checked using
fluoroscopy with contrast media. Prior to the end of the procedure,
the catheter tip position and angle were adjusted if it was not
appropriately placed.
Complications at the chamber insertion site included
infection and erosion. Infection of the chamber insertion site
comprised erythema, tenderness and occasional discharge (25). In the present study, 5 patients with
chamber insertion site infection presented with redness, swelling
and pain, and they were administered antibiotics.
Skin erosion at the chamber insertion site is a rare
long-term complication. The skin overlying the chamber generally
breaks down, exposing the device in the subcutaneous space
(26,27). Skin erosion is a gradual process,
which results in infection. This may manifest systemically as a
fever with chills and/or locally with discharge or abscess
(28). However, erosion without
infection has also been documented (29). Incision site tension, repeated
abrasion or repeated needle puncture may also result in skin
erosion.
A recent study suggested that the incidence of skin
erosion was 1% (30). The incidence
of skin erosion in the present study was 0.7% (6/843). The pocket
was bigger than the chamber (semicircle, ~2 cm in diameter) to
reduce the tension of the skin incision after chamber insertion. A
thick skin flap was also created to withstand repeated puncture and
weight loss. Patients undergoing chemotherapy are more likely to
lose weight due to chemotherapy-associated side effects, and
therefore, the use of a thick skin flap is appropriate in these
patients. The subcutaneous skin incision site was sutured with
monocryl by a well-trained surgeon and reinforced with an aseptic
Steri strip to reduce skin infections and dehiscence. A previous
study reported that subcutaneous suture closure of the incision
site reduced wound disruption (31).
The methods mentioned above may have resulted in a lower incidence
of skin erosion and lower complication rates in the present
study.
In the present study, all patients underwent TICVPS
in a hybrid operation room, which fulfilled aseptic criteria for a
standard surgical room and imaging equipment from a mobile C arm or
angio suite (32), which may have
contributed to reducing sources of infection.
Catheter-associated thrombosis may occur
spontaneously or from a prothrombotic state associated with an
underlying malignancy or treatment (25). The association between cancer and
thrombosis arises as a consequence of cancer treatment and direct
vessel trauma, which is a result of long-term central venous
catheter placement (30). Thrombosis
may cause several symptoms associated with loss of catheter
function, including an increased risk of infection, pulmonary
embolism and postphlebitic syndrome; it is also associated with
greater cost (33).
Catheter-associated thrombosis has a reported incidence of
0.3–28.3% (17,34,35). In
the present study, the incidence of catheter-associated thrombosis
was 0.5% (4/843). At our center, the catheter angle was constantly
checked using fluoroscopy and it was attempted to adjust the
catheter angle to >60° during the procedure. A sharp catheter
angle causes poor blood flow in the catheter, and thrombosis may
easily occur. At our center, the right internal jugular vein was
primarily selected as the access vein, as the jugular vein has a
lower risk for catheter-associated thrombosis than the subclavian
vein (36), and the right internal
jugular vein provides direct access to the superior vena cava
(37). The left internal jugular
vein was considered as the secondary access vein if the right
internal jugular vein had an anatomical abnormality or in patients
with right breast cancer, due to radical axillary lymph node
dissection and postoperative radiotherapy (38). The creation of a catheter angle of
>60° and selection of the appropriate access vein during the
procedure may have reduced the occurrence of catheter-associated
thrombosis in our center.
Patients were diagnosed with catheter-associated
infections if they had at least 2 positive blood culture results,
obtained from at least 2 separate sites at different times, with
evidence of colonization of the catheter with the same organism.
The fulfillment of the latter part of the definition may only be
determined by removing the catheter (25). In the present study, 7 (0.8%) cases
of catheter-associated infection occurred. In a previous study, the
overall incidence of catheter-associated infection was reported as
0–6.8% (39). To reduce
infection-associated complications, TICVPS insertion was performed
in the operating room under aseptic conditions. All healthcare
professionals who participated in the procedure wore surgical gowns
and it was attempted to minimize the length of the surgery.
In the present study, no periprocedural
complication, e.g., pneumothorax, was recorded. Ultrasound-guided
puncture reduces the rate of these complications. Port site
discomfort was recorded in 1 patient, which may have been caused by
nerve injury. Potential early complications, including
pneumothorax, air embolism or arterial puncture may be fatal, but
these did not occur in the present study (40,41).
In conclusion, low complication rates of TICVPS
insertion were observed in the present, large, retrospective study.
Complication rates may be reduced by using a well-designed
procedure, experienced vascular surgeons, an aseptic environment,
ultrasound-guided puncture and fluoroscopy with contrast media.
Acknowledgements
The content of this study was previously presented
at the 17th Congress of the Asian Society for Vascular Surgery,
11th Asian Venous Forum, 20–23 October 2016 in Singapore. The
abstract was published in the Annals of Vascular Diseases, Volume 9
(2016), issue supplement pages S1-S160.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SSL and HJJ devised the project, the main conceptual
ideas and proof outline. DHK and DYR collected and analyzed
patients' data. DHK and DYR wrote the manuscript in consultation
with SSL and HJJ. The final version of the manuscripts has been
read and approved by all authors, and each author believes that the
manuscript represents honest work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niederhuber JE, Ensminger W, Gyves JW,
Liepman M, Doan K and Cozzi E: Totally implanted venous and
arterial access system to replace external catheters in cancer
treatment. Surgery. 92:706–712. 1982.PubMed/NCBI
|
|
2
|
Kock HJ, Pietsch M, Krause U, Wilke H and
Eigler FW: Implantable vascular access systems: Experience in 1500
patients with totally implanted central venous port systems. World
J Surg. 22:12–16. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schenck M and Jäger T: What is practically
important when carrying out a chemotherapy? Urologe A. 45:572,
574–576. 578–579. 2006.(In German).
|
|
4
|
Broviac JW, Cole JJ and Scribner BH: A
silicone rubber atrial catheter for prolonged parenteral
alimentation. Surg Gynecol Obstet. 136:602–606. 1973.PubMed/NCBI
|
|
5
|
Hickman RO, Buckner CD, Clift RA, Sanders
JE, Stewart P and Thomas ED: A modified right atrial catheter for
access to the venous system in marrow transplant recipients. Surg
Gynecol Obstet. 148:871–875. 1979.PubMed/NCBI
|
|
6
|
Torramadé JR, Cienfuegos JA, Hernández JL,
Pardo F, Benito C, González J, Balén E and de Villa V: The
complications of central venous access systems: A study of 218
patients. Eur J Surg. 159:323–327. 1993.PubMed/NCBI
|
|
7
|
Biffi R, de Braud F, Orsi F, Pozzi S,
Mauri S, Goldhirsch A, Nolè F and Andreoni B: Totally implantable
central venous access ports for long-term chemotherapy. A
prospective study analyzing complications and costs of 333 devices
with a minimum follow-up of 180 days. Ann Oncol. 9:767–773. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Funaki B and Zaleski GX: Re: Long-term
follow-up of upper extremity implanted venous access devices in
oncology patients. J Vasc Interv Radiol. 10:12811999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teichgräber UK, Gebauer B, Benter T and
Wagner J: Long-term central venous lines and their complications.
Rofo. 176:944–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeste S, ánchez L, Galbis Caravajal JM,
Fuster Diana CA and Moledo Eiras E: Protocol for the implantation
of a venous access device (Port-A-Cath System). The complications
and solutions found in 560 cases. Clin Transl Oncol. 8:735–741.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yaacob Y, Nguyen DV, Mohamed Z, Ralib AR,
Zakaria R and Muda S: Image-guided chemoport insertion by
interventional radiologists: A single-center experience on
periprocedural complications. Indian J Radiol Imaging. 23:121–125.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGee DC and Gould MK: Preventing
complications of central venous catheterization. N Engl J Med.
348:1123–1133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beheshti MV: A concise history of central
venous access. Tech Vasc Interv Radiol. 14:184–185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dede D, Akmangit I, Yildirim ZN, Sanverdi
E and Sayin B: Ultrasonography and fluoroscopy-guided insertion of
chest ports. Eur J Surg Oncol. 34:1340–1343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Carlo I, Pulvirenti E, Mannino M and
Toro A: Increased use of percutaneous technique for totally
implantable venous access devices. Is it real progress? A 27-year
comprehensive review on early complications. Ann Surg Oncol.
17:1649–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto N, Arai Y, Takeuchi Y, Takahashi
M, Tsurusaki M and Sugimuta K: Ultrasound-guided radiological
placement of central venous port via the subclavian vein: A
retrospective analysis of 500 cases at a single institute.
Cardiovasc Intervent Radiol. 33:989–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teichgräber UK, Kausche S, Nagel SN and
Gebauer B: Outcome analysis in 3,160 implantations of
radiologically guided placements of totally implantable central
venous port systems. Eur Radiol. 21:1224–1232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahn SJ, Kim HC, Chung JW, An SB, Yin YH,
Jae HJ and Park JH: Ultrasound and fluoroscopy-guided placement of
central venous ports via internal jugular vein: Retrospective
analysis of 1254 port implantations at a single center. Korean J
Radiol. 13:314–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goltz JP, Janssen H, Petritsch B and
Kickuth R: Femoral placement of totally implantable venous power
ports as an alternative implantation site for patients with central
vein occlusions. Support Care Cancer. 22:383–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ, Yun J, Kim HJ, Kim KH, Kim SH, Lee
SC, Bae SB, Kim CK, Lee NS, Lee KT, et al: Safety and effectiveness
of central venous catheterization in patients with cancer:
Prospective observational study. J Korean Med Sci. 25:1748–1753.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar AH, Srinivasan NM, Thakkar JM and
Mathew S: A prospective observational study of the outcome of
central venous catheterization in 100 patients. Anesth Essays Res.
7:71–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Babu KG, Suresh Babu MC, Lokanatha D and
Bhat GR: Outcomes, cost comparison, and patient satisfaction during
long-term central venous access in cancer patients: Experience from
a Tertiary Care Cancer Institute in South India. Indian J Med
Paediatr Oncol. 37:232–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Son JT, Min SY, Kim JI, Choi PW, Heo TG,
Lee MS, Kim CN, Kim HY, Yi SY, Lee HR and Roh YN: Thrombolytic
therapy using urokinase for management of central venous catheter
thrombosis. Vasc Specialist Int. 30:144–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schenck M, Schneider T, Rubben H and
Eisenhardt A: Central venous port implantations via the cephalic
vein applying an intravasal electrographic control of the catheter
tip position: A single-center experience of 316 cases. World J
Urol. 30:399–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bishop L, Dougherty L, Bodenham A, Mansi
J, Crowe P, Kibbler C, Shannon M and Treleaven J: Guidelines on the
insertion and management of central venous access devices in
adults. Int J Lab Hematol. 29:261–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brothers TE, Von Moll LK, Niederhuber JE,
Roberts JA, Walker-Andrews S and Ensminger WD: Experience with
subcutaneous infusion ports in three hundred patients. Surg Gynecol
Obstet. 166:295–301. 1988.PubMed/NCBI
|
|
27
|
Whitman ED: Complications associated with
the use of central venous access devices. Curr Probl Surg.
33:309–378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harish K: Chemoport-skin erosion: Our
experience. Int J Angiol. 23:215–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Almhanna K, Pelley RJ, Thomas Budd G,
Davidson J and Moore HC: Subcutaneous implantable venous access
device erosion through the skin in patients treated with
anti-vascular endothelial growth factor therapy: A case series.
Anticancer Drugs. 19:217–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee AY: Cancer and thromboembolic disease:
Pathogenic mechanisms. Cancer Treat Rev. 28:137–140. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chelmow D, Rodriguez EJ and Sabatini MM:
Suture closure of subcutaneous fat and wound disruption after
cesarean delivery: A meta-analysis. Obstet Gynecol. 103:974–980.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hertault A, Sobocinski J, Spear R, Azzaoui
R, Delloye M, Fabre D and Haulon S: What should we expect from the
hybrid room? J Cardiovasc Surg (Torino). 58:264–269.
2017.PubMed/NCBI
|
|
33
|
Frank DA, Meuse J, Hirsch D, Ibrahim JG
and van den Abbeele AD: The treatment and outcome of cancer
patients with thromboses on central venous catheters. J Thromb
Thrombolysis. 10:271–275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verso M and Agnelli G: Venous
thromboembolism associated with long-term use of central venous
catheters in cancer patients. J Clin Oncol. 21:3665–3675. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biffi R, Orsi F, Pozzi S, Pace U, Bonomo
G, Monfardini L, Della Vigna P, Rotmensz N, Radice D, Zampino MG,
et al: Best choice of central venous insertion site for the
prevention of catheter-related complications in adult patients who
need cancer therapy: A randomized trial. Ann Oncol. 20:935–940.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saber W, Moua T, Williams EC, Verso M,
Agnelli G, Couban S, Young A, De Cicco M, Biffi R, van Rooden CJ,
et al: Risk factors for catheter-related thrombosis (CRT) in cancer
patients: A patient-level data (IPD) meta-analysis of clinical
trials and prospective studies. J Thromb Haemost. 9:312–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Carlo I, Cordio S, La Greca G,
Privitera G, Russello D, Puleo S and Latteri F: Totally implantable
venous access devices implanted surgically: A retrospective study
on early and late complications. Arch Surg. 136:1050–1053. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Araujo C, Silva JP, Antunes P, Fernandes
JM, Dias C, Pereira H, Dias T and Fougo JL: A comparative study
between two central veins for the introduction of totally
implantable venous access devices in 1201 cancer patients. Eur J
Surg Oncol. 34:222–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Safdar N and Maki DG: Risk of
catheter-related bloodstream infection with peripherally inserted
central venous catheters used in hospitalized patients. Chest.
128:489–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ballarini C, Intra M, Pisani Ceretti A,
Cordovana A, Pagani M, Farina G, Perrone S, Tomirotti M, Scanni A
and Spina GP: Complications of subcutaneous infusion port in the
general oncology population. Oncology. 56:97–102. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wolosker N, Yazbek G, Nishinari K,
Malavolta LC, Munia MA, Langer M and Zerati AE: Totally implantable
venous catheters for chemotherapy: Experience in 500 patients. Sao
Paulo Med J. 122:147–151. 2004. View Article : Google Scholar : PubMed/NCBI
|