Introduction
Heart surgery with cardiopulmonary bypass (CPB) is a
primary treatment strategy for patients with coronary artery
disease. As blood circulation in the myocardium is avoided during
heart surgery, ischaemia-reperfusion (I/R) injury may occur during
cardioplegic arrest.
A prominent characteristic of ischaemic injury is a
reduced vascular endothelium-dependent vasodilation. Nitric oxide
(NO) (1) and endothelin-1 (ET-1)
(2) are two critical
endothelium-derived factors. NO has a fundamental biological role
in protecting organs (such as the heart) against I/R injury
(3–5). In particular, the protective role of NO
in the heart (6) and kidney
(7) have been proven. Furthermore,
the generation of ET-1 is aggravated under ischaemic conditions
(8). In addition, substantial
evidence has indicated that I/R injury associated with CPB is in
closely linked with the systemic inflammatory response (SIRS)
(9,10). The important roles of inflammation
have also been reported in the pathogenesis of brain ischemia
(11–13). Various inflammatory factors,
including soluble intercellular adhesion molecule-1 (sICAM-1) and
ET-1 (14), participate in
inflammatory processes. Furthermore, oxidative stress contributes
to the pathogenesis of I/R injury (15).
It has been proved that the production of oxygen
radicals is directly associated with major tissue and organ damage
(16). Furthermore, toxic oxygen
metabolites, including the lipid peroxidation product
malondialdehyde (MDA) (17), exert
damaging effects on multiple pathophysiological processes.
Peri-operative myocardial injury (PMI) is a type of
injury that typically occurs in patients who received valve surgery
(18). Furthermore, due to the
effects of anesthetic drugs and mechanical ventilation, pulmonary
compliance of the patients gradually decreases with the time of
ventilation progressing. During CPB, the pulmonary function is
impaired by the continuous low perfusion of the lungs and
pre-flush-mediated blood dilution (19). Such lung I/R injury may affect the
functions of other organs in the patients after the operation.
Based on these investigations, it is necessary to
develop effective therapeutic interventions so as to protect
against tissue injury (20). Remote
ischaemic pre-conditioning (RIPC) has been recognized as a
low-cost, non-invasive intervention method by applying brief
ischaemia and reperfusion on an arm or a leg. RIPC exerts
protective effects on remote tissue or organs against lethal acute
I/R injury (21–24). RIPC may be achieved by performing a
standard blood-pressure cuff (25).
While the effect is not obvious under certain conditions (25–27),
application of RIPC has produced beneficial outcomes in patients
who received open-heart surgery (27–30) or
coronary intervention (31). In
addition, the protective effect of RIPC on the kidney has been
previously demonstrated (32).
However, whether RIPC has the capacity to prevent myocardial and
lung I/R injury has remained to be fully demonstrated.
The overall objective of the present study was to
investigate the protective effect of RIPC on myocardial and lung
I/R injury. Furthermore, the present study aimed to elucidate the
possible underlying mechanisms.
Materials and methods
Study design
The present randomized controlled trial was approved
by the Ethics Committee of the First Affiliated Hospital of Wenzhou
Medical University (Wenzhou, China). Written informed consent was
received from each patient included in the study. Patients who
received valve surgery at the First Affiliated Hospital of Wenzhou
Medical University (Wenzhou, China) between July 2012 and July 2015
were recruited. The inclusion criteria were mitral valve disease,
aortic valve disease or combined valvular disease and patients with
stable hemodynamic blood. The exclusion criteria were, infection,
chronic lung disease, medications that may interfere with RIPC,
pregnancy, renal disease, cardiac arrest during hospital admission
and peripheral arterial disease affecting the limbs, complicated
coronary heart disease, complicated hypertension, congenital heart
valve disease, preoperative stroke, simultaneous radiofrequency
ablation of atrial fibrillation and reoperate. The recruited
patients were randomly divided into two groups. In the grouping
process, the information regarding treatment allocation was
delivered by a nurse who was not involved in the study. The
investigators who analyzed the data were blinded to the treatment
allocation.
Intervention
In the RIPC and control groups, surgery was
initiated after anaesthesia and completed prior to sternotomy. An
intense multi-limb method was performed consisting of two 5-min
cycles of simultaneous upper arm and thigh cuff inflation and
deflation (simultaneous inflation to 200 mmHg, left inflation for 5
min and then deflation to 0 mmHg and left deflated for 5 min)
(32). In the control group,
patients were not subjected to any preconditioning. The
intervention was performed without any arterial line on the arm,
and the blood-pressure cuffs on the arms were bound up.
Anesthesia and surgical protocol
Patients were intramuscularly injected with 0.3
mg/kg scopolamine and 0.2 mg/kg morphine at 0.5 h prior to the
surgery. All patients were routinely monitored via
electrocardiogram, non-invasive blood pressure, invasive radial
arterial pressure, heart rate and respiration using a
multifunctional monitor. Anaesthesia was induced with imidazole
valium (0.1 mg/kg), sufentanyl (0.5 µg/kg), vucuronium bromide
(0.15 mg/kg) and propofol (2.0 mg/kg). Mechanical ventilation was
maintained by a Datex-Ohmeda Aestiva/5 anaesthesia machine (GE
Healthcare, Little Chalfont, UK) with the tidal volume set at 8–10
ml/kg and the suction/call ratio set at 1:2. The normal-end tidal
carbon dioxide pressure was maintained at 26–32 mmHg by setting the
respiratory frequency at 11–13 breaths/min. Myocardium was
protected by perfusion of cold blood cardioplegia. The
concentration of K+ was 23–24 mmol/l. Surgery was
performed with a median sternal incision. The distal ascending
aorta was inserted into the arterial infusion tube. The superior
and inferior venas cava were inserted into the vena cava drainage
tube. The aortic valve was replaced with the atrial cavity tube,
and the right superior pulmonary vein was placed in the left
cardiac drainage to establish extracorporeal circulation. Mitral
valve replacement was performed through the right atrial septal
incision, with continuous or intermittent sutures. Aortic valve
replacement was performed through the aortic root incision with
intermittent suture. If the tricuspid valve has a lesion, it may be
shaped or replaced at the same time. A standard CPB was performed
using the Stöckert SIII perfusion system (Stöckert GmbH,
Munich, Germany), which was followed by valve replacement. The
surgery was completed and protamine was employed to achieve heparin
reversal (protamine/heparin, 1-1.2:1).
Primary and secondary endpoints
The primary endpoint of the present study was PMI.
Highly sensitive cardiac troponin T (hsTnT) was detected as a
marker for PMI. Furthermore, the present study had two secondary
endpoints, one of which were the blood gas indexes, acute lung
injury (ALI) and length of intensive care unit (ICU) stay, while
the other one was length of hospital stay and major adverse
cardiovascular events at 90 days (death, myocardial infarction or
stroke).
Detection of serum markers
Blood samples were collected pre-operatively (T1)
and at 5 min (T2), 2 h (T3), 6 h (T4) and 24 h (T5) after CPB.
hsTnT was quantitated by one-step enzyme immunoassay technology
(Elecsys 2010; Roche Diagnostics, Basel, Switzerland) as described
previously (33). hsTnT levels of
≥14 ng/l were considered to indicate severe myocardial injury. The
content of sICAM-1 was determined by ELISA (sICAM-1; cat. no.
48T96T; Xitang Biotechnology, Shanghai, China) and the optical
density value was recorded by a microplate reader (Multiskan
Spectrum; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Furthermore, the level of ET-1 was detected using an immunoassay
(ET-1; cat. no. 990826; Beijing Institute of East Asian Institute
of Immunology, Beijing, China) according to the manufacturer's
protocol. The contents of MDA and NO were measured using
spectrophotometrical assays (MDA, cat. no. A003-1; NO, cat. no.
A013-2; Nanjin Jiancheng Bioengineering Institute, Jiangsu,
China).
Blood gas analysis and ALI
estimation
Alveolar-arterial oxygen pressure difference
[P(A-aDO2)] and respiratory index (RI) were considered
as blood gas indexes. The partial oxygen pressure
(PaO2), partial CO2 pressure
(PaCO2) and fraction of inspired oxygen
(FiO2) were recorded using an i-STAT (Abbott, Princeton,
NJ, USA) and used to calculate the P(A-aDO2) and RI
using the following formulas:
P(A-aDO2)=(Patm-PH2O) ×
FiO2-PaCO2/R-PaO2 and
RI=P(A-aDO2)/PaO2, where Patm is the
atmospheric pressure of 760 mmHg and PH2O is the water
vapor pressure of 47 mmHg. ALI was estimated according to the
diagnostic criteria of American-European Consensus Conference on
the acute respiratory distress syndrome/ALI (34): i) PaO2/FiO2
<300 mmHg; ii) no atelectasis, no pleural effusion and no
pneumothorax; and iii) no congestive heart failure.
Statistical analysis and sample size
estimation
Values are expressed as the mean ± standard
deviation. Comparison between groups was performed using Student's
t-test or Wilcoxon Mann Whitney test for continuous variables that
were normally or distributed or not, respectively. The Chi-squared
and Fisher's Exact test were used for discontinuous variables.
Two-way analysis of variance followed by Bonferroni's post-hoc test
was used to analyze differences among groups for serum markers
collected at different time-points. Assuming a statistical power of
90% and a type I error rate of 5%, this required a sample size of
120 subjects (which accommodated withdrawal or missing
data-points). SPSS 20.0 (IBM Corp., Armonk, NY, USA) and GrahPad
Prism 5 (GraphPad Inc., La Jolla, CA, USA) were used to analyze the
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients
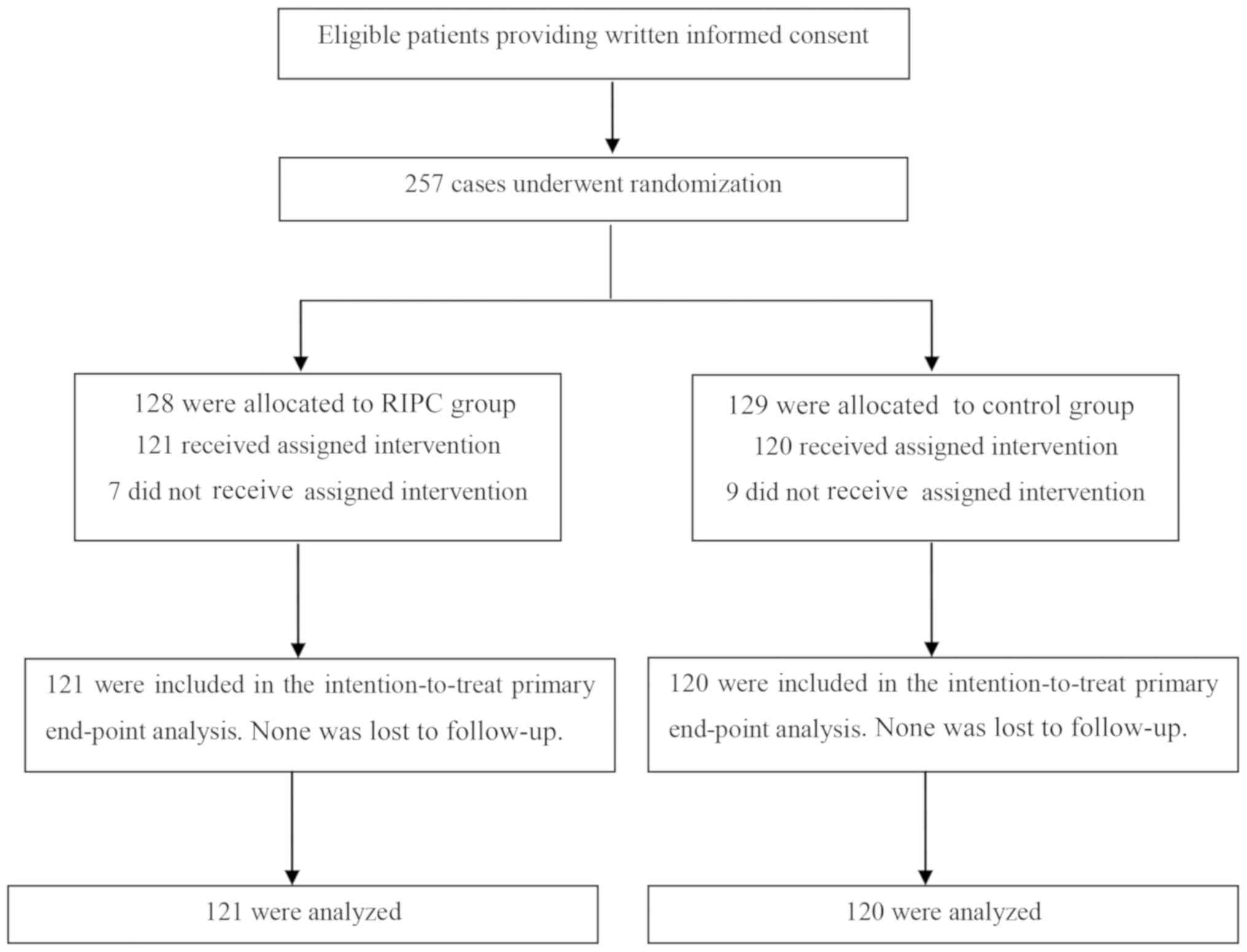
A total of 280 patients were assessed for
recruitment eligibility, and 241 patients were finally enrolled and
assigned to the RIPC (n=121) or control (n=120) group (Fig. 1). With regard to the basic
characteristics, no significant difference was identified between
the two groups (Table I).
Furthermore, no adverse events (death, myocardial infarction or
stroke) associated with the RIPC protocol were observed.
 | Table I.Comparison of clinicopathological
characteristics between the two groups. |
Table I.
Comparison of clinicopathological
characteristics between the two groups.
| Characteristic | RIPC (n=121) | Control group
(n=120) |
|---|
| Age (years) | 45.2±10.06 | 48.2±9.89 |
| Male sex (%) | 65 (53.7) | 62 (55.0) |
| Weight (kg) | 57.6±11.36 | 55.3±9.86 |
| Single/double
valve | 72/49 | 80/40 |
| Left ventricular
ejection fraction (%) |
|
|
|
>55 | 93 (76.7) | 91 (75.8) |
|
<55 | 29 (23.9) | 29 (24.1) |
| NYHA class |
|
|
| I | 27 (22.7) | 25 (20.8) |
| II | 58 (47.9) | 62 (51.7) |
|
III | 31 (25.6) | 33 (27.5) |
| IV | 2 (1.6) | 2 (1.7) |
| AVR | 22 (18.1) | 25 (20.8) |
| DVR | 47 (38.8) | 47 (39.2) |
| MVR | 53 (43.8) | 48 (40.0) |
| Aortic clamp time
(min) | 77.87±28.09 | 80.53±26.32 |
| CPB time (min) | 114.07±31.04 | 112.80±33.87 |
| Mechanical
ventilation time (h) | 8.8±3.64 | 9.2±5.7 |
Effect of RIPC on myocardial injury
and lung injury
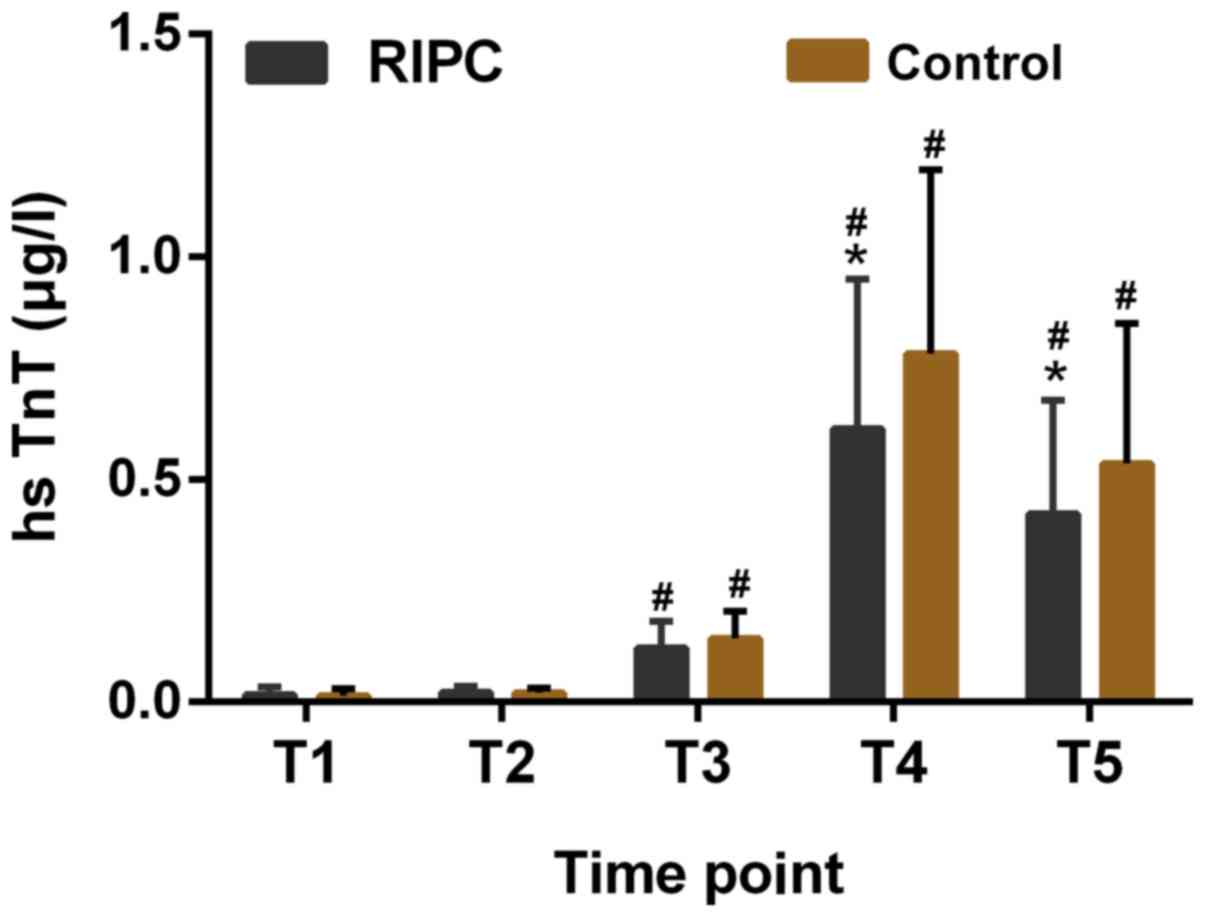
The baseline hsTnT levels in the two groups were
similar and no significant difference was observed. It was
identified that the levels of hsTnT in the RIPC group were reduced
at 6 and 24 h post-CPB as compared with those in the control group
(P<0.05, Fig. 2).
P(A-aDO2) and RI are direct indicators of pulmonary
ventilation and oxygenation function (35), and these two parameters exhibited an
increasing trend at first, followed by a gradual decline gradual
after CPB was performed in each of the two groups [the decline
occurred: P(A-aDO2), T4; RI, RIPC, T5, Control, T4].
After CPB, the P(A-aDO2) was identified to be
significantly lower in the RIPC group compared with that in the
control group at the same time-points (Table II, Fig.
3A). The RI in the control group was significantly higher than
that in the RIPC group at 2, 6 and 24 h after CPB (Table II, Fig.
3B). Furthermore, RIPC achieved a reduction in the incidence of
ALI from 54.1 to 41.3% (P=0.053 vs. control group, Table II).
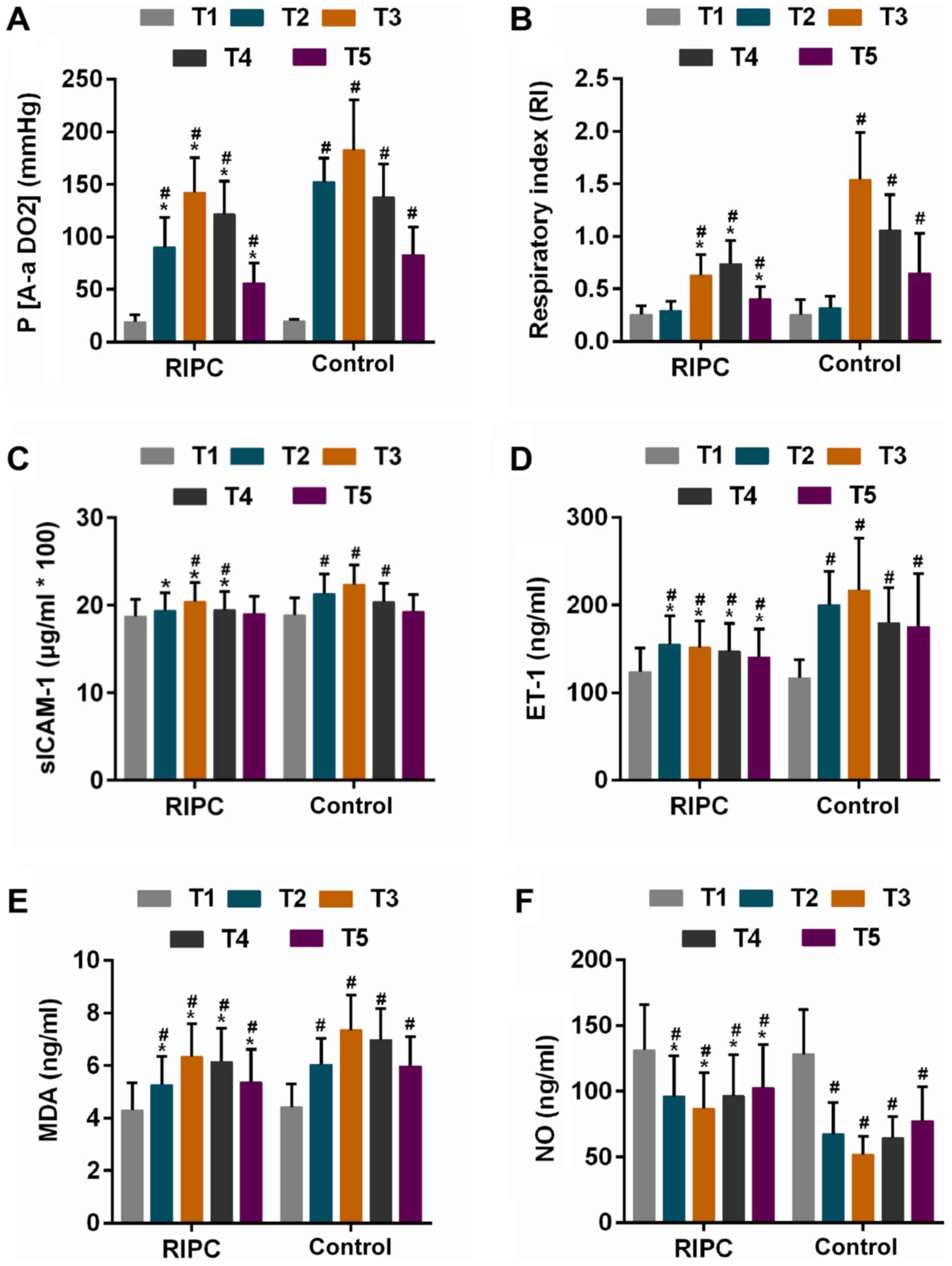 | Figure 3.Parameters in the two groups at
different time-points. (A and B) Oxygen supply represented by (A)
P(A-aDO2) and (B) RI. (C and D) Alteration of serum
markers for inflammation represented by (C) sICAM-1 and (D) ET-1.
(E) Estimation of oxidative stress via determination of MDA
content. (F) Release of NO in RIPC and control group groups
Time-points: T1, prior to surgery; T2, 5 min post-surgery; T3, 2 h
post-surgery; T4, 6 h post-surgery; T5, 24 h post-surgery.
#P<0.01 vs. T1, *P<0.01 vs. Control group at
corresponding time-point. P(A-aDO2), alveolar-arterial
oxygen pressure difference; RI, respiratory index; sICAM-1, soluble
intercellular adhesion molecule-1; ET-1, endothelin-1; MDA,
malondialdehyde; NO, nitric oxide; RIPC, remote ischaemic
pre-conditioning. |
 | Table II.Summary of study endpoints. |
Table II.
Summary of study endpoints.
| Endpoint | Control group
(n=120) | RIPC group
(n=121) | Mean difference
(95% CI) | P-value |
|---|
| hsTnT (µg/l) |
|
|
|
|
| T1 | 0.014±0.016 | 0.016±0.018 | −0.002 (−0.060 to
0.064) | >0.999 |
| T2 | 0.020±0.011 | 0.022±0.013 | −0.001 (−0.061 to
0.063) | >0.999 |
| T3 | 0.143±0.061 | 0.122±0.059 | −0.021 (−0.083 to
0.041) | >0.999 |
| T4 | 0.783±0.412 | 0.614±0.336 | −0.169 (−0.231 to
−0.106) | <0.001 |
| T5 | 0.536±0.314 | 0.423±0.254 | −0.113 (−0.175 to
−0.050) | <0.001 |
|
P(A-aDO2) (mmHg) |
|
|
|
|
| T1 | 19.96±1.47 | 19.09±6.61 | −0.8600 (−10.14 to
8.424) | >0.999 |
| T2 | 152.16±23.80 | 89.98±28.70 | −62.18 (−71.46 to
−52.90) | <0.001 |
| T3 | 182.70±47.74 | 142.3±33.17 | −40.32 (−49.60 to
−31.04) | <0.001 |
| T4 | 137.94±31.15 | 121.6±31.54 | −16.29 (−25.57 to
−7.006) | <0.001 |
| T5 | 82.83±26.60 | 56.02±18.89 | −26.81 (−36.09 to
−17.53) | <0.001 |
| RI |
|
|
|
|
| T1 | 0.255±0.14 | 0.258±0.08 | 0.003 (−0.079 to
0.085) | >0.999 |
| T2 | 0.318±0.11 | 0.292±0.09 | −0.026 (−0.108 to
0.056) | >0.999 |
| T3 | 1.538±0.75 | 0.629±0.20 | −0.909 (−0.991 to
−0.826) | <0.001 |
| T4 | 1.057±0.34 | 0.739±0.22 | −0.318 (−0.400 to
−0.235) | <0.001 |
| T5 | 0.646±0.38 | 0.403±0.12 | −0.243 (−0.325 to
−0.160) | <0.001 |
| ALI | 65 (54.1) | 50 (41.3) | NA | 0.053a |
| ICU stay (h) | 72.28±10.5 | 53.59±8.45 | NA |
<0.001b |
| Hospital stay
(days) | 17.56±3.64 | 16.98±4.01 | NA | 0.241b |
| Clinical outcome at
90 days |
|
|
|
|
|
Death | 4 (3.3) | 2 (1.65) | NA | 0.446c |
|
Myocardial infarction | 2 (1.67) | 1 (0.83) | NA | 0.662c |
|
Stroke | 1 (0.83) | 1 (0.83) | NA | 1.000c |
Effect of RIPC on other endpoints
The length of ICU stay was shortened by the RIPC
treatment (P<0.05, Table II).
The duration of the hospital stay in the RIPC group was also short,
but not significant compared with that in the control group
(P=0.24, Table II). In addition, no
significant difference in the occurrence rate of death, myocardial
infarction and stroke was identified between the RIPC and the
control group (Table II).
Effect of RIPC on inflammatory factors
and oxidative stress
The release of sICAM-1 and ET-1, as well as the
content of MDA increased at first in the two groups at 5 min after
CPB and was further enhanced at 2 h (except for ET-1 in RIPC
group), and declined thereafter. However, the extent of the
increase of these factors was lower in the RIPC group compared with
that in the control group at each corresponding time-point
(Fig. 3C-E). Furthermore, the NO
levels were increased by the RIPC treatment compared with that in
the control group at each corresponding time-point (Fig. 3F).
Discussion
In the present prospective study, it was
demonstrated that RIPC decreased the PMI of patients receiving
valve replacement. Certain studies have proved that RIPC has
beneficial effects in terms of reducing PMI (27,28,30),
which has also been demonstrated in a recent meta-analysis
(36). However, no significant
cardioprotective effect of RIPC was indicated in certain other
previous studies (25,37). Notably, RIPC may not reduce hsTnT
levels, renal injury or ICU-support requirements in high-risk
cardiac surgery in patients receiving generous doses of opioids as
well as propofol and volatile anaesthesia, which differed from the
effective trials. The intense technique used in the present study
was more rapid (requires only 20 min) than the standard single-limb
RIPC protocol (requires 40 min). Thus, it was possible to perform
multi-limb RIPC prior to sternotomy. Furthermore, the different
relative timing of RIPC and the concomitant therapy in patients
undergoing cardiac surgery may contribute to the conflicting
results among studies (25,26,32).
Another conclusion of the present study was that
RIPC treatment elicited protective effects on the lung. Pulmonary
artery blood flow was completely disrupted under CPB, and lung I/R
injury was induced during this process. Post-operative pulmonary
dysfunction has been identified as one of the most important
factors contributing to the cardiac surgery-associated mortality
(38). Pulmonary oxygenation, an
important indicator for evaluating lung function when lung injury
occurs, may be directly reflected by the P(A-aDO2) and
RI (35). In the present study, RIPC
was indicated to achieve a reduction of the P(A-aDO2)
and RI after CPB compared with that in the control group,
suggesting an improvement in the oxygenation of the patients in
RIPC group. In addition, ALI may be triggered by valve replacement
surgery (39). Although no
significant difference was noted in comparison with the control
group, the incidence of ALI was slightly reduced in the RIPC group.
Furthermore, the length of ICU and hospital stays following cardiac
surgery was shortened by RIPC. This result was in line with a
previous study (32). In the present
study, RIPC treatment also reduced kidney injury in patients after
cardiac surgery (32,40). All of these results proved the
protective effect of RIPC on various organs.
Although the mechanisms underlying the protective
effect of RIPC remain to be fully elucidated, a mechanistic model
for the interaction between the pre-conditioned limb and the remote
organ has been proposed (22,41).
Previous studies have demonstrated that ischemic pre-conditioning
suppressed the inflammatory response and improved the anti-oxidant
capacity of tissues (42,43). In addition, the lung is highly
susceptible to oxidative stress due to its large surface area
(44). The effect of RIPC on the
inflammation status and oxidation was then investigated in the
present study. The results indicated that the release of sICAM-1
and ET-1 was mitigated and the content of lipid peroxidation
product MDA after CPB was decreased by RIPC. These results
indicated that RIPC produced a protective effect through inhibiting
SIRS and oxidative stress in lung tissues. Furthermore, the
decreased ET-1 in the RIPC group also suggested that the strength
of myocardial constriction was closely associated with blood
vessels. NO is a vasoactive factor and has relaxation effects,
which were contrary to ET-1 (45).
Consistently, it increased production of NO in the RIPC group,
pointing to the improvement of pulmonary vascular tension. Taken
together, it was concluded that RIPC elicits a protective effect by
reducing the inflammatory status and improving the anti-oxidant
capacity.
A limitation of the present study was that the
effect of RIPC was not assessed in children, as all subjects were
adult patients. The small-scale cohort and single-center design of
the present study were further limitations of this study.
Undoubtedly, the effect of RIPC should be explored on a larger
scale and subjects should be recruited from multiple medical
centers.
In conclusion, the present study demonstrated that
RIPC alleviated PMI and lung I/R injury and may improve clinical
outcomes, including shortened ICU stay, decreased hsTnT level at 6
and 24 h post-surgery, decreased P(A-aDO2) level
beginning from 5 min post-surgery and decreased RI level beginning
from 2 h post-surgery, in adult patients undergoing valve
replacement. The protective effect of RIPC may be associated with
the reduction of inflammation and oxidative stress. However,
large-scale and multi-center randomized controlled trials should be
performed in order to confirm the precise effects of RIPC.
Acknowledgements
None.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XL and LW made substantial contributions to the
conception and design of the present study. LL and XZ were
responsible for acquisition, analysis and interpretation of data.
XJ and XZ were responsible for drafting the article and critically
revising it for important intellectual content. All authors
provided final approval of the version to be published.
Ethical approval and consent to
participate
The present randomized controlled trial was approved
by the Ethics Committee of the First Affiliated Hospital of Wenzhou
Medical University (Wenzhou, China). Written informed consent was
provided by each of the patients included.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ignarro LJ, Buga GM, Wood KS, Byrns RE and
Chaudhuri G: Endothelium-derived relaxing factor produced and
released from artery and vein is nitric oxide. Proc Natl Acad Sci
USA. 84:9265–9269. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yanagisawa M, Kurihara H, Kimura S, Tomobe
Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K and Masaki T: A novel
potent vasoconstrictor peptide produced by vascular endothelial
cells. Nature. 332:411–415. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jalowy A, Schulz R and Heusch G: AT1
receptor blockade in experimental myocardial ischemia/reperfusion.
J Am Soc Nephrol. 10 Suppl 11:S129–S136. 1999.PubMed/NCBI
|
|
4
|
Kubota I, Han X, Opel DJ, Zhao YY, Baliga
R, Huang P, Fishman MC, Shannon RP, Michel T and Kelly RA:
Increased susceptibility to development of triggered activity in
myocytes from mice with targeted disruption of endothelial nitric
oxide synthase. J Mol Cell Cardiol. 32:1239–1248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bolli R: Cardioprotective function of
inducible nitric oxide synthase and role of nitric oxide in
myocardial ischemia and preconditioning: An overview of a decade of
research. J Mol Cell Cardiol. 33:1897–1918. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang XM, Proctor JB, Cui L, Krieg T,
Downey JM and Cohen MV: Multiple, brief coronary occlusions during
early reperfusion protect rabbit hearts by targeting cell signaling
pathways. J Am Coll Cardiol. 44:1103–1110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Chen H, Zhan B, Xing B, Zhou J, Zhu
H and Chen Z: Attenuation of reperfusion injury by renal ischemic
postconditioning: The role of NO. Biochem Biophys Res Commun.
359:628–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasdai D, Kornowski R and Battler A:
Endothelin and myocardial ischemia. Cardiovasc Drugs Ther.
8:589–599. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lang SC, Elsässer A, Scheler C, Vetter S,
Tiefenbacher CP, Kübler W, Katus HA and Vogt AM: Myocardial
preconditioning and remote renal preconditioning-identifying a
protective factor using proteomic methods? Basic Res Cardiol.
101:149–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu
P and Zhou X: Limb ischemic preconditioning reduces heart and lung
injury after an open heart operation in infants. Pediatr Cardiol.
31:22–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuttolomondo A, Pecoraro R, Casuccio A, Di
Raimondo D, Buttà C, Clemente G, Della Corte V, Guggino G, Arnao V,
Maida C, et al: Peripheral frequency of CD4+ CD28- cells in acute
ischemic stroke: Relationship with stroke subtype and severity
markers. Medicine (Baltimore). 94:e8132015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tuttolomondo A, Pedone C, Pinto A, Di
Raimondo D, Fernandez P, Di Sciacca R and Licata G; Gruppo Italiano
di Farmacoepidemiologia dell'Anziano (GIFA) researchers, :
Predictors of outcome in acute ischemic cerebrovascular syndromes:
The GIFA study. Int J Cardiol. 125:391–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Raimondo D, Tuttolomondo A, Buttà C,
Miceli S, Licata G and Pinto A: Effects of ACE-inhibitors and
angiotensin receptor blockers on inflammation. Curr Pharm Des.
18:4385–4413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Przepiera-Będzak H, Fischer K and Brzosko
M: Serum interleukin-18, Fetuin-A, soluble intercellular adhesion
molecule-1, and endothelin-1 in ankylosing spondylitis, psoriatic
arthritis and SAPHO syndrome. Int J Mol Sci. 17(pii): E12552016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lønborg J, Kelbaek H, Vejlstrup N,
Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L,
Treiman M, Jensen JS and Engstrøm T: Cardioprotective effects of
ischemic postconditioning in patients treated with primary
percutaneous coronary intervention, evaluated by magnetic
resonance. Circ Cardiovasc Interv. 3:34–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hashmi MA, Ahsan B, Shah SIA and Khan MIU:
Antioxidant capacity and lipid peroxidation product in pulmonary
tuberculosis. Al Ame en J Med Sci. 5:313–319. 2012.
|
|
18
|
Muehlschlegel JD, Perry TE, Liu KY,
Nascimben L, Fox AA, Collard CD, Avery EG, Aranki SF, D'Ambra MN,
Shernan SK, et al: Troponin is superior to electrocardiogram and
creatinine kinase MB for predicting clinically significant
myocardial injury after coronary artery bypass grafting. Eur Heart
J. 30:1574–1583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erdil N, Eroglu T, Akca B, Disli OM,
Yetkin O, Colak MC, Erdil F and Battaloglu B: The effects of
N-acetylcysteine on pulmonary functions in patients undergoing
on-pump coronary artery surgery: A double blind placebo controlled
study. Eur Rev Med Pharmacol Sci. 20:180–187. 2016.PubMed/NCBI
|
|
20
|
Bonservizi WG, Koike MK, Saurim R, Felix
GA, da Silva SM, Montero EF and Taha MO: Ischemic preconditioning
and atenolol on lung injury after intestinal ischemia and
reperfusion in rats. Transplant Proc. 46:1862–1866. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Przyklenk K, Bauer B, Ovize M, Kloner RA
and Whittaker P: Regional ischemic ‘preconditioning’ protects
remote virgin myocardium from subsequent sustained coronary
occlusion. Circulation. 87:893–899. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hausenloy DJ and Yellon DM: Remote
ischaemic preconditioning: Underlying mechanisms and clinical
application. Cardiovasc Res. 79:377–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sivaraman V, Pickard JM and Hausenloy DJ:
Remote ischaemic conditioning: Cardiac protection from afar.
Anaesthesia. 70:732–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heusch G, Bøtker HE, Przyklenk K,
Redington A and Yellon D: Remote ischemic conditioning. J Am Coll
Cardiol. 65:177–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Young PJ, Dalley P, Garden A, Horrocks C,
La Flamme A, Mahon B, Miller J, Pilcher J, Weatherall M, Williams
J, et al: A pilot study investigating the effects of remote
ischemic preconditioning in high-risk cardiac surgery using a
randomised controlled double-blind protocol. Basic Res Cardiol.
107:2562012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman IA, Mascaro JG, Steeds RP,
Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN,
Green D and Bonser RS: Remote ischemic preconditioning in human
coronary artery bypass surgery: From promise to disappointment?
Circulation. 122:9266672010. View Article : Google Scholar
|
|
27
|
Venugopal V, Hausenloy DJ, Ludman A, Di
Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J and Yellon DM:
Remote ischaemic preconditioning reduces myocardial injury in
patients undergoing cardiac surgery with cold-blood cardioplegia: A
randomised controlled trial. Heart. 95:1567–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hausenloy DJ, Mwamure PK, Venugopal V,
Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C,
Kolvekar S, et al: Effect of remote ischaemic preconditioning on
myocardial injury in patients undergoing coronary artery bypass
graft surgery: A randomised controlled trial. Lancet. 370:575–579.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ali N, Rizwi F, Iqbal A and Rashid A:
Induced remote ischemic pre-conditioning on ischemia-reperfusion
injury in patients undergoing coronary artery bypass. J Coll
Physicians Surg Pak. 20:427–431. 2010.PubMed/NCBI
|
|
30
|
Thielmann M, Kottenberg E, Boengler K,
Raffelsieper C, Neuhaeuser M, Peters J, Jakob H and Heusch G:
Remote ischemic preconditioning reduces myocardial injury after
coronary artery bypass surgery with crystalloid cardioplegic
arrest. Basic Res Cardiol. 105:657–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoole SP, Heck PM, Sharples L, Khan SN,
Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM,
O'Sullivan M and Dutka DP: Cardiac remote ischemic preconditioning
in coronary stenting (CRISP Stent) study: A prospective, randomized
control trial. Circulation. 119:820–827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Candilio L, Malik A, Ariti C, Barnard M,
Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, et
al: Effect of remote ischaemic preconditioning on clinical outcomes
in patients undergoing cardiac bypass surgery: A randomised
controlled clinical trial. Heart. 101:185–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lopez-Calle E, Espindola P, Spinke J, Lutz
S, Nichtl A, Tgetgel A, Herbert N, Marcinowski M, Klepp J, Fischer
T, et al: A new immunochemistry platform for a guideline-compliant
cardiac troponin T assay at the point of care: proof of principle.
Clin Chem Lab Med. 55:1798–1804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernard GR, Artigas A, Brigham KL, Carlet
J, Falke K, Hudson L, Lamy M, Legall JR, Morris A and Spragg R: The
American-European consensus conference on ARDS. Definitions,
mechanisms, relevant outcomes and clinical trial coordination. Am J
Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Gong W, Liu H, Guo Z and Ge S:
Inhibition of matrix metalloproteinase-9 with low-dose doxycycline
reduces acute lung injury induced by cardiopulmonary bypass. Int J
Clin Exp Med. 7:4975–4982. 2014.PubMed/NCBI
|
|
36
|
Pilcher JM, Young P, Weatherall M, Rahman
I, Bonser RS and Beasley RW: A systematic review and meta-analysis
of the cardioprotective effects of remote ischaemic preconditioning
in open cardiac surgery. J R Soc Med. 105:436–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karuppasamy P, Chaubey S, Dew T, Musto R,
Sherwood R, Desai J, John L, Shah AM, Marber MS and Kunst G: Remote
intermittent ischemia before coronary artery bypass graft surgery:
A strategy to reduce injury and inflammation? Basic Res Cardiol.
106:511–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adabag AS, Wassif HS, Rice K, Mithani S,
Johnson D, Bonawitz-Conlin J, Ward HB, McFalls EO, Kuskowski MA and
Kelly RF: Preoperative pulmonary function and mortality after
cardiac surgery. Am Heart J. 159:691–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mazzeffi M, Kassa W, Gammie J, Tanaka K,
Roman P, Zhan M, Griffith B and Rock P: Preoperative aspirin use
and lung injury after aortic valve replacement surgery: A
retrospective cohort study. Anesth Analg. 121:271–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zimmerman RF, Ezeanuna PU, Kane JC,
Cleland CD, Kempananjappa TJ, Lucas FL and Kramer RS: Ischemic
preconditioning at a remote site prevents acute kidney injury in
patients following cardiac surgery. Kidney Int. 80:861–867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karu I, Tahepold P, Ruusalepp A, Reimann
E, Koks S and Starkopf J: Exposure to sixty min of hyperoxia
upregulates myocardial humanins in patients with coronary artery
disease-a pilot study. J Physiol Pharmacol. 66:899–906.
2015.PubMed/NCBI
|
|
42
|
Pinheiro DF, Fontes B, Shimazaki JK,
Heimbecker AM, Jacysyn Jde F, Rasslan S, Montero EF and Utiyama EM:
Ischemic preconditioning modifies mortality and inflammatory
response. Acta Cir Bras. 31:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ucar G, Topaloglu E, Kandilci HB and
Gümüsel B: Effect of ischemic preconditioning on reactive oxygen
species-mediated ischemia-reperfusion injury in the isolated
perfused rat lung. Clin Biochem. 38:681–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Imai Y, Kuba K, Neely GG,
Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen
R, Leung YH, Wang H, et al: Identification of oxidative stress and
Toll-like receptor 4 signaling as a key pathway of acute lung
injury. Cell. 133:235–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Victorino GP, Wisner DH and Tucker VL:
Basal release of nitric oxide and its interaction with endothelin-1
on single vessel hydraulic permeability. J Trauma. 50:535–539.
2001. View Article : Google Scholar : PubMed/NCBI
|