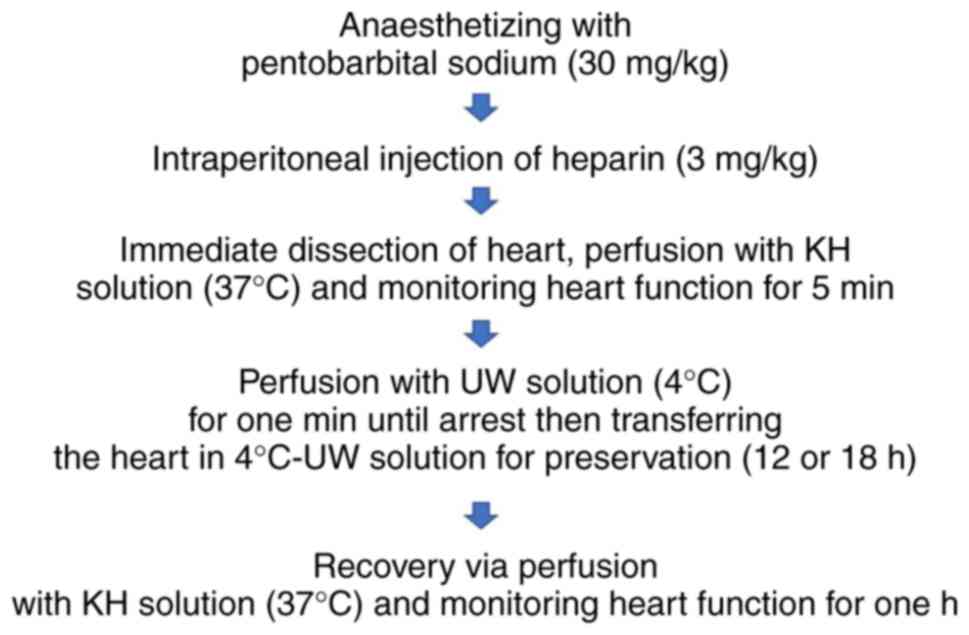
Introduction
Heart transplantation is a widely accepted treatment
for end stage heart disease (1).
Currently, safe heart storage is restricted to 6 h (2), making it impossible to supply donor
hearts in emergencies to patients who are a large distance away
from donors. Thus, it is necessary to explore approaches that may
extend the storage time (3).
The heart consumes a large amount of oxygen to
support cardiomyocytes, which can become hypoxic within 8 min of
the heart stopping (4). Thus, during
preservation and transplantation, cardiomyocytes suffer
pathological changes, including acidosis, autolysis and aggravated
myocardial damage. These changes are caused by free radicals and
calcium overload following reperfusion, leading to deteriorations
in metabolism, function and the ultrastructure of the cells
(5). An improved heart preservation
solution with ATP precursors could be effective in preventing
cellular edema, acidosis, and intercellular edema and injury.
Currently, effective preservation methods, including University of
Wisconsin solution (UW), histidine-tryptophan-ketoglutarate
solution and Celsior solution, cannot extend effective heart
preservation beyond 6 h (6–8).
Previous studies have revealed that various
traditional Chinese medicines are effective in protecting organs
from reperfusion-induced injury, including root of red-rooted
salvia, astragalus saponin and ligustrazine (9–15).
Luteolin (3′,4′,5,7-tetrahydroxyflavone,
C15H10O6) is a widely distributed
natural flavonoid and the main active ingredient in various
medicinal plants (16). Luteolin has
exhibited anti-oxidative, anti-bacterial, anti-inflammatory and
anti-viral effects; it has been demonstrated to lower blood fat and
cholesterol, and inhibit intracellular calcium elevation (17–20).
However, whether luteolin may be used in heart preservation remains
unknown.
In the current study, the effect of luteolin on
heart preservation and the underlying mechanisms of luteolin were
explored. The present study may provide a theoretical basis for
preserving hearts with luteolin.
Materials and methods
Animal and heart dissection
Specific pathogen free-grade Sprague Dawley rats
(n=80, 40 females and 40 males; age, 12–13 weeks; weight, 250 g)
were obtained from the Animal Center of Xi'an Jiaotong University
(Xian, China). Rats were given access to a standard diet of
Animalabo A 04 and water ad libidum, maintained under
controlled conditions of light (12-h light/dark cycle), temperature
(22±1°C) and humidity (35±5%) and. The animals were separated into
four groups (control, low luteolin 7.5 µM, medium luteolin 15 µM
and high luteolin 30 µM; n=20/group). In each group, dissected
hearts were stored for 12 (n=10) or 18 h (n=10). Prior to heart
dissection, rats were fasted for 12 h, anesthetized with
pentobarbital sodium (30 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and then administered heparin (3 mg/kg) by
intraperitoneal injection. Breathing was observed for 5 min before
additional doses of pentobarbital sodium (10–30 mg/kg) were
administered by intraperitoneal injection, and sacrifice by
cervical dislocation. The chest cavity was immediately opened, the
main arteries were cut and the hearts were transferred into 37°C
Krebs-Henseleit (KH) solution (118 mM NaCl, 4.7 mM KCl, 0.9 mM
KH2PO4, 1.2 mM
MgSO4·7H2O, 1.5 mM CaCl2, 25 mM
NaHCO3 and 11 mM
C6H12O6, pH=7.4). Following the
washing-out of residual blood, an aorta perfusion tube was set up
and the hearts were transferred to a Langendorff system
(custom-made) for perfusion with 37°C filtered KH solution balanced
with 95% O2 and 5% CO2 (perfusion pressure of
75 cm H2O). The interval between the opening of the
chest and the start of perfusion was within 50 sec. After a 1-min
perfusion, left ventricular functional assessments can be made
using a left ventricular balloon. One end of a latex balloon was
fixed to polyethylene tubing (devoid of air bubbles) and inserted
into the left ventricle, via the mitral valve through a small
opening in the left atrium, whilst the other end was connected to a
multi-channel recorder for data collection. The ventricular latex
sphere (balloon) was filled with saline (~0.15 ml) to maximize
signal detection. The left ventricular end-diastolic pressure was
maintained at 4 mmHg for 5 min and then the heart was perfused with
15 ml University of Wisconsin (UW) preservation solution (100 mM
potassium lactose, 25 mM KH2PO4, 5 mM
MgSO4, 30 mM raffinose, 5 mM adenosine, 3 mM
glutathione, 1 mM allopurinol, 50 g/l hydroxyethyl starch, 100 U
insulin, 40 U penicillin and 8 mg dexamethasone, pH=7.4 and osmotic
pressure=320±5 mOsm/l) with or without luteolin (Hangzhou FST
Pharmaceutical Co., Ltd., Hangzhou, China) for 1 min at 4°C. The
dissected hearts were incubated in UW preservation solution for 12
or 18 h (Fig. 1). All surgical tools
were purchased from Shanghai Medical Instruments Co., Ltd.
(Shanghai, China). All protocols were approved by the Ethics
Committee of Hainan Medical University (Haikou, China) and
procedures conformed to the Directive 2010/63/EU of the European
Parliament and the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (21). All animal experiments were performed
in an animal facility at Hainan Medical University.
Monitoring heart function
Heart function was measured following reperfusion in
the Langendorff system. Heart rate (HR), left ventricle peak
systolic pressure (LVPSP), maximal rate of rise of LVPSP
(dP/dtmax) and coronary flow (CF) were measured for 1
min at 3, 5, 15 and 30 min after reperfusion. Rate pressure product
(RPP) was calculated as LVPSP × HR. Residual liquid was wiped away
with filter papers and wet weight was measured. Following
incubation at 80°C for 48 h, dry weight was measured. Water content
(%) was calculated as: 1-(dry weight/wet weight) ×100.
Histological monitoring
Following reperfusion, 2–3 cm3 tissue
samples of the left ventricle were dissected and fixed in 10%
neutral formaldehyde solution at room temperature for 24 h,
embedded in paraffin and cut into 4–5 µm-thick sections for
hematoxylin and eosin staining. Paraffin-embedded tissue samples
were deparaffinised in xylene at 25°C at room temperature and
rehydrated through graded concentrations of ethanol (100% ethanol
for 5 min, 95% ethanol for 1 min, 80% ethanol for 5 min, 75%
ethanol for 5 min and distilled water for 2 min) at room
temperature prior to staining with hematoxylin and eosin for 12 min
at room temperature. Images were captured using an optical
microscope (magnification, ×400). In addition, 1 mm3
tissue samples of the left ventricle were dissected and fixed with
2.5% glutaraldehyde for 24 h. The fixed samples were washed three
times with PBS, post-fixed with 1% osmium tetroxide, dehydrated
with acetone, and embedded in epoxy resin. Ultrathin sections were
cut and double-stained with 1% lead citrate and 0.5% uranyl
acetate. Images were captured using a transmission electron
microscope (H-7700; Hitachi, Ltd., Tokyo, Japan).
Immunohistochemistry
Immunohistochemical staining was performed on
paraffin-embedded left ventricular tissue samples using the
Avidin-Biotin-Peroxidase kit (Boster Biological Technology, Ltd.,
Wuhan, China). Antigen retrieval was performed using a citrate
buffer (pH 6.0) for 20 min. Paraffin-embedded tissue sections were
cut into 3-µm-thick sections using a SM200R microtome (Leica
Microsystems GmbH, Wetzlar, Germany) and mounted onto silane-coated
glass slides. The dried tissue sections were subsequently
deparaffinized in xylene and rehydrated in a descending alcohol
series. Deparaffinised sections were incubated with 3% hydrogen
peroxide for 10 min at room temperature to inhibit endogenous
peroxidase activity. Tissue sections were incubated with primary
antibodies directed against Bax (1:300; cat. no. BM3964) or Bcl-2
(1:100; cat. no. BA0412; both Boster Biological Technology, Ltd.)
for 2 h at room temperature. Following the primary incubation,
tissue sections were incubated with a biotinylated secondary
antibody (1:200; cat. no. BA1005) for 20 min at room temperature
followed by incubation with peroxidase-labeled streptavidin (1:500;
cat. no. BA1088; Boster Biological Technology, Ltd.) in a
humidified box at 37°C for 20 min. Peroxidase activities were
visualized using 3,3-diaminobenzidene peroxidase substrate.
Sections were counterstained with 1% haematoxylin for 2–3 min at
room temperature. Negative and positive controls were run on all
sections. The intensity of Bax and Bcl-2 immunostaining was
assessed using a light microscope (magnification, ×400), by two
independent observers. Cytoplasmic staining was considered as
positive staining. Immunostaining was scored as follows: (−)
negative, (+) weak, (++) moderate and (+++) strong.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The left ventricular tissue sections were analyzed
using a TUNEL assay kit (cat. no. MK1025; Boster Biological
Technology, Ltd.), according to the manufacturer's protocol.
Paraffin-embedded tissue sections were subsequently deparaffinized
in xylene at 58–60°C for 30 min and rehydrated in a descending
ethanol series (100, 95 and 70% ethanol) for 5 min, respectively.
Antigen retrieval was performed with 100 µl of 20 µg/ml proteinase
K (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 10
min and subsequently washed twice with PBS, according to Gavrieli's
method (22). Terminal
deoxynucleotidyl transferase (TdT) was used to incorporate
digocigenin (DIG)-conjugated dUTP to the ends of DNA fragments. The
sections were incubated with 20 µl TdT reaction solution in a dark
humidified chamber at 37°C for 120 min. Following TdT incubation,
sections were incubated with anti-DIG-Biotin antibody (1:100;
Boster Biological Technology, Ltd.) for 30 min at 37°C. The signal
of TdT-mediated TUNEL was detected by incubation with 100 µl
streptavidin biotin-peroxidase complex (Boster Biological
Technology, Ltd.) in a dark humidified chamber for 30 min at 37°C.
The tissue sample slides were incubated with diaminobenzidine (DAB;
Boster Biological Technology, Ltd.) solution for color development
and observed using a light microscope (magnification, ×400). DAB
precipitates as a dark brown pigment allowing easy visualization of
positively stained cells. Apoptotic cells with condensed nuclei
were stained brown, whilst non-apoptotic cells were not stained.
Cells with clear nuclear labeling were defined as TUNEL-positive
cells. The number of TUNEL-positive cells from four randomly
selected fields from each tissue section were used to determine the
extent of myocardial tissue injury.
Monitoring enzymatic activities
Extracellular lactate dehydrogenase (LDH; cat. no.
A020-2) and creatine kinase (CK; cat. no. A032) activity in the
perfusion solution, as well as superoxide dismutase (SOD; cat. no.
A001-3) and malondialdehyde (MDA; cat. no. A003-1) activity in
ventricular homogenates were examined using kits purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China),
according to the manufacturer's protocols.
Recording L-type calcium channels in
cardiomyocytes
Dissected hearts were placed into 4°C high potassium
Tyrode's solution [143 mM NaCl, 140 mM KC1, 1.8 mM
CaCl2, 0.5 mM MgCl2, 0.3 mM
NaH2PO4, 5 mM
4-(2-hydroxyethyl)1-piperazineethanesulphonic acid (HEPES) and 5 mM
glucose, pH=7.4]. The aorta was rapidly cannulated and connected to
a Langendorf perfusion apparatus. The hearts were perfused
retrogradely at 37°C for 5 min with normal Tyrode's solution (143
mM NaCl, 5.4 mM KC1, 1.8 mM CaCl2, 0.5 mM
MgCl2, 0.3 mM NaH2PO4, 5 mM HEPES
and 5 mM glucose, pH=7.4) followed by 3–5 min perfusion with
calcium-free Tyrode's solution (143 mM NaCl, 5.4 mM KC1, 0.5 mM
MgCl2, 0.3 mM NaH2PO4, 5 mM HEPES
and 5 mM glucose, pH=7.4) and 15–18 min perfusion with calcium-free
Tyrode's solution containing Type-II collagenase (2 mg/ml). The
hearts were then washed with Krebs solution (70 mM L-glutamic acid,
25 mM KC1, 3 mM MgC12, 20 mM taurine, 10 mM
NaH2PO4, 0.5 mM EGTA, 10 mM HEPES and 10 mM
glucose, pH=7.4) until the heart expanded to increase tension, and
then the tension decreased and the heart became soft. The left
ventricular wall was dissected and sheared in Krebs solution;
following filtration with a 200-mesh filter and incubation in Krebs
solution at room temperature for 10 min, cardiomyocytes were
separated following sedimentation. The supernatant was replaced
with Krebs solution supplemented with bovine serum albumin (1
mg/ml, Sigma-Aldrich) and incubated at room temperature for 40 min,
the myocardial cells were resuspended in Tyrode's solution
supplemented with the calcium concentration gradually increased to
1.8 mmol/l. Then the cells were stored in normal Tyrode's solution
at room temperature for 1 h. The myocardial cells were cultured in
glucose-free Krebs solution and hypoxia was induced by bubbling the
gas mixture (90% N2 and 10% O2) in the cell
medium for 3 h. The level of hypoxia was measured using a
commercial oxygen needle electrode (Strathkelvin Instruments Ltd.,
North Lanarkshire, UK). Hypoxic myocardiocytes were placed in an
experimental perfusion chamber mounted on an inverted microscope
for 5 min to allow cell adhesion. The chamber was perfused with
external solution (135 mM NaCl, 1.8 mM CaCl2, 4.6 mM CsCl, 0.5 mM
MgCl2, 5 mM HEPES, 10 mM glucose, pH=7.4) at a rate of
1.8 ml/min at 37°C. Cells with a rod-like appearance, good marginal
refraction, clear striations and no contraction activity were
selected for recording.
Action potentials were recorded using an Axopatch
200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Data
acquisition and analysis was performed by pCLAMP™ 10
Electrophysiology Data Acquisition & Analysis software
(Molecular Devices). Micropipettes (resistance 2.5–3.5 MΩ) were
pulled using a two-step vertical microelectrode puller (pp-83,
Narisige, Japan). Whole-cell recording micropipettes were filled
with external solution (140 mM CsCl, 0.5 mM MgCl2, 4 mM
Na2ATP, 1 mM EGTA, 5 mM HEPES and 5.5 mM glucose,
pH=7.2). After the whole-cell configuration was achieved, action
potential was recorded in current clamp mode and membrane current
recorded in voltage clamp mode. Positive pressure (10 cm
H2O) was applied when electrodes approached each cell,
while negative pressure was applied following the attachment of
each electrode. Cell membranes were broken by negative pressure
(~100 cm H2O), which allowed recording in whole-cell
mode. Whole-cell recording started 5 min after the cell membranes
were broken and membrane potential was gradually increased from −40
mV to +50 mV in 10 mV steps (300 msec duration, 0.5 Hz) with 10 sec
intervals. Cells were incubated with 7.5, 15 or 30 µM luteolin
(Hangzhou FST Pharmaceutical Co., Ltd., Hangzhou, China) dissolved
in external solution for perfusion and membrane potential was
clamped at −40 mV, depolarized to 0 mV and further clamped for 300
msec.
Statistical analysis
Data were presented as mean ± standard deviation.
SPSS 12.0 (SPSS, Inc., Chicago, IL, USA) was used for analysis.
Differences between groups were evaluated using a two-way analysis
of variance followed by Bonferroni's correction. P<0.05 was
considered to indicate a statistically significant difference.
Results
Luteolin improves heart function
following preservation
Heart function was monitored prior to preservation.
No significant differences were identified in the monitored
parameters among the different groups (Table I).
 | Table I.Heart function prior to
preservation. |
Table I.
Heart function prior to
preservation.
| Group | Left ventricle peak
systolic pressure (mmHg) | +dP/dtmax
(mmHg/sec) | –dP/dtmax
(mmHg/sec) | Heart rate
(beats/min) | Rate pressure
product (mmHg/min) |
|---|
| Control | 85.4±9.7 | 1,815.2±321.9 | 1,429.6±320.6 | 344.8±45.4 | 30,819.5±501.0 |
| Lu low (7.5
µmol/l) | 87.3±8.3 | 1,785.1±283.5 | 1,586.5±349.3 | 326.1±53.1 | 30,973.1±410.2 |
| Lu medium (15
µmol/l) | 85.7±7.7 | 1,708.2±335.4 | 1,681.4±278.7 | 354.1±49.2 | 30,701.3±545.2 |
| Lu high (30
µmol/l) | 86.8±8.4 | 1,828.7±132.0 | 1,646.7±295.4 | 340.9±43.6 | 30,740.8±617.4 |
To characterize heart preservation under different
conditions, several parameters reflecting heart function were
monitored. All parameters significantly improved in the presence of
luteolin at 12 h (Fig. 2A-F) and 18
h (Fig. 3A-F) after preservation in
what appeared to be a dose-dependent manner. No significant
difference was identified in tissue water content among different
groups (Figs. 2G and 3G). Thus, luteolin may improve heart
function following long term heart storage.
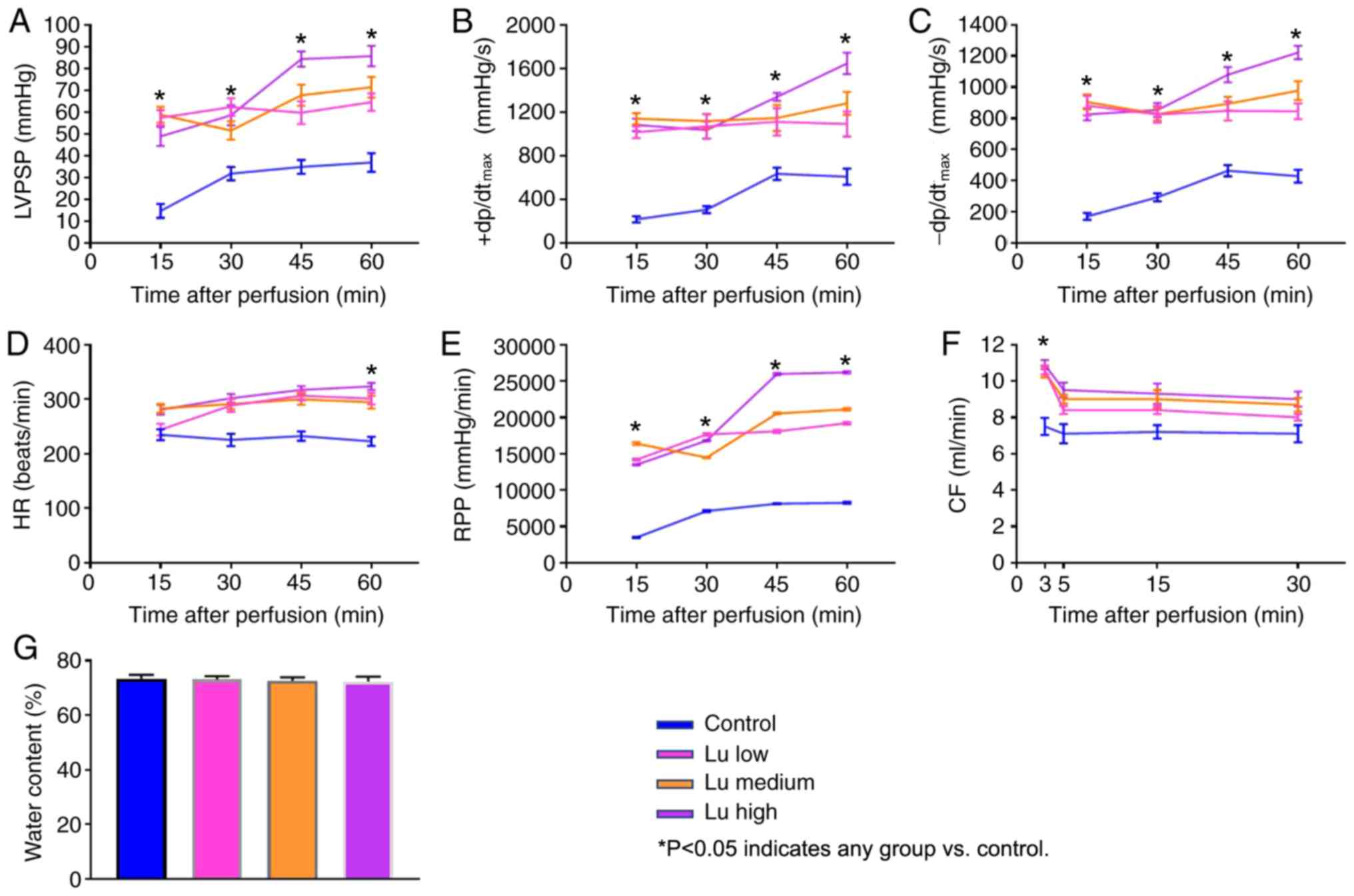 | Figure 2.Lu improves heart function 12 h after
preservation. (A) LVPSP, (B) +dP/dtmax, (C)
-dP/dtmax, (D) HR, (E) RPP and (F) CF were measured 15,
30, 45 and 60 min after a 12-h preservation in a low, medium or
high Lu solution. (G) Water content was measured after a 12-h
preservation in a low, medium or high Lu solution. *P<0.05 vs.
Control. Lu, luteolin; low, 7.5 µmol/l; medium, 15 µmol/l; high, 30
µmol/l; LVPSP, left ventricle peak systolic pressure;
dP/dtmax, maximal rate of rise of LVPSP; HR, heart rate;
RPP, rate pressure product; CF, coronary flow. |
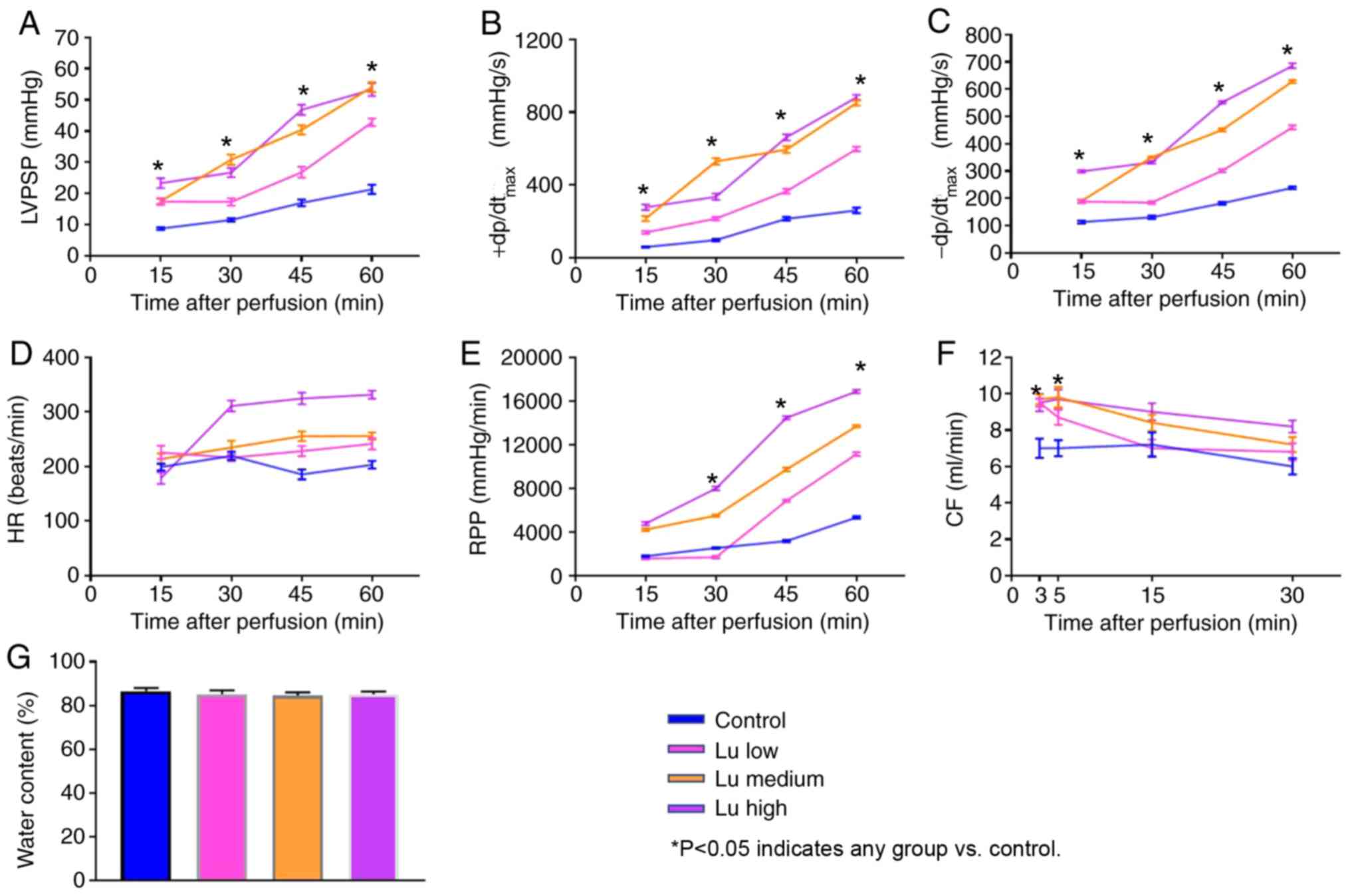 | Figure 3.Lu improves heart function 18 h after
preservation. (A) LVPSP, (B) +dP/dtmax, (C)
-dP/dtmax, (D) HR, (E) RPP and (F) CF were measured 15,
30, 45 and 60 min after an 18-h preservation in a low, medium or
high Lu solution. (G) Water content was measured after an 18-h
preservation in a low, medium or high Lu solution. *P<0.05 vs.
control. Lu, luteolin; low, 7.5 µmol/l; medium, 15 µmol/l; high, 30
µmol/l; LVPSP, left ventricle peak systolic pressure;
dP/dtmax, maximal rate of rise of LVPSP; HR, heart rate;
RPP, rate pressure product; CF, coronary flow. |
Luteolin protects hearts from
structural damage
Structural analysis using a light microscope
demonstrated that, prior to preservation, cardiomyocytes were
arranged in an orderly manner and branches extended to form a
network (Fig. 4A). Intercalated
disks were clear and no edema was evident. Blood vessels were
normal with intact endothelia and stratified structures. At 12 h
after preservation, vacuoles and irregular structures were evident
in the cytosol of cardiomyocytes, and cardiac muscle fibers were
interrupted. (Fig. 4B). In the Lu
low group (Fig. 4C), there was some
evidence of necrosis as necrotic cardiomyoctes exhibited
eosinophilic hyperchromatism. In the Lu medium group (Fig. 4D) and Lu high group (Fig. 4E), 12 h after preservation, there
were some areas of the myocardial cytoplasm that showed a small
number of vacuoles. In addition, the myocardial fibers became thin
and elongated, forming waves of parallel arrangement. Necrosis was
evident in cardiomyocytes, the interstitial space was slightly
enlarged and the endothelia were interrupted. The aforementioned
pathological alterations were more evident 18 h after preservation
compared with the control group (Fig.
4F). A small number of different-sized vacuoles were observed
in the cytosol of cardiomyocytes, and cardiac muscle fibers were
elongated, forming waves of parallel arrangement. In some regions,
myocardial fibers were broken. Some necrotic myocardiocytes
exhibited eosinophilic hyperchromatism in luteolin groups 18 h
after preservation (Fig. 4G-I).
However, the pathological alterations observed were improved by
luteolin in what appeared to be a dose dependent manner (Fig. 4G-I).
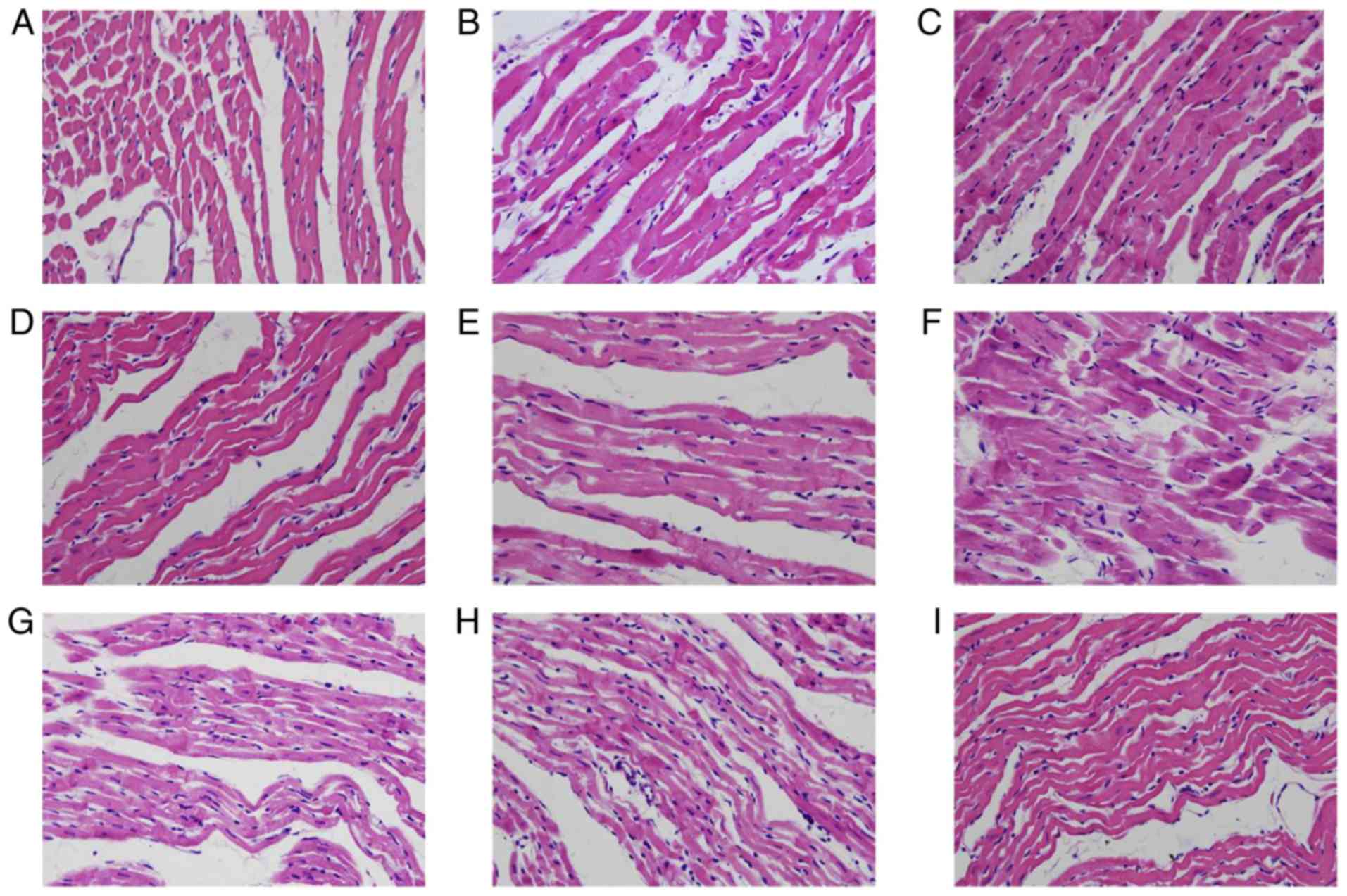 | Figure 4.Lu protects rat cardiac muscle
morphology from long-term storage-induced damage. The morphology of
rat cardiac muscle (A) prior to preservation, in the (B) control,
(C) Lu low, (D) Lu medium and (E) Lu high groups 12 h after
preservation, and in the (F) control, (G) Lu low, (H) Lu medium,
(I) Lu high groups 18 h after preservation. Magnification, ×400.
Lu, luteolin; low, 7.5 µmol/l; medium, 15 µmol/l; high, 30
µmol/l. |
In structural analysis using TEM, cardiomyocytes
exhibited a fusiform and large oval nucleus, the long axis of
nucleus was parallel to muscle fibers, and chromatin was
distributed normally prior to preservation (Fig. 5A). In cytosol, slight mitochondrial
edema was evident, but with uniform crista. Sparse sarcoplasmic
reticulum (SR), dense rough endoplasmic reticulum and Golgi complex
were around each nucleus. The enlargement of SR was evident, and
formed diad and triad complexes with T tubules. Intercalated disks,
which form intercellular connections, were observed between
neighboring cardiomyocytes. Prior to preservation it was revealed
that microvasculature networks were within intercellular spaces,
which exhibited flattened endothelia and intact base membranes. At
12 h after preservation, some myofibrils and sarcomeres exhibited
distortions. Mitochondrial membranes were unclear, and mitochondria
exhibited edema and reduced crista; Vacuoles were observed in a
number of crista (Fig. 5B). In the
Lu low, medium and high groups, most of the cardiac muscle fibers
were arranged orderly with clear striations. There were no
significant changes observed in the nucleus of the myocardiocytes,
however there were some broken muscle fibers. The arrangement of
myofibrils was slightly disordered, but the light and dark bands
were still clear. Mitochondria appeared swollen and vacuolated 12 h
after preservation, compared with the control group (Fig. 5C-E). In addition, the observed
alterations were more pronounced in cells 18 h after preservation
compared with the control group (Fig.
5G-I). However, those alterations could be ameliorated by
luteolin in what appeared to be a dose dependent manner (Fig. 5G-I). Thus, luteolin may protect
hearts from structural damage during long-term preservation.
 | Figure 5.Lu protects rat cardiac muscle
ultrastructure from long-term storage-induced damage. (A) The
morphology of rat cardiac muscle prior to preservation
(magnification, ×10,000). The morphology of rat cardiac muscle in
the (B) control (magnification, ×10,000), (C) Lu low
(magnification, ×10,000), (D) Lu medium (magnification, ×10,000)
and (E) Lu high (magnification, ×10,000) groups 12 h after
preservation. The morphology of rat cardiac muscle in the (F)
control (magnification, ×10,000), (G) Lu low (magnification,
×10,000), (H) Lu medium (magnification, ×10,000), (I) Lu high
(magnification, ×10,000) groups 18 h after preservation. Lu,
luteolin; low, 7.5 µmol/l; medium, 15 µmol/l; high, 30 µmol/l. |
Luteolin protects cardiomyocytes from
apoptosis
As apoptosis of cardiomyocytes is related to
functional damage during long-term storage, inhibiting the
apoptotic process may serve a role in organ transplantation
(23). The effect of luteolin on
apoptosis in cardiomyocytes was assessed. According to the TUNEL
assay, no apoptosis was observed in isolated hearts prior to
preservation (Fig. 6A). The
morphology of rat cardiac muscle was examined in the control, Lu
low, Lu medium and Lu high groups 12 and 18 h after preservation.
In addition, the number of apoptotic cells was examined at 12 and
18 h after preservation. Following long-term storage, apoptotic
cells were sparsely distributed. In cardiomyoctyes at 12 h after
preservation there were a few apoptotic cells distributed below the
epicardium (Fig. 6B). Additionally,
in the presence of luteolin there was a decrease in the number of
apoptotic cell observed at 12 h after preservation (Fig. 6C-E). In cardiomyoctyes at 18 h after
preservation there were a few apoptotic cells distributed near the
endocardium (Fig. 6F). Similarly, in
the presence of luteolin there was a decrease in the number of
apoptotic cell observed at 18 h after preservation (Fig. 6G-I). The results of TUNEL test
demonstrated that luteolin administration significantly decreased
the number of apoptotic cells 12 h (Fig.
6J) and 18 h (Fig. 6K) after
preservation, compared with their respective control groups.
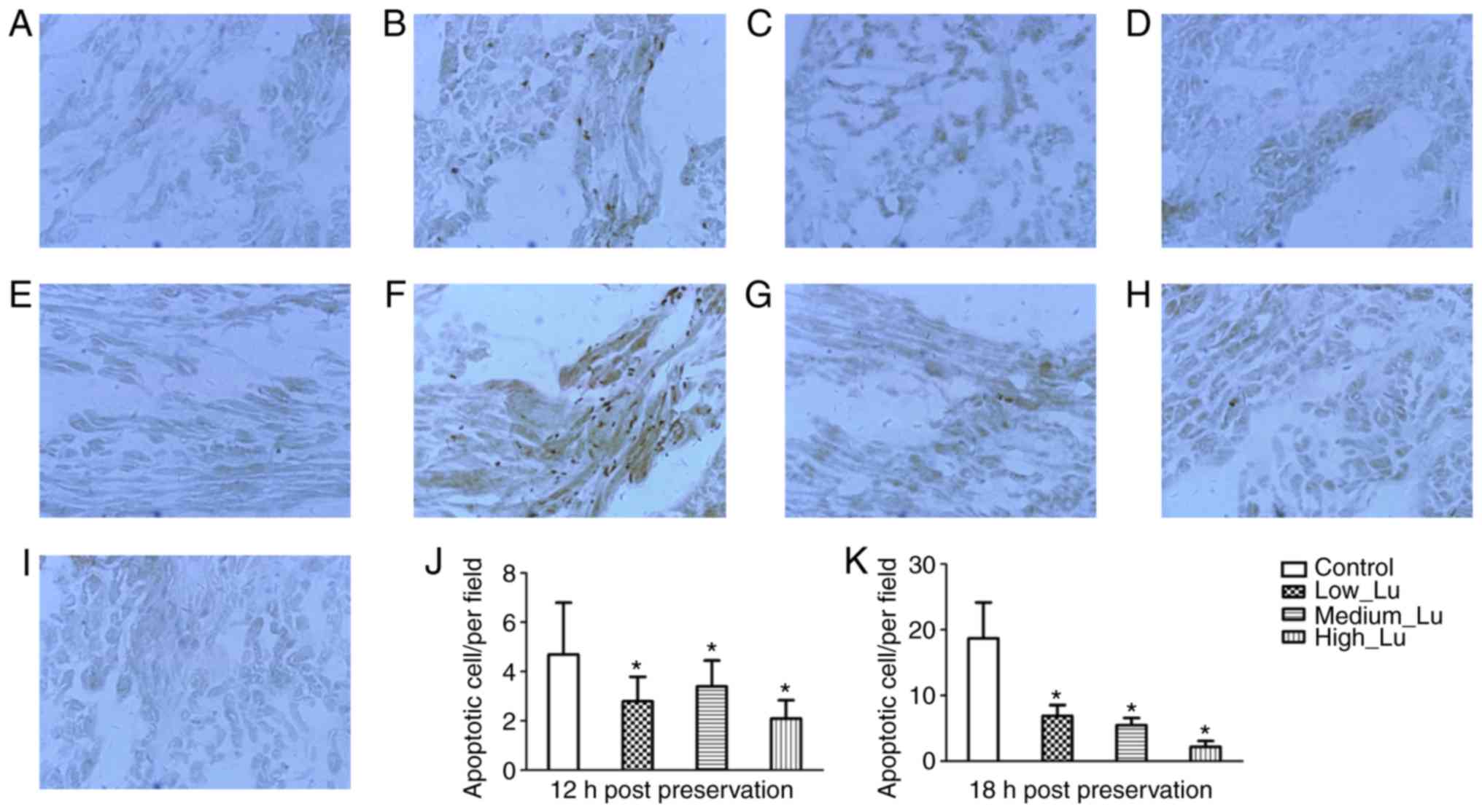 | Figure 6.Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
staining reveals that Lu protects rat cardiac muscle from
apoptosis. Rat cardiac muscle (A) prior to preservation, in the (B)
control, (C) Lu low, (D) Lu medium and (E) Lu high groups 12 h
after preservation, and in the (F) control, (G) Lu low, (H) Lu
medium, (I) Lu high groups 18 h after preservation (magnification,
×400). Quantification of the number of apoptotic cells (J) 12 h and
(K) 18 h after preservation. *P<0.05 vs. control. Lu, luteolin;
low, 7.5 µmol/l; medium, 15 µmol/l; high, 30 µmol/l. |
Key factors involved in anti- and pro-apoptotic
signaling were examined. The endogenous expression of Bcl-2 in
myocardiocytes at 12 h after preservation was very low (Fig. 7A). The expression of Bcl-2 in cardiac
myocardium increased in luteolin groups compared with the control
group at 12 h after preservation (Fig.
7B-D). Similarly, the endogenous expression of Bcl-2 in
myocardiocytes at 18 h after preservation was very low (Fig. 7E). The expression of Bcl-2 in cardiac
myocardium increased in luteolin groups compared with the control
group at 18 h after preservation (Fig.
7F-H).
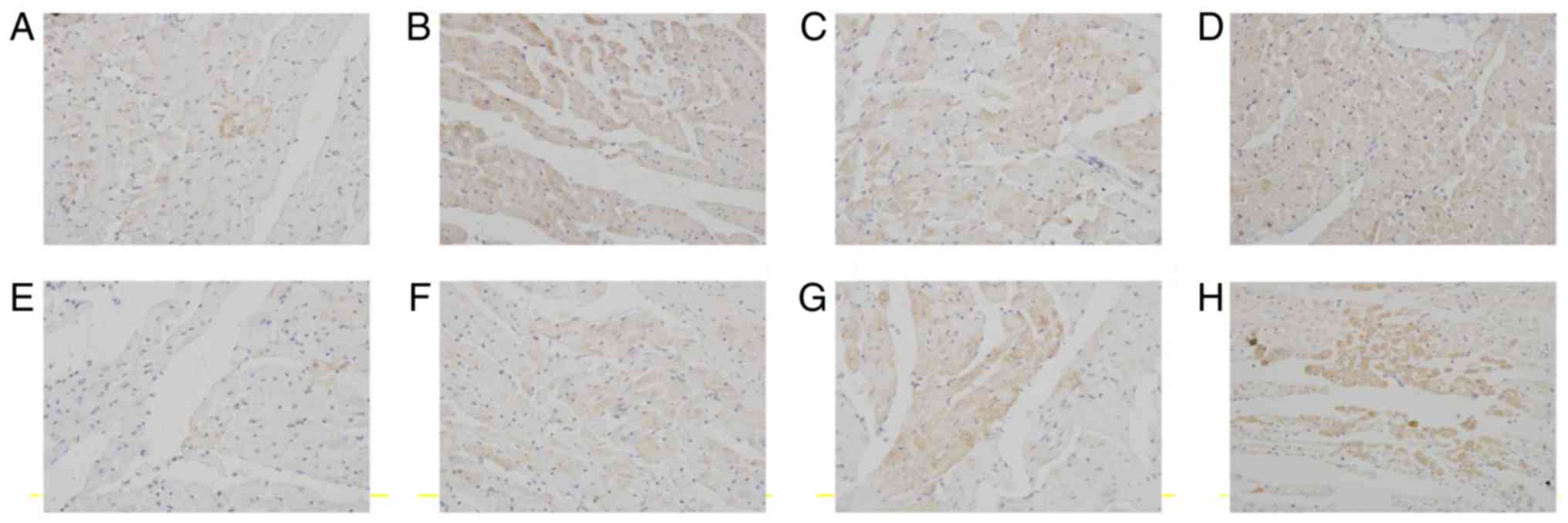 | Figure 7.Lu ameliorates long-term
storage-induced increases in apoptosis regulator Bcl-2 expression.
Rat cardiac muscle in the (A) control, (B) Lu low, (C) Lu medium
and (D) Lu high groups 12 h after preservation, and in the (E)
control, (F) Lu low, (G) Lu medium, (H) Lu high groups 18 h after
preservation. Magnification, ×400. Lu, luteolin; low, 7.5 µmol/l;
medium, 15 µmol/l; high, 30 µmol/l. |
The positive staining of Bax protein was mainly
located in the cytoplasm of myocardiocytes and smooth muscle cells.
The endogenous expression of Bax in myocardiocytes at 12 h after
preservation was very high (Fig.
8A). The expression of Bax in cardiac myocardium decreased in
luteolin groups compared with the control group at 12 h after
preservation (Fig. 8B-D). Similarly,
the endogenous expression of Bax in myocardiocytes at 18 h after
preservation was very high (Fig.
8E). The expression of Bax in cardiac myocardium decreased in
luteolin groups compared with the control group at 18 h after
preservation (Fig. 8F-H). The
immunostaining results suggest that luteolin enhanced the
storage-induced increase of Bcl-2 and decrease of Bax expression in
what appeared to be a dose-dependent manner according to the
semi-quantitative results presented in Tables II and III. The results indicated that luteolin
may protect cardiomyocytes from apoptosis via increasing Bcl-2 and
decreasing Bax.
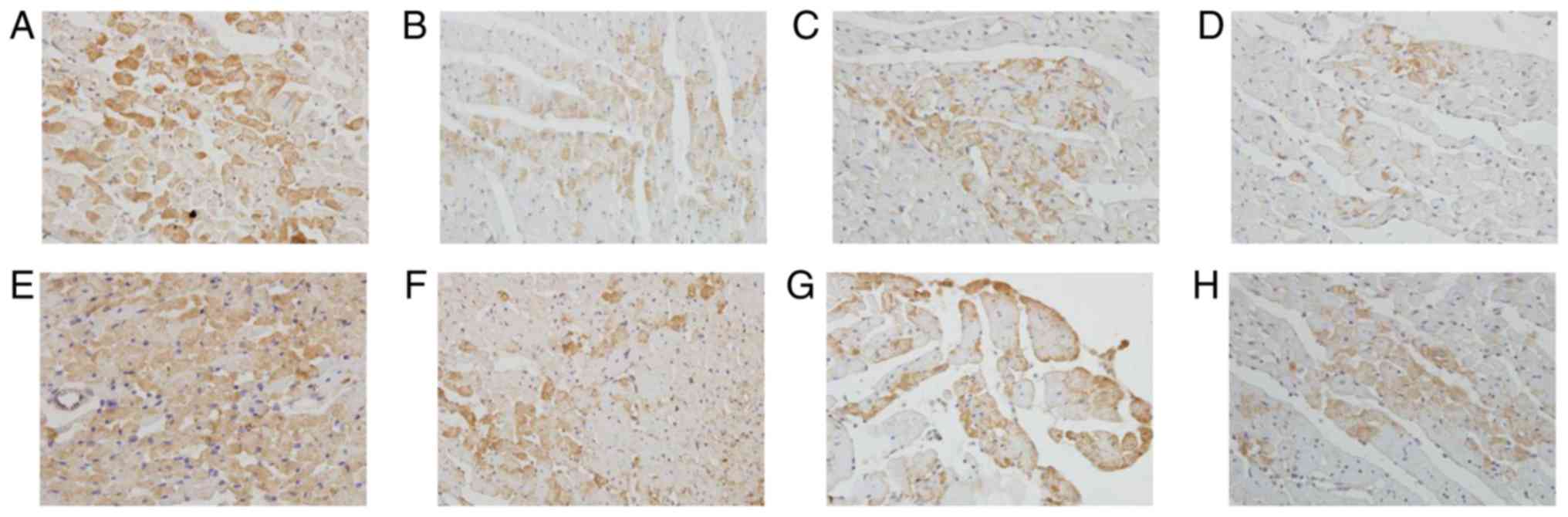 | Figure 8.Lu enhances long-term storage-induced
decreases in apoptosis regulator BAX expression. Rat cardiac muscle
in the (A) control, (B) Lu low, (C) Lu medium (D) Lu high groups 12
h after preservation, and in the (E) control, (F) Lu low, (G) Lu
medium, (H) Lu high groups 18 h after preservation. Magnification,
×400. Lu, luteolin; low, 7.5 µmol/l; medium, 15 µmol/l; high, 30
µmol/l. |
 | Table II.Semi-quantification of apoptosis
regulator Bcl-2 protein expression following long-term storage. |
Table II.
Semi-quantification of apoptosis
regulator Bcl-2 protein expression following long-term storage.
| Group | 12-h
preservation | 18-h
preservation |
|---|
| Control | + | + |
| Lu low (7.5
µmol/l) | ++ | ++ |
| Lu medium (15
µmol/l) | ++ | ++ |
| Lu high (30
µmol/l) | ++ | ++ |
 | Table III.Semi-quantification of apoptosis
regulator Bax protein expression following long-term storage. |
Table III.
Semi-quantification of apoptosis
regulator Bax protein expression following long-term storage.
| Group | 12-h
preservation | 18-h
preservation |
|---|
| Control | +++ | +++ |
| Lu low (7.5
µmol/l) | ++ | ++ |
| Lu medium (15
µmol/l) | ++ | ++ |
| Lu high (30
µmol/l) | + | + |
Luteolin inhibits the activity of LDH,
CK and MDA, and promotes the activity of SOD
The enzymatic activity of LDH, CK, SOD and MDA may
reflect damage in cardiomyocytes (24). The extracellular LDH and CK activity
significantly decreased after 12 h (Fig.
9A and B) and 18 h (Fig. 9C and
D) of storage in all three luteolin groups in what appeared to
be a time-dependent manner. Therefore, heart damage may be
positively associated with storage duration and luteolin may be
involved in reducing myocardial damage during long-term storage. By
contrast, SOD activity, which is negatively associated with cell
damage (25), increased after 12 an
18 h of storage in all three luteolin groups in what appeared to be
a dose-dependent manner (Fig. 9E).
In addition, MDA activity decreased after 12 and 18 h of storage in
all three luteolin groups in what appeared to be a dose-dependent
manner Fig. 9F). Thus, luteolin may
protect hearts from long-term storage-induced damage.
 | Figure 9.Luteolin inhibits the enzymatic
activity of LDH, CK and MDA, and promotes the enzymatic activity of
SOD. The enzymatic activities of (A) LDH and (B) CK were measured
15, 30, 45 and 60 min after a 12-h preservation in a low, medium or
high Lu solution. The enzymatic activities of (C) LDH and (D) CK
were measured 15, 30, 45 and 60 min after an 18-h preservation in a
low, medium or high Lu solution. The enzymatic activities of (E)
SOD and (F) MDA were measured 15, 30, 45 and 60 min after a 12 and
18-h preservation in a low, medium or high Lu solution. *P<0.05
vs. control. Lu, luteolin; low, 7.5 µmol/l; medium, 15 µmol/l;
high, 30 µmol/l; LDH, lactate dehydrogenase; CK, creatine kinase;
MDA, malondialdehyde; SOD, superoxide dismutase. |
Luteolin inhibits L-type calcium
channels during hypoxia
As hypoxia is one of the main causes for heart
damage during long-term storage and L-type calcium channels, which
mediate calcium influx, are involved (26), the effect of luteolin on L-type
calcium channels was assessed in cardiomyocytes experiencing
hypoxia. Calcium currents mediated through L-type calcium channels
exhibited typical voltage-dependency. Original current traces were
elicited by depolarizing voltage to −40, 0, and 50 mV from a
holding potential of −40 mV with a peak potential of 0 mV (Fig. 10A and B). A comparison of the
current densities at 0 mV demonstrated that the percentage of
calcium current significantly decreased in all three luteolin
groups in what appeared to be a dose-dependent manner (Fig. 10C and D). Thes results of this study
demonstrated that luteolin can inhibit L-type calcium channels
during hypoxia.
Discussion
The present study demonstrated that luteolin may
protect hearts from damage induced by long-term storage (12–18 h),
including heart dysfunction, structural damage observed using a
light microscope and TEM, increased apoptosis and disrupted cell
membranes. The amelioration of this damage may result from the
inhibitory effect of luteolin on L-type calcium channels. The
current study has provided experimental evidence demonstrating that
luteolin may be applied to heart preservation solutions,
particularly during long-term storage.
Heart transplantation is the only effective
treatment for patients with end stage cardiopathy (27). However, donor heart preservation has
been a major obstacle in clinical practice (28). Currently, storage duration has been
limited to 6 h (2,29–32),
despite studies attempting to extend storage by supplementing
preservation solutions with additional substances that are able to
block calcium channels (29,32–36),
making it impossible to transport donor hearts over long distances.
Luteolin may be a plausible supplement to improve long-term heart
preservation.
It is well known that ischemia and
reperfusion-induced injuries are the main cause for heart injury;
free radical oxygen species and calcium overload are two widely
recognized factors leading to heart injury (37). Free radical oxygen species can result
in the over-oxidation of membrane lipids and cause heart damage
(38); thus, scavenging free radical
oxygen species and blocking oxidation are effective strategies to
prevent heart injury. Previous studies have revealed that luteolin
is capable of clearing superoxide anion, inhibiting lipid
peroxidation and blocking oxidation of low-density lipoprotein
(39–42), making luteolin a promising candidate
for heart protection. On the other hand, there are a large number
of calcium channels on cardiomyocytes. During excitation under
physiological conditions, calcium influx through L-type calcium
channels is important and responsible for further calcium influx
from internal stores (43–45). However in the presence of ischemia
and hypoxia, calcium influx through L-type calcium channels
increases and leads to further calcium overload from internal
stores during reperfusion, leading to irreversible pathological
changes, including cell necrosis and apoptosis (46,47).
Thus, the inhibition of L-type calcium channels may also contribute
to the protection of the heart. Additionally, luteolin can lead to
vasculature dilation (39), which
may be involved in the inhibition of voltage-gated calcium
channels, ligand-gated calcium channels, internal calcium release
and potassium channel activation; this is consistent with the
results of the current study, which demonstrate an increase in CF
in the presence of luteolin. Luteolin-induced dilation may minimize
endothelial cell injury caused by UW-induced vessel contraction and
improve the penetration of protective components in UW, thereby
improving heart protection (31,48).
Thus, during long term-storage, luteolin may protect against heart
injury through its effect on anti-oxidation, and by preventing
calcium overload and increasing vessel dilation.
In conclusion, the current study demonstrated that
luteolin may improve long-term (≤18 h) heart preservation. However,
whether the stored hearts are suitable enough for transplantation
requires verification. For longer storage, it is hypothesized that
ultra-low or low temperature storage may be better alternative
approaches (29). A recent study
demonstrated that luteolin mitigated the myocardial inflammatory
response induced by a high-carbohydrate/high-fat diet, thus
supporting the conclusion of the current study (49). Although there are several technical
obstacles, such as irreversible injury during freezing,
developments in cellular and molecular techniques may provide
breakthroughs in long-term heart preservation in the near
future.
Acknowledgements
The authors would like to thank Dr Hui Liu from the
Department of Anatomy, Hainan Medical College (Haikou, China) for
his technical assistance and for reading the final manuscript.
Funding
The present study was supported by grants from the
National Natural Science Fund (grant no. 81360029), Natural Science
Foundation of Hainan province (grant no. 809042) and the Research
Project Start Fund of Hainan Tropical Ocean University (grant no.
BHDXB201701).
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
QY and JY prepared the manuscript. JY designed the
study. YLi and YLiu performed the experiments and collected the
data. YZ analyzed the data. SL made substantial contributions to
the study conception.
Ethics approval and consent to
participate
All protocols were approved by the Ethics Committee
of Hainan Medical University and procedures conformed to the
Directive 2010/63/EU of the European Parliament and the Guide for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH publication no. 85-23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Milaniak I, Wilczek-Rużyczka E, Wierzbicki
K, Piatek J, Kapelak B and Przybyłowski P: Relationship between
satisfaction with social support and self-efficacy and the
occurrence of depressive symptoms and stress in heart transplant
recipients. Transplant Proc. 50:2113–2118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minasian SM, Galagudza MM, Dmitriev YV,
Karpov AA and Vlasov TD: Preservation of the donor heart: From
basic science to clinical studies. Interact Cardiovasc Thorac Surg.
20:510–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunt SA: Taking heart-cardiac
transplantation past, present, and future. N Engl J Med.
355:231–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosky LP and Rodman T: Medical aspects of
open-heart surgery. N Engl J Med. 274:833–840. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrera R and Benhabbouche S: Improving
donor heart preservation ex vivo. Bull Acad Natl Med. 195:861–881.
2011.(In French). PubMed/NCBI
|
|
6
|
Belzer FO and Southard JH: Principles of
solid-organ preservation by cold storage. Transplantation.
45:673–676. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jamieson NV, Sundberg R, Lindell S,
Claesson K, Moen J, Vreugdenhil PK, Wight DG, Southard JH and
Belzer FO: Preservation of the canine liver for 24–48 h using
simple cold storage with UW solution. Transplantation. 46:517–522.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeevanandam V, Auteri JS, Marboe CC, Hsu
D, Sanchez JA, Smith CR and Rose EA: Extending the limits of donor
heart preservation: A trial with University of Wisconsin solution.
Transplant Proc. 23:697–698. 1991.PubMed/NCBI
|
|
9
|
Experimental study on long-term
preservation of heart of Danshen Xinma liquid. Henan Yi, Ke Da Xue
and Xue Bao: 31:72–75. 1996.(In Chinese).
|
|
10
|
Xu P: Study on the application of Salvia
miltiorrhiza in heart preservation. Guangdong Yi Xue. 20:247–248.
1999.(In Chinese).
|
|
11
|
Chu LS, Shi XJ and Xi SF: Protective
effect of Astragalus Saponin on heart storage. Chin J Integr Trad
West Med. 19:31999.
|
|
12
|
Chu LS and Shi XJ: Experimental study on
the effect of ligustrazine on improving cardiac preservation.
Zhongyao Xin Yao Yu Lin Chang Yao Li. 2:80–83+125. 2000.(In
Chinese).
|
|
13
|
Ye H, Zhang R and Shen W: Comparison of
the effects of HX-3 solution and UW solution on rat liver. Zhonghua
Qi Guan Yi Zhi Za Zhi. 18:50–53. 1997.(In Chinese).
|
|
14
|
Bosetti C, Bravi F, Talamini R, Parpinel
M, Gnagnarella P, Negri E, Montella M, Lagiou P, Franceschi S and
La Vecchia C: Flavonoids and prostate cancer risk: A study in
Italy. Nutr Cancer. 56:123–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y and Foo LY: Antioxidant activities of
polyphenols from sage (Salvia officinalis). Food Chem. 75:197–202.
2001. View Article : Google Scholar
|
|
16
|
Abu Bakar FI, Abu Bakar MF, Rahmat A,
Abdullah N, Sabran SF and Endrini S: Anti-gout potential of
malaysian medicinal plants. Front Pharmacol. 9:2612018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Odontuya G, Hoult JR and Houghton PJ:
Structure-activity relationship for antiinflammatory effect of
luteolin and its derived glycosides. Phytother Res. 19:782–786.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ooi LS, Wang H, He Z and Ooi VE: Antiviral
activities of purified compounds from Youngia japonica (L.)
DC (Asteraceae, Compositae). J Ethnopharmacol. 106:187–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harris GK, Qian Y, Leonard SS, Sbarra DC
and Shi X: Luteolin and chrysin differentially inhibit
cyclooxygenase-2 expression and scavenge reactive oxygen species
but similarly inhibit prostaglandin-E2 formation in RAW 264.7
cells. J Nutr. 136:1517–1521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong YJ, Choi YJ, Kwon HM, Kang SW, Park
HS, Lee M and Kang YH: Differential inhibition of oxidized
LDL-induced apoptosis in human endothelial cells treated with
different flavonoids. Br J Nutr. 93:581–591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
NIH to adopt new guide January 1 2012.
Physiologist. 55:21–22. 2012.PubMed/NCBI
|
|
22
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou PY, Zhang Z, Guo YL, Xiao ZZ, Zhu P,
Mai MJ and Zheng SY: Protective effect of antiapoptosis potency of
prolonged preservation by desiccation using high-pressure carbon
monoxide on isolated rabbit hearts. Transplant Proc. 47:2746–2751.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mo X, Zhao N, Du X, Bai L and Liu J: The
protective effect of peony extract on acute myocardial infarction
in rats. Phytomedicine. 18:451–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Li Z, Shi C, Li J, Yao M and Chen
X: Trichostatin A attenuates oxidative stress-mediated myocardial
injury through the FoxO3a signaling pathway. Int J Mol Med.
40:999–1008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arslantas A: Development of functional
models for a SOD. Met Based Drugs. 9:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oz MC, Pinsky DJ, Koga S, Liao H, Marboe
CC, Han D, Kline R, Jeevanandam V, Williams M, Morales A, et al:
Novel preservation solution permits 24-hour preservation in rat and
baboon cardiac transplant models. Circulation. 88:II291–II297.
1993.PubMed/NCBI
|
|
28
|
Puehler T, Ensminger S, Schulz U, Fuchs U,
Tigges-Limmer K, Börgermann J, Morshuis M, Hakim K, Oldenburg O,
Niedermeyer J, et al: Heart and combined heart-lung
transplantation. Indications, chances and risks. Herz. 39:66–73.
2014.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guibert EE, Petrenko AY, Balaban CL, Somov
AY, Rodriguez JV and Fuller BJ: Organ preservation: Current
concepts and new strategies for the next decade. Transfus Med
Hemother. 38:125–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozcinar E, Okatan EN, Tuncay E, Eryilmaz S
and Turan B: Improvement of functional recovery of donor heart
following cold static storage with doxycycline cardioplegia.
Cardiovasc Toxicol. 14:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jahania MS, Sanchez JA, Narayan P, Lasley
RD and Mentzer RM Jr: Heart preservation for transplantation:
Principles and strategies. Ann Thorac Surg. 68:1983–1987. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tolba RH, Akbar S, Müller A, Glatzel U and
Minor T: Experimental liver preservation with Celsior: A novel
alternative to University of Wisconsin and
histidine-tryptophan-alpha-ketoglutarate solutions? Eur Surg Res.
32:142–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer JH, Kuhn-Régnier F, Jeschkeit S,
Switkowski R, Bardakcioglu O, Sobottke R and Rainer de Vivie E:
Excellent recovery after prolonged heart storage by preservation
with coronary oxygen persufflation: Orthotopic pig heart
transplantations after 14-hr storage. Transplantation.
66:1450–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wheeler TJ, McCurdy JM, denDekker A and
Chien S: Permeability of fructose-1,6-bisphosphate in liposomes and
cardiac myocytes. Mol Cell Biochem. 259:105–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dzeja PP, Bast P, Ozcan C, Valverde A,
Holmuhamedov EL, Van Wylen DG and Terzic A: Targeting
nucleotide-requiring enzymes: Implications for diazoxide-induced
cardioprotection. Am J Physiol Heart Circ Physiol. 284:H1048–H1056.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kevelaitis E, Oubénaissa A, Mouas C,
Peynet J and Menasché P: Ischemic preconditioning with opening of
mitochondrial adenosine triphosphate-sensitive potassium channels
or Na/H exchange inhibition: Which is the best protective strategy
for heart transplants? J Thorac Cardiovasc Surg. 121:155–162. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Furuichi K, Wada T, Yokoyama H and
Kobayashi KI: Role of cytokines and chemokines in renal
ischemia-reperfusion injury. Drug News Perspect. 15:477–482. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bagchi D, Wetscher GJ, Bagchi M, Hinder
PR, Perdikis G, Stohs SJ, Hinder RA and Das DK: Interrelationship
between cellular calcium homeostasis and free radical generation in
myocardial reperfusion injury. Chem Biol Interact. 104:65–85. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang HD and Ru HL: Study on the
relaxation effect of luteolin on rat aorta and its related
mechanisms. Zhongguo Yao Xue Za Zhi. 40:427–430. 2005.(In
Chinese).
|
|
40
|
Xie P and Zhang MH: Advances in research
on bacteriostatic action of flavonoids. Zhongguo Dong Wu Bao Jian.
12:31–33. 2004.(In Chinese).
|
|
41
|
He LN, Ma QY and Gao YM: Extraction,
purification and study of antibacterial active components from
luteolin in peanut shell. Shipin Ke Xue. 24:84–88. 2003.(In
Chinese).
|
|
42
|
Kimata M, Shichijo M, Miura T, Serizawa I,
Inagaki N and Nagai H: Effects of luteolin, quercetin and baicalein
on immunoglobulin E-mediated mediator release from human cultured
mast cells. Clin Exp Allergy. 30:501–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang S, Binder P, Fang Q, Wang Z, Xiao W,
Liu W and Wang X: Endoplasmic reticulum stress in the heart:
Insights into mechanisms and drug targets. Br J Pharmacol.
175:1293–1304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zalk R and Marks AR: Ca2+
release channels join the ‘Resolution Revolution’. Trends Biochem
Sci. 42:543–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tankeu AT, Ndip Agbor V and Noubiap JJ:
Calcium supplementation and cardiovascular risk: A rising concern.
J Clin Hypertens (Greenwich). 19:640–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prieto-Moure B, Lloris-Carsí JM,
Barrios-Pitarque C, Toledo-Pereyra LH, Lajara-Romance JM,
Berda-Antolí M, Lloris-Cejalvo JM and Cejalvo-Lapeña D:
Pharmacology of ischemia-reperfusion. Translational research
considerations. J Invest Surg. 29:234–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lejay A, Fang F, John R, Van JA, Barr M,
Thaveau F, Chakfe N, Geny B and Scholey JW: Ischemia reperfusion
injury, ischemic conditioning and diabetes mellitus. J Mol Cell
Cardiol. 91:11–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mohara J, Tsutsumi H, Takeyoshi I,
Tokumine M, Aizaki M, Ishikawa S, Matsumoto K and Morishita Y: The
optimal pressure for initial flush with UW solution in heart
procurement. J Heart Lung Transplant. 21:383–390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abu-Elsaad N and El-karef A: The falconoid
luteolin mitigates the myocardial inflammatory response induced by
high-carbohydrate/high-fat diet in wistar rats. Inflammation.
41:221–231. 2018. View Article : Google Scholar : PubMed/NCBI
|