Introduction
Missed abortion (MA), also known as abnormal
intrauterine pregnancy, refers to the embryo or fetus dying in the
uterine cavity but failing to spontaneously excrete in time prior
to 20 weeks of gestation (1). MA is
a specific type of spontaneous abortion (SA). In the process of MA,
with embryonic death, amniotic fluid absorption, embryonic tissue
organization, increasingly tight adhesion between the embryonic
tissue and the uterine wall occurs, causing difficulty in curettage
or induced abortion (2). MA may
result in a coagulation dysfunction leading to disseminated
intravascular coagulation (DIC), which results in severe bleeding
and may threaten the life of affected patients (3). With the accelerated pace of work,
increased social pressure and increased gestational age, the
proportion of patients with MA has significantly increased among
all cases of abortion (4). In the
past six years, the proportion of MA among the total abortions
encountered at Guangzhou Women and Children's Medical Center
(Guangzhou, China) has increased from 24.14 to 30.50% (Fig. 1), and the average increase was 1% per
year, indicating that MA is increasingly becoming a vital factor
impairing family planning. The recent relaxation of the two-child
policy in China has also contributed to this phenomenon. Therefore,
investigating the etiology and pathogenesis of MA has become of
great interest in the field of pre-natal diagnosis in China and
abroad (5,6).
Studies have indicated that the causes of MA are
multiple and complex, including abnormalities in chromosome number
and structure (7,8), immune dysfunction (9,10),
endocrine dysfunction (11),
abnormal intrauterine environment (12), hereditary thrombophilia (13), systemic infectious diseases (14,15) and
environmental factors (3). However,
in ~50% of affected patients, the reason for the occurrence of MA
and the associated pathogenesis remain elusive (16). By analyzing the decidua tissue of 11
SA cases and 9 cases of selective termination of pregnancy by
immunohistochemistry and the terminal deoxynucleotidyl-transferase
mediated dUTP nick end labeling (TUNEL) assay, it was revealed that
the rate of positive staining for M30 in decidual tissue cells from
the SA was 2.5-fold higher than that in the selective termination
group, while the apoptotic index was up to 5-fold higher (17). TUNEL-positive cells were occasionally
observed in chorionic villi of women with a normal
pregnancy-induced abortion but significantly increased in those of
MA patients, which mainly concentrated in the nourishment layer
cells and extravillous trophoblast cells of villous tissue.
However, the factors involved in the apoptosis of trophoblast cells
remain unknown and an association with the occurrence of MA remains
to be determined.
Apoptosis was also detected in placenta tissue
during the early stages of pregnancy (<12 weeks) via the
expression of pro-apoptotic genes (18). Numerous other studies have
demonstrated this phenomenon (17,19,20). The
presence of apoptosis is associated with placental development,
including trophoblast invasion, spiral arterial transformation and
trophoblast cell differentiation, as well as during birth (20). During pregnancy, the degree of
apoptosis of placental tissue gradually increases until delivery
(21). However, an imbalance of the
‘inhibition-induction’ equilibrium leads to a pathological
pregnancy (22). Hence, the present
study hypothesized that apoptosis of villous trophoblast cells may
be associated with the occurrence of MA. Apoptosis-inducing and
-inhibiting factors have a potential regulatory role during
pregnancy.
Galectin-3, a multifunctional protein, belongs to
the family of galectins. Its unique chimeric structure enables it
to interact with a plethora of ligands and modulate diverse
functions, including cell growth, adhesion, migration, invasion,
angiogenesis, immune function, apoptosis and endocytosis, and it
has significance in the process of tumor progression (23). Studies have indicated that galectin-3
is involved in embryo implantation, embryogenesis and placental
formation, and is closely associated with the success and
maintenance of pregnancy (24,25).
Numerous studies suggest that high expression of galectin-3 exerts
inhibitory effects on apoptotic responses of various cell types
(26–29); of note, intracellular galectin-3 has
anti-apoptotic effects, while extracellular galectin-3 may induce
apoptosis (26,29). Galectin-3 has been reported to
inhibit the release of cytochrome C by translocating to the
mitochondrial membrane. It is was reported to inhibit nitric
oxide-induced apoptosis of BT547 human breast cancer cells by
activating caspase-9 and caspase-3 (30). Furthermore, Annexin 7, a
Ca2+-mediated phospholipid binding protein, binds
galectin-3 to inhibit the release of cytochrome C to activate the
caspase cascade, which prevented mitochondrial damage in
cisplatin-treated BT549 cells (29).
By contrast, tumor cells have an important role in immune escape
mechanisms during tumor progression by secreting soluble galectin-3
to promote T lymphocyte apoptosis (31). Extracellular galectin-3 forms
complexes by binding to the polysaccharide CD29/CD7 on the cell
surface, which increases cytochrome C release to activate
intracellular mitochondrial apoptotic signaling pathways (31,32).
Studies have indicated that extracellular galectin-3 secreted by
macrophages participates in the apoptosis of neutrophils, and an
increase in galectin-3 levels enhances macrophage removal of
inflammatory cells (33). Increasing
galectin-3 levels also increases macrophage removal of inflammatory
cells.
A striking similarity is apparent between the
‘pseudo-malignant’ blastocyst trophoblastic cells and malignant
tumor cells in certain biological aspects, including growth and
development, and the abnormal expression of galectin-3 appears to
be involved in the regulation of excessive apoptosis of trophoblast
cells in MA patients (34).
Although the functional studies confirm that
galectin-3 has an important role in the regulation of apoptosis in
endometrial cells (35), the
association between abnormal expression of galectin-3 and excessive
apoptosis of trophoblast cells in MA patients has not been
reported. Thus, in the present study, the apoptosis of placental
cells, the expression of galectin-3 at the mRNA and protein level,
and the levels of macrophages in the blood were assessed in
patients with MA and patients with normal pregnancy induced
abortions (spontaneously induced abortions). The possible
mechanisms of MA were explored from the perspective of placental
apoptosis, and the results may contribute to the clinical treatment
as well as the prevention of MA.
Materials and methods
Patient sample collection
After approval by the Institutional Review Board of
Guangzhou Women and Children's Medical Center (Guangzhou, China),
32 women with MA and 13 women with normal pregnancy induced
abortion (spontaneously induced abortions) were enrolled in the
clinical trial (registry no. 20181022) from January 2010 to
December 2016. Written informed consent was obtained from each
participant. Prior to inclusion in the study, all subjects
underwent a standard diagnostic analysis to rule out any abnormal
causes for disease, which included the following: i) Hepatitis B or
C virus, human immunodeficiency virus, human papilloma virus,
ureaplasma urealyticum, Chlamydia trachomatis and human
cytomegalovirus; ii) medical comorbidities, blood group antibodies,
anti-sperm antibodies, anti-cardiolipin antibodies and
anti-endometrial antibodies; iii) abnormalities in chromosome
number and structure; iv) no unnatural pregnancy, number of
gestational weeks of 12, use of sex hormones in the past 6 months,
history of miscarriage treatment.
Fresh chorionic villous samples were collected.
Briefly, in the lithotomy position a probe was used to detect the
direction and depth of the uterine cavity. A thin plastic tube is
used to slowly enter the cavity and according to the week of
conception and the size of the cavity, a continuous or
discontinuous negative pressure (400–500 mmHg) aspiration system
was used to obtain samples. The aspiration system is filtered and
rinsed with PBS. Each sample was divided into three parts: One part
was immediately stored at −80°C for ELISA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
detection of galectin-3, and the other two parts were fixed in
formalin solution and paraffin-embedded to detect galectin-3 by
immunohistochemistry (IHC) and apoptosis by using the TUNEL assay.
Blood samples were collected from patients in anti-coagulant tubes
to determine the macrophage content.
Patients provided consent for the inclusion of their
data and samples to the ‘Pregnancy tissue sample bank and patient
clinical database for MA patients’ at the Guangzhou Women and
Children Medical Center.
TUNEL assay
Apoptosis of villi was determined using the TUNEL
apoptosis detection kit (cat. no. C1098; Beyotime Institute of
Biotechnology, Haimen, China). Paraffin sections of villous tissue
were de-waxed with xylene, and the rehydrated with graded ethanol
and water. Proteinase K (20 µg/ml; cat. no. A5104530; Sangon
Biotech Co., Ltd., Shanghai, China) without DNase was added
dropwise to the tissue samples followed by incubation at 37°C for
30 min. Subsequently, the samples were incubated in PBS with 3%
hydrogen peroxide for 20 min at room temperature and washed three
times with PBS. The tissue was then covered with 50 µl TUNEL assay
solution and incubated at 37°C for 60 min in the dark. Following
washing with PBS, 0.1 ml labeled reaction stop solution was added
and samples were incubated for 10 min at room temperature.
Streptavidin-horseradish peroxidase (HRP) working solution (50 µl)
was added, followed by incubation for 30 min at room temperature
and washing for three times with PBS. Diaminobenzidine (DAB)
coloring solution (0.2 ml) was added, samples were incubated for 5
min at room temperature and washed 3 times with PBS. Following
staining with hematoxylin for 2 min, slides were washed with pure
water, dried and sealed with neutral resin.
Under a microscope, dark brown-yellow granules
appeared in the cytoplasm of apoptotic cells. For each slice, 3
non-overlapping higher-power fields (magnification, ×250) in the
same position were selected. The number of apoptotic cells per 200
cells was counted in each field of view. The apoptotic rate/index
was the average of the percentage of positive cells, which
represented the degree of apoptosis (apoptotic index=number of
TUNEL-positive cells/total count of nuclei ×100%).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tissue samples (10 mg) frozen in liquid nitrogen
were ground into powder. Total RNA was extracted from powdered
tissue samples using the RNAprep pure Tissue kit (cat. no. DP431;
Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using the FastQuant RT kit (cat. no. KR106; Tiangen Biotech
Co., Ltd.), according to the manufacturer's protocol. qPCR was
performed using 2X Talent qPCR PreMix (cat. no. FP209; Tiangen
Biotech Co., Ltd.). The following primer pairs were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China) and used for qPCR:
Galectin-3 forward, 5-TGCCTTTGCCTGGGGGAGT-3 and reverse,
5-CTGTTGTTCTCATTGAAGCGTGGG-3′; β-actin forward,
5-AGCGAGCATCCCCCAAAGTT-3 and reverse, 5-GGGCACGAAGGCTCATCATT-3′.
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 3 min; 40 cycles of 95°C for 5 sec, 60°C
for 10 sec and 72°C for 15 sec. Galectin-3 mRNA levels were
quantified using the 2−ΔΔCq method and
normalized to the internal reference gene β-actin (36).
ELISA
Galectin-3 protein levels in villous tissue were
detected using a Galectin-3 Human SimpleStep ELISA® Kit
(cat. no. ab188394; Abcam, Cambridge, UK). Following grinding of
100 mg tissue in dry ice, 500 µl pre-cooled 1X Cell Extraction
Buffer PTR containing 100 mM phenylmethane sulfonyl fluoride was
added, followed by mixing and sonication in an ice-water bath. The
sample was incubated on ice for 20 min and then centrifuged at
18,000 × g for 20 min at 4°C. The supernatant was transferred to a
new tube and the tissue protein concentration was detected using
the Pierce BCA Protein Assay Kit (cat. no. 23225; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Determination of galectin-3
protein concentration was then performed according to the
manufacturer's protocol for the Galectin-3 Human Simple Step
ELISA® kit. Each sample was assessed in three
replicates. The protein concentration of galectin-3 in the sample
was determined by measuring the absorbance at a wavelength of 450
nm using a microplate reader (ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA).
Immunohistochemistry
Paraffin-embedded slides were baked at 60°C for 1 h.
Dewaxing and rehydration were performed using xylene, ethanol and
tap water. Following incubation in 3% H2O2 at
room temperature for 10 min, samples were washed three times with
distilled water. The slides were immersed in 0.01 M citrate buffer,
heated to boil in a microwave and then allowed to cool to room
temperature, followed by blocking with 5% bovine serum albumin
(BSA; cat. no. V900933; Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany).
The samples were incubated with the primary antibody
anti-galectin 3 (1:200; cat. no. PB9081; Boster Biotechnology,
Wuhan, China) overnight at 4°C. Following washing with PBS, they
were incubated with goat anti-rabbit immunoglobulin G (H+L)
secondary antibody, biotin conjugate (1:200; cat. no. BA1003;
Boster Biotechnology) at 37°C for 1 h. Subsequent to washing with
PBS, samples were incubated with HRP-streptavidin (1:500) for 30
min at 37°C and washed again with PBS, followed by addition of DAB
color reagent and incubation for 2 h at room temperature. The
slides were counterstained with hematoxylin, rinsed, air-dried and
slides were sealed with neutralresinsize and examined under a
fluorescent microscope (TE2000-E; Nikon Corporation, Tokyo, Japan).
The appearance of brown-yellow granules indicated a positive
result. From each slide, 5 high-power fields (magnification, ×400)
were randomly chosen. The percentage of positive cells was scored
as follows: <10%, 0 points; 10–25%, 1 point; 26–50%, 2 points;
and >50%, 3 points. The staining intensity was scored as
follows: Pale yellow, 1 point; brownish yellow, 3 points; and an
intermediate between the two colours, 2 points. The percentage
score and staining intensity score were multiplied to obtain the
final score: 0, negative (−); 1–3, weakly positive (+); 4–6,
positive (++); and 7–9, strongly positive (+++).
Macrophage assay
A blood analyzer (Sysmex XE-5000; Sysmex Corp.,
Kobe, Japan) was used to detect the macrophage content of blood
samples according to the manufacturer's protocol.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism statistical software (version 6.0; GraphPad Inc., La
Jolla, CA, USA). Data presented as the mean ± standard error of the
mean. Student's t-test was used to analyze differences between two
groups. One-way analysis of variance followed by
Student-Newman-Keuls post-hoc test was used to analyze differences
among multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical information of patients with
MA
Since 2013, the proportion of MA among the total
abortions encountered at Guangzhou Women and Children's Medical
Center (Guangzhou, China) has increased from 24.14 to 30.50%, with
an average 1% per year, indicating that MA is an increasing
concern.
The age, BMI, number of abortions, number of births,
history of miscarriage, history of surgery (gynecological surgery),
number of pregnant menopause weeks and the number of weeks
gestation at time of miscarriage were recorded for all patients
with MA (Table I). Of note, none of
the patients with MA had any history of miscarriage treatment,
premature rupture of membranes, endometriosis, drug allergies,
mental illness, hypertension, thrombosis or trauma during
pregnancy. All conceptions were natural without any assistance of
reproductive technology. As the embryos stopped developing and
remained in the uterus, it was required to perform a negative
pressure aspiration.
 | Table I.Clinical information of patients with
MA. |
Table I.
Clinical information of patients with
MA.
| Age (years) | BMI
(kg/m2) | Number
abortion | Gravidity | History of MA | History of
surgery | Pregnant menopause
week | Gestational
week | Missed week |
|---|
| 39 | 23.7 | 0 | 2 | N | N | 11 | 7 | 4 |
| 31 | 24.2 | 1 | 2 | N | N | 13 | 5 | 8 |
| 31 | 21.9 | 0 | 2 | N | N | 15 | 6 | 9 |
| 27 | 22.5 | 0 | 1 | N | N | 17 | 8 | 9 |
| 31 | 21.2 | 0 | 1 | N | N | 10 | 9 | 1 |
| 21 | 19.8 | 0 | 1 | N | N | 18 | 11 | 7 |
| 28 | 18.8 | 0 | 1 | N | N | 11 | 9 | 2 |
| 26 | 22.2 | 0 | 1 | N | N | 14 | 7 | 7 |
| 20 | 24.1 | 1 | 2 | N | N | 11 | 6 | 5 |
| 28 | 26.5 | 1 | 1 | N | N | 12 | 11 | 1 |
| 40 | 17.7 | 1 | 1 | N | Y | 14 | 7 | 7 |
| 29 | 19.4 | 1 | 1 | N | N | 20 | 9 | 11 |
| 35 | 19.3 | 0 | 2 | N | N | 11 | 11 | 0 |
| 30 | 27.1 | 3 | 4 | Y | N | 8 | 7 | 1 |
| 36 | 23.1 | 1 | 3 | N | N | 18 | 8 | 10 |
| 46 | 26.6 | 1 | 1 | N | N | 10 | 7 | 3 |
| 29 | 22.2 | 0 | 0 | N | N | 12 | 9 | 3 |
| 40 | 20.7 | 0 | 3 | N | N | 11 | 8 | 3 |
| 28 | 25 | 1 | 2 | N | N | 13 | 8 | 5 |
| 26 | 19.8 | 1 | 1 | N | N | 12 | 6 | 6 |
| 28 | 23.3 | 3 | 3 | Y | N | 12 | 7 | 5 |
| 39 | 26.9 | 0 | 2 | N | Y | 14 | 11 | 3 |
| 29 | 22.1 | 2 | 2 | Y | N | 13 | 9 | 4 |
| 34 | 20.5 | 3 | 6 | N | N | 11 | 6 | 5 |
| 42 | 19.9 | 0 | 2 | N | Y | 15 | 7 | 8 |
| 36 | 18.5 | 2 | 2 | Y | N | 13 | 8 | 5 |
| 26 | 18.2 | 1 | 2 | Y | N | 15 | 8 | 7 |
| 46 | 40.9 | 1 | 1 | N | Y | 12 | 6 | 6 |
| 34 | 34.8 | 0 | 1 | N | N | 13 | 8 | 5 |
| 30 | 33.3 | 0 | 1 | N | N | 13 | 7 | 6 |
| 33 | 29.2 | 2 | 4 | N | Y | 12 | 5 | 7 |
| 31 | 33.0 | 1 | 2 | N | N | 17 | 8 | 9 |
Apoptotic index of chorionic
villi
Apoptosis of villi was determined using TUNEL
(Fig. 2A and B). The apoptotic index
in the SA group was 2.93±0.44%, while that in the MA group was
significantly higher at 4.80±0.60% (P<0.05; Fig. 2C).
RT-qPCR detection of galectin-3 mRNA
in villous tissue
The melting curves for galectin-3 and β-actin had a
single peak and the dissolution temperature was ~80°C. The results
of the RT-qPCR analysis indicated that the relative expression of
galectin-3 mRNA in the MA group was significantly higher than that
in the SA group (2−∆∆Cq=1.20±0.02 vs. 1.00±0.05;
P<0.05; Fig. 3A). In terms of
different time-points of MA occurring, the MA patients were divided
into two groups, MA occurring <4 weeks gestation and MA
occurring >4 weeks gestation. Compared with the SA group, the
galectin-3 mRNA levels in the MA at <4 weeks subgroup were
significantly lower (2−∆∆Cq=1.00±0.00 vs. 0.47±0.02;
P<0.05; Fig. 3B). However, in the
MA at >4 weeks subgroup, the galectin-3 mRNA levels were
significantly higher than those in the SA group
(2−∆∆Cq=1.00±0.05; P<0.05; Fig. 3B). Those results suggested that that
the expression level of galectin-3 was significantly increased in
the MA group compared with the SA group. The expression of
galectin-3 in early MA group (<4 weeks) is decreased compared
with the SA group. The expression level of galectin-3 in the MA
group gradually increases with time.
Protein levels of galectin-3 in
chorionic villi detected by ELISA
As presented in Fig.
4A, the mean protein level of galectin-3 detected by ELISA in
the SA group was 19.12±3.43 ng/ml and that in the MA group was
21.6±3.15 ng/ml. The protein level of galectin-3 in the MA group
was slightly higher than that in the SA group and the difference
was statistically significant (P<0.05). Regarding the different
time-points of MA occurring, the protein levels of galectin-3 in
the subgroup with MA at <4 weeks (18.26±3.67 ng/ml) were
slightly lower than those in the SA group (19.3±3.43 ng/ml), and in
the subgroup with MA at >4 weeks, the protein levels of
galectin-3 (22.04±3.68 ng/ml) were higher than those in the SA
group; however, the differences were not statistically significant
(P>0.05; Fig. 4B).
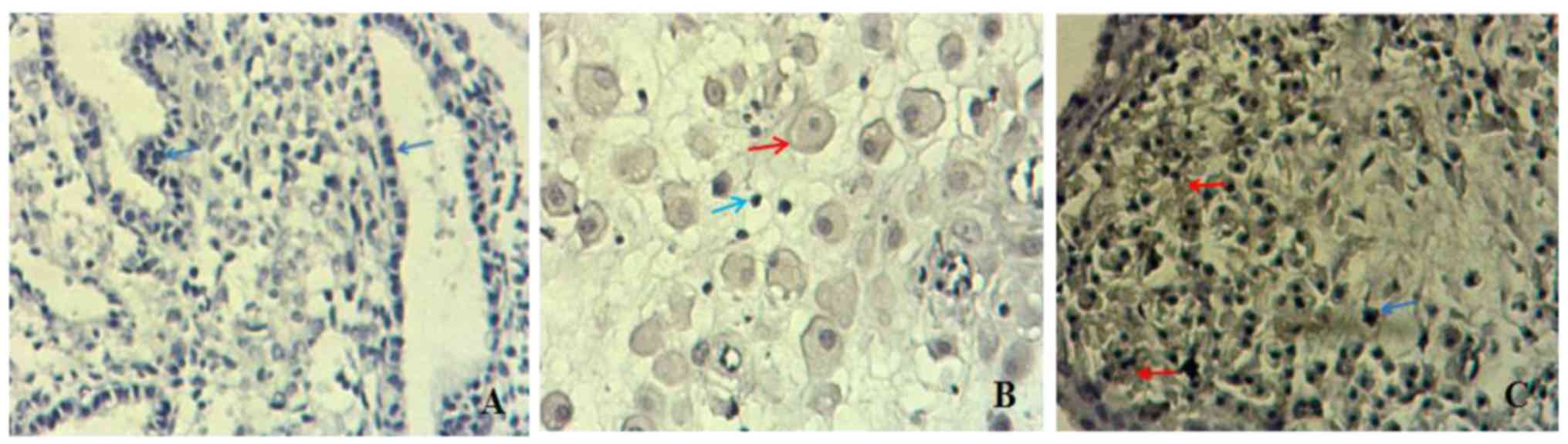
IHC detection of galectin-3 protein
levels in villi
In villous trophoblasts, galectin-3 protein was
mainly expressed in the cell membrane, cytoplasm and nuclear
membrane. Galectin-3 expression was indicated as brown DAB staining
in the cytoplasm (red arrows; Fig.
5A-C). The score of galectin-3 expression in the SA group was
4.84±1.44 and the positive (++) rate was 100%. The score of
galectin-3 expression in the MA group was 5.48±1.40. The rate of
weakly positive (++), positive (++) and strongly positive (+++)
staining was 20, 60 and 20%, respectively. As presented in Fig. 6, the difference between the SA and MA
in the overall galectin-3 staining score was not statistically
significant (P>0.05).
Macrophage levels in the blood
The mean concentration of macrophages detected in in
the MA group (0.45±0.19 109/l) was slightly, but not
significantly higher than that in the SA group (0.40±0.11
109/l; P>0.05; Fig.
7A). Regarding the different time-points of MA, the macrophage
levels in the subgroup with MA at <weeks (0.35±0.12
109/l) were lower than those in the SA group (0.40±0.11
109/l), while those in the subgroup with MA at >4
weeks (0.57±0.19 109/l) were higher than those in the SA
group (P<0.05; Fig. 7B). These
results support the current study hypothesis that galectin-3
accumulation is associated with alternative activation of
macrophages following extensive apoptosis.
Discussion
The present study assessed samples from patients
with unexplained MA and SA. Neither the patients with MA nor SA
included in the present study had any history of pre-mature rupture
of membranes, endometriosis, drug allergies, mental illness,
hypertension, thrombosis or trauma during pregnancy. RT-qPCR, ELISA
and IHC were used to detect the mRNA and protein levels of
galectin-3 in villous tissue, and it was indicated that the protein
expression of galectin-3 in the MA group was higher than that in
the SA group. Furthermore, the apoptotic indices of chorionic villi
in the MA group were higher than those in the SA group. Of note, it
was observed that galectin-3 mRNA and protein expression levels
were decreased in those patients with the time-point of MA of <4
weeks (post-menstruation) compared with those in the SA group.
However, in those patients with MA occurring at >4 weeks, the
expression of galectin-3 was higher than that in the SA group.
Galectin-3 is a β-galactoside binding protein that
has roles in various biological processes, including cell growth,
differentiation, cell adhesion and apoptosis (37). It has been reported that galectin-3
is highly expressed in chorionic villi and decidua during the third
trimester of pregnancy (38).
17β-estradiol (E2), progesterone and human chorionic gonadotropin
(hCG) were indicated to induce the expression and secretion of
galectin-3 in BeWo trophoblast cells and 17β-E2 was also
demonstrated to participate in the differentiation process of
trophoblast cells through regulating galectin-3 (24). Furthermore, hCG was reported to
regulate galectin-3 to prepare for embryo implantation into the
endometrium (25). Previous studies
demonstrated that galectin-3 may have an important role in embryo
implantation through regulation of macrophage levels in the blood,
and this was also observed in the current study. Comparison among
pregnant women with surgical and medical abortion (gestation age
<13 weeks) revealed that galectin-3 expression in the placental
villi was significantly reduced. The present study also indicated
that galectin-3 expression was decreased in the placental villi of
MA patients with a time-point of MA of <4 weeks compared with
those in SA patients with a time-point of MA <4 weeks. A
reduction of galectin-3 affects the normal development of the
embryo, as well as the interaction between the villi and the
endometrium, which impairs the invasive ability of trophoblast
cells (39). Studies have indicated
that overexpression of galectin-3 in tumor cells increases the
degree of malignancy of the tumor, while galectin-3 acts as an
anti-apoptotic factor to regulate apoptotic responses (40,41).
Based on the striking similarity in the growth and developmental
biological processes between the ‘pseudo-malignant’ trophoblastic
cells of blastocyst and malignant tumor cells, it may be speculated
that the low expression of galectin-3 in the villous trophoblast
cells led to cell apoptosis. Early MA was associated with
downregulation of galectin-3 expression compared with the SA group,
which likely contributes to apoptosis. In addition, galectin-3
expression increased with the advancement of the time-point of
MA.
Furthermore, it was reported that BeWo trophoblast
cells secrete galectin-3 to inhibit the proliferation of RL95-2
endometrial cells and to mediate endometrial apoptosis by
activating RL95-2 cells to secrete integrin β1 (34). It has been indicated that during
embryo implantation, endometrial cells undergo proliferation and
apoptosis under the regulation of estrogen, progesterone and hCG
and the action of galectin-3, which is secreted by trophoblast
cells, to facilitate this process. Although galectin-3 secreted by
BeWo cells was observed to induce apoptosis in endometrial cells,
under induction by 17β-E2, RL95-2 cells exhibit and upregulated
galectin-3 expression and reduced apoptosis induced by
staurosporine (34). High expression
and secretion of galectin-3 may selectively activate macrophages,
which have important roles in tissue damage and repair, while it
depends on the degree of damage and the effect time whether their
role is pathogenic or reparative (42). An effective endometrial environment
for embryo implantation is established on the basis of the balance
between extracellular galectin-3 with pro-apoptotic and endometrial
cell galectin-3 with anti-apoptotic effects. In the present study,
it was revealed that galectin-3 was highly expressed in villous
trophoblast tissue of MA patients with a time-point of MA of >4
weeks. It was speculated that due to a compensatory mechanism
during early MA, trophoblast cells secreted excessive galectin-3 to
cause massive apoptosis of endometrial cells. Via this process, the
normal development of the endometrium and villi in early pregnancy
was affected and the interaction between the villi and the
endometrium further promoted MA (34). A limitation of the present study was
that galectin-3 was not detected in extracellular fluid or decidual
tissues. Detection of the intracellular and extracellular levels of
galectin-3 may have had a greater scope of revealing the mechanism
of villous trophoblast cells causing apoptosis of endometrial cells
by excessive secretion of galectin-3. It may also further prove
that extracellular galectin-3 promotes apoptosis, while galectin-3
inside of endometrial cells inhibits apoptosis. An imbalance of
intra- and extracellular galectin-3 may trigger embryonic
developmental abnormalities, which ultimately result in MA.
In summary, the present study suggested that early
MA is associated with apoptosis of placental villi due to
down-regulation of galectin-3. With the advancement of the
time-point of MA, galectin-3 in placental villi was increased and
placental survival may have been extended via compensatory
protective mechanisms. Excessive galectin-3 secreted from
trophoblast cells likely caused excessive apoptosis of endometrial
cells, which affected the normal development of endometrium and
villi in early pregnancy. The interaction between villi and
endometrium further contributed to the eventual occurrence of MA.
As a likely underlying mechanism of MA, it is important to
elucidate the exact association between apoptosis and galectin-3 in
the corresponding cell types, which will be further assessed in
detail in a future study. The present study provided a potential
mechanism of MA from a perspective of apoptosis and also provided
potential therapeutic approaches to prevent MA.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of Guangdong Province (grant no.
2015A030313689).
Availability of data and materials
Not applicable.
Authors' contributions
QX, SQ, QS and HXL designed the experiments. QX,
FLZ, XXZ, XLL and BLP carried out the experiments. QX, FLZ, GYT and
CYL collected and analyzed the data. QX and FLZ prepared the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Guangzhou Women and Children's Medical Center (Guangzhou, China)
and written informed consent was obtained from each participant
(registry no. 20181022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Petersen SG, Perkins AR, Gibbons KS,
Bertolone JI and Mahomed K: The medical management of missed
miscarriage: Outcomes from a prospective, single-centre, Australian
cohort. Med J Aust. 199:341–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Xiao X, Luo F, Shi G, He Y, Yao Y
and Xu L: Acute disseminated intravascular coagulation developed
after dilation and curettage in an adenomyosis patient: A case
report. Blood Coagul Fibrinolysis. 24:771–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Huijun: Epidemiological analysis on
hazards of missed abortion. Zhongguo Bing An. 8:71–72. 2012.(In
Chinese).
|
|
4
|
Zhang X, Li J, Gu Y, Zhao Y, Wang Z and
Jia G: A pilot study on environmental and behavioral factors
related to missed abortion. Environ Health Prev Med. 16:273–278.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Leung PC, Chung TK and Wang CC:
Systematic review of Chinese medicine for miscarriage during early
pregnancy. Evid Based Complement Alternat Med.
2014:7538562014.PubMed/NCBI
|
|
6
|
Kang MH: The research progress and
analysis on pathogenic factor relative to missed abortion. Medical
Recapitulate. 21:3300–3302. 2011.(In Chinese).
|
|
7
|
Chatzimeletiou K, Makrydimas G and
Nicolaides KH: Aneuploidy screening in a coelomic sample from a
missed abortion using sequential fluorescence in situ
hybridization. Fertil Steril. 85:1059.e13–e16. 2006. View Article : Google Scholar
|
|
8
|
Halder A and Fauzdar A: Skewed sex ratio
and low aneuploidy in recurrent early missed abortion. Indian J Med
Res. 124:41–50. 2006.PubMed/NCBI
|
|
9
|
Nagima M and Alija A: Immune status
parameters significance in pregnancy interruption on type of missed
abortion in first trimester. Med Health Sci J. 12:25–30. 2012.
View Article : Google Scholar
|
|
10
|
Cao W, Xu W, Chen T, Wang X, Wang X, Qiu
J, Chen N and Mao Y:
CD4+CD25+FoxP3+ regulatory T cells
and cytokines interact with estradiol in cases of missed abortion.
Exp Ther Med. 7:417–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang XX: Predictive value of
pregnancy-associated plasma protein A and free β-HCG in missed
abortion and ectopic pregnancy. Chinese Rural Health Service
Administration. 9:980–981. 2012.(In Chinese).
|
|
12
|
Aboujaoude R, Alvarez JR, Alvarez M and
Al-Khan A: Management of missed abortion in a patient with
congenital uterine anomalies. Arch Gynecol Obstet. 275:137–139.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosus N, Kosus A, Yildirim M, Duran M and
Turhan NO: Mean platelet volume as a marker of thrombosis in
patients with missed abortion. Acta Haematol. 125:208–209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horn LC, Nenoff P, Ziegert M and Höckel M:
Missed abortion complicated by Candida infection in a woman with
rested IUD. Arch Gynecol Obstet. 264:215–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang JH, Kim JW, Hwang JY, Lee KM, Shim
HM, Bae YK, Paik SS and Park H: Coxsackievirus B infection is
highly related with missed abortion in Korea. Yonsei Med J.
55:1562–1567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerra Shinohara EM, Pereira PM, Kubota
AM, Silva TA, Reis JL, Miyashita GS, D'Almeida V, Allen RH and
Stabler SP: Increased MMA concentration and body mass index are
associated with spontaneous abortion in Brazilian women: A pilot
study. Clin Chim Acta. 411:423–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guenther S, Vrekoussis T, Heublein S,
Bayer B, Anz D, Knabl J, Navrozoglou I, Dian D, Friese K,
Makrigiannakis A and Jeschke U: Decidual macrophages are
significantly increased in spontaneous miscarriages and
over-express FasL: A potential role for macrophages in trophoblast
apoptosis. Int J Mol Sci. 13:9069–9080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neslon DM: Apoptosis changes occur in
syncytiontrophoblast of human placenta villi where fibrin type
fibrinoin is deposite at discontinuities in the villous trophblast.
Placenta. 17:387–391. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Deng X, Yang Y, Shen Y, Chao L,
Wen Y and Sun Y: Expression of GRIM-19 in missed abortion and
possible pathogenesis. Fertil Steril. 103:138–146.e3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayhew TM, Leach L, McGee R, Ismail WW,
Myklebust R and Lammiman MJ: Proliferation, differentiation and
apoptosis in villous trophoblast at 13–41 weeks of gestation
(including observations on annulate lamellae and nuclear pore
complexes). Placenta. 20:407–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith SC, Baker PN and Symonds EM:
Increased placental apoptosis in intrauterine growth restriction.
Am J Obstet Gynecol. 177:1395–1401. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei D, Wu Q and Shi H: Apoptosis and p53
expression in the placental villi of females with unexplained
recurrent spontaneous abortion. Exp Ther Med. 7:191–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Funasaka T, Raz A and Nangia-Makker P:
Galectin-3 in angiogenesis and metastasis. Glycobiology.
24:886–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang H, Taylor HS, Lei C, Cheng C and
Zhang W: Hormonal regulation of galectin 3 in trophoblasts and its
effects on endometrium. Reprod Sci. 18:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang H, Lei CX and Zhang W: Human
chorionic gonadotropin (hCG) regulation of galectin-3 expression in
endometrial epithelial cells and endometrial stromal cells. Acta
Histochem. 115:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang RY, Hsu DK and Liu FT: Expression of
galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad
Sci USA. 93:6737–6742. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akahani S, Nangia-Makker P, Inohara H, Kim
HR and Raz A: Galectin-3: A novel antiapoptotic molecule with a
functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res.
57:5272–5276. 1997.PubMed/NCBI
|
|
28
|
Kim HR, Lin HM, Biliran H and Raz A: Cell
cycle arrest and inhibition of anoikis by galectin-3 in human
breast epithelial cells. Cancer Res. 59:4148–4154. 1999.PubMed/NCBI
|
|
29
|
Yu F, Finley RL Jr, Raz A and Kim HR:
Galectin-3 translocates to the perinuclear membranes and inhibits
cytochrome c release from the mitochondria. A role for synexin in
galectin-3 translocation. J Biol Chem. 277:15819–15827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moon BK, Lee YJ, Battle P, Jessup JM, Raz
A and Kim HR: Galectin-3 protects human breast carcinoma cells
against nitric oxide-induced apoptosis: Implication of galectin-3
function during metastasis. Am J Pathol. 159:1055–1060. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fukumori T, Takenaka Y, Yoshii T, Kim HR,
Hogan V, Inohara H, Kagawa S and Raz A: CD29 and CD7 mediate
galectin-3-induced type II T-cell apoptosis. Cancer Res.
63:8302–8311. 2003.PubMed/NCBI
|
|
32
|
Demetriou M, Granovsky M, Quaggin S and
Dennis JW: Negative regulation of T-cell activation and
autoimmunity by Mgat5 Nglycosylation. Nature. 409:733–739. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karlsson A, Christenson K, Matlak M,
Björstad A, Brown KL, Telemo E, Salomonsson E, Leffler H and Bylund
J: Galectin-3 functions as an opsonin and enhances the macrophage
clearance of apoptotic neutrophils. Glycobiology. 19:16–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sengupta J and Ghosh D: Role of
progesterone on peri-implantation stage endometrium-embryo
interaction in the primate. Steroids. 65:753–762. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang H, Lei C, Cheng C, Feng Y, Zhang W,
Petracco RG and Sak S: The antiapoptotic effect of galectin-3 in
human endometrial cells under the regulation of estrogen and
progesterone. Biol Reprod. 87:392012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao L and Fang AH: Expression and
influence of galectin-3 on missed abortion. J Reprod Contracep.
25:227–234. 2014.
|
|
38
|
Takenaka Y, Fukumori T, Yoshii T, Oka N,
Inohara H, Kim HR, Bresalier RS and Raz A: Nuclear export of
phosphorylated galectin-3 regulates its antiapoptotic activity in
response to chemotherapeutic drugs. Mol Cell Biol. 24:4395–4406.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Califice S, Castronovo V, Bracke M and van
den Brûle F: Dual activities of galectin-3 in human prostate
cancer: Tumor suppression of nuclear galectin-3 vs.tumor promotion
of cytoplasmic galectin-3. Oncogene. 23:7527–7536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Honjo Y, Inohara H, Akahani S, Yoshii T,
Takenaka Y, Yoshida J, Hattori K, Tomiyama Y, Raz A and Kubo T:
Expression of cytoplasmic galectin-3 as a prognostic marker in
tongue carcinoma. Clin Cancer Res. 6:4635–4640. 2000.PubMed/NCBI
|
|
41
|
Sokolov DI, Kolobov AV, Lesnichija MV,
Kostiouchek IN, Stepanova OI, Kvetnoy IM and Selkov SA: Regulatory
mechanisms for apoptosis in placental tissue during normal
pregnancy and gestosis-complicated pregnancy. Bull Exp Biol Med.
148:766–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
MacKinnon AC, Farnworth SL, Hodkinson PS,
Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes
SJ and Sethi T: Regulation of alternative macrophage activation by
galectin-3. J Immunol. 180:2650–2658. 2008. View Article : Google Scholar : PubMed/NCBI
|