Introduction
Osteoporosis termed systemic osteopathy
characterized as bone loss and bone microstructure destruction is
correlated with aging and menopause, which becomes a primary
disturbing issue common in postmenopausal women and the elderly
worldwide (1,2). In spite of several marked advances in
early diagnosis, work-up and therapeutic managements (3,4), there
are still demands for emerging strategies with postmenopausal
osteoporosis (PMOP).
Occurrence and progress of PMOP comprise a
sophisticated biological process involving a series of genomic
changes and diverse molecular pathogenesis. With the development of
various bioinformatics, a wide spectrum of disease prediction and
investigation of molecular mechanism have come to light. Mounting
evidence indicates that several pathways are tightly associated
with PMOP, including the Wnt/β-catenin pathway,
(RANKL)/RANK/osteoprotegerin (OPG) signaling pathway, and NF-κB
signaling pathway (5–7). Previous findings demonstrated that
numerous protein biomarkers with PMOP were selected, for instance,
SOD, A1AT and TRIM63 (8–10). It is well known that genes contain
most of the genetic information that is closely relevant with
phenotype of human beings, old, sick, and dead. Thus, genetic
expression variations in disease development are more likely to
affect a series of biomarker behaviors and signaling transductions.
In addition, a large body of previous studies identified that
multiple genes relative to PMOP were screened out using gene
expression profiling (11,12). Nevertheless, studies on key genes and
crucial pathway in PMOP are limited.
Gibbs sampling is one of the Markov chain Monte
Carlo (MCMC) algorithms, used to construct a random sample with
multivariable probability distribution (13). Notably, on the basis of the
probabilities, it is probable that crucial pathways and key genes
of importance to uncover various pathogenesis of disorder are
identified (14,15). Thus, in our research, Gibbs sampling
was applied to investigate the significance of a pathway gene set
and their functions in postmenopausal osteoporosis.
In the present study, we selected 280 pathways based
on gene intersection greater than 5. The pathways were then
transformed to MC, and Gibbs sampling was performed to gain a new
MC. Moreover, the mean probability of gene expression in each
pathway was calculated using the MCMC algorithm and then
differential pathways were identified on the basis of the
probability of pathway expression more than 0.7. Furthermore, the
emergence times of genes in differential pathway were counted, the
average probability of gene expression in pathway was calculated
and the hub genes were gained by the probability of gene expression
more than 0.7. Our findings are useful in the investigatioin of
potential molecular biomarkers for the diagnosis, therapy and
monitoring progression of PMOP.
Materials and methods
Microarray data capturing and data
preprocessing
The profile E-MEXP-1618 (16) was downloaded from ArrayExpress
(http://www.ebi.ac.uk/arrayexpress/)
serving as a public archive of functional genomics data. In the
gene microarray data of E-MEXP-1618, there were 84 trans-iliacal
bone biopsy samples from postmenopausal female patients (50–85
years), comprising 45 patients with osteoporosis and 39 patients
with no osteoporosis as normal controls. The downloaded microarray
data and probe annotation files were used for further analysis.
Based on the annotation of platform, the probe data were
transformed into the gene symbol level. Gene symbols were obtained
for further analysis.
Pathway enrichment analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) is
one of the most commonly used biological information databases
worldwide, characterized by associating large set of genome
information with higher level functional information of cell,
species and ecosystems (17). In the
present study, gene symbols were enriched to KEGG pathways, from
which we chose pathways with gene interaction in pathways
>5.
Gibbs sampling
According to the enrichment condition of the gene
expression profile in each pathway, we calculated the average gene
expression of each pathway under the first state (normal) and the
second state (osteoporosis) of samples, and regarded this average
gene expression as the pathway expression value. At this point, the
first state was acted as the final state and the second state was
acted as a prior state. After having converted all the pathway
expression values into MCs, their posterior inference was used to
identify probability distributions of pathway expression values for
PMOP. During the pathway system, the initial transition probability
was obtained from expression values of the first state and the
second state, the third state was reckoned from the second
state.
Gibbs sampling is MCMC algorithm which aims to gain
a sequence of samples approximated from a specified
multi-dimensional probability distribution. To perform Gibbs
sampling, the above pathway expression value should be converted
into Markov chains. Firstly, an empty Gibbs sampling set containing
an M-dimensional (M = pathway samples) random vector was defined.
Secondly, N samples Markov chain data set including the initial
value and prior value were put into the empty Gibbs sampling set.
Thirdly, an M-dimensional vector was initialized, M-1 elements of
this vector were fixed, the remaining elements were extracted, like
this cycled M times which amounts to refresh the whole vectors and
generate a new sample. The third state was acquired. Finally,
through n cycles of Gibbs sampling, a Markov chain was
constructed.
Differential pathway analysis
Based on the posterior value of the pathway
generated by the Markov chain and using the probability calculation
formula alfa.pi, the probability of each pathway was obtained.
alfa.pi=∑i=200010000Pi10000-2000+1
where ‘alfa.pi’ is ‘posterior value of a
pathway’.
Thereinto, Pi represented the posterior value
of the pathway in subsample i. According to pathway expression
values in different states, P-values of pathways were calculated
using t-test. Subsequently, P-values were ranked, combining
P-values with probability alfa.pi, the correction coefficients
(Rvalue) were calculated and then the adjusted probability of the
pathway (alfa-adj) was obtained. Pathways of which alfa-adj was
>0.7 were regarded as differential pathway. The formula used
was:
Rvalue=1-rankin
where n stands for the number of pathway, and
ranki stands for i ranking.
Hub gene screening
Following the analysis of genes in different
pathways, a pathway gene set were identified, and the frequencies
of appearance of pathway genes were counted. Pathway genes were
transformed into MC and Gibbs sampling was executed based on the
above theory. Finally, differential pathway genes with alfa-adj
>0.7 were regarded as hub genes.
Results
Identification of differential
pathways
There were a total of 20,544 genes that were
determined after preprocessing. Then, using KEGG enrichment
analysis containing 287 pathways and 6,894 genes, 20,544 genes were
enriched to KEGG pathway, and 280 pathways >5 were identified.
Furthermore, by means of Gibbs sampling, the probabilities
distribution of all the pathways were gained via utilizing the
alfa.pi formula, as presented in Fig.
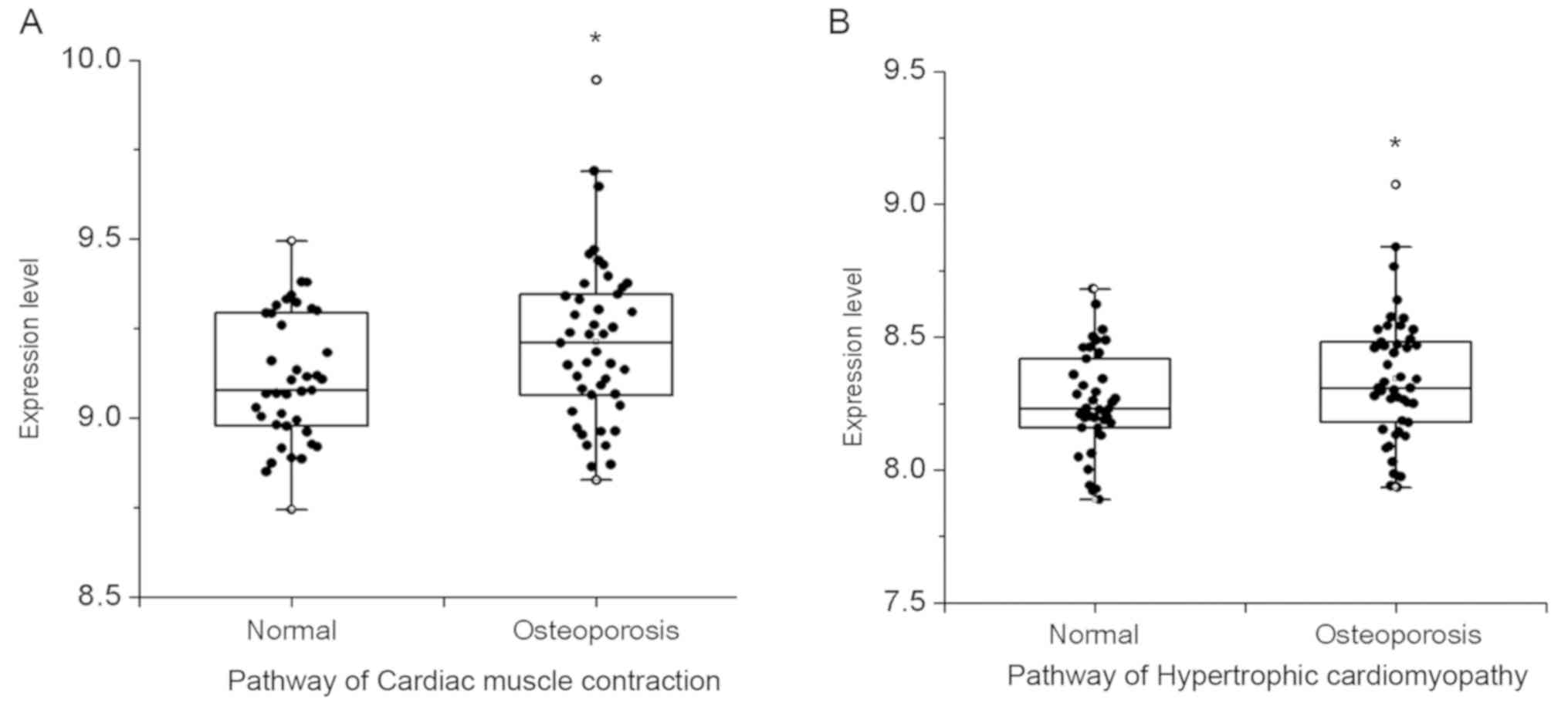
1. As shown, there were 2 differential pathways that were
obtained based on the alfa-adj >0.7, comprising cardiac muscle
contraction and hypertrophic cardiomyopathy. As presented in the
box scatter diagram, the results of these differential pathways
expressed in normal and PMOP state showed that expression levels in
pathways of cardiac muscle contraction and hypertrophic
cardiomyopathy in PMOP patients were higher than that in the normal
population (Fig. 2).
Screening out pathway gene set
To determine the hub genes, the sequence of pathway
gene sets in the differential pathway was analysed. Pathway gene
set of 2 differential pathways included 74 genes in cardiac muscle
contraction and 83 genes in hypertrophic cardiomyopathy, in which
122 genes were expressed, respectively, in each differential
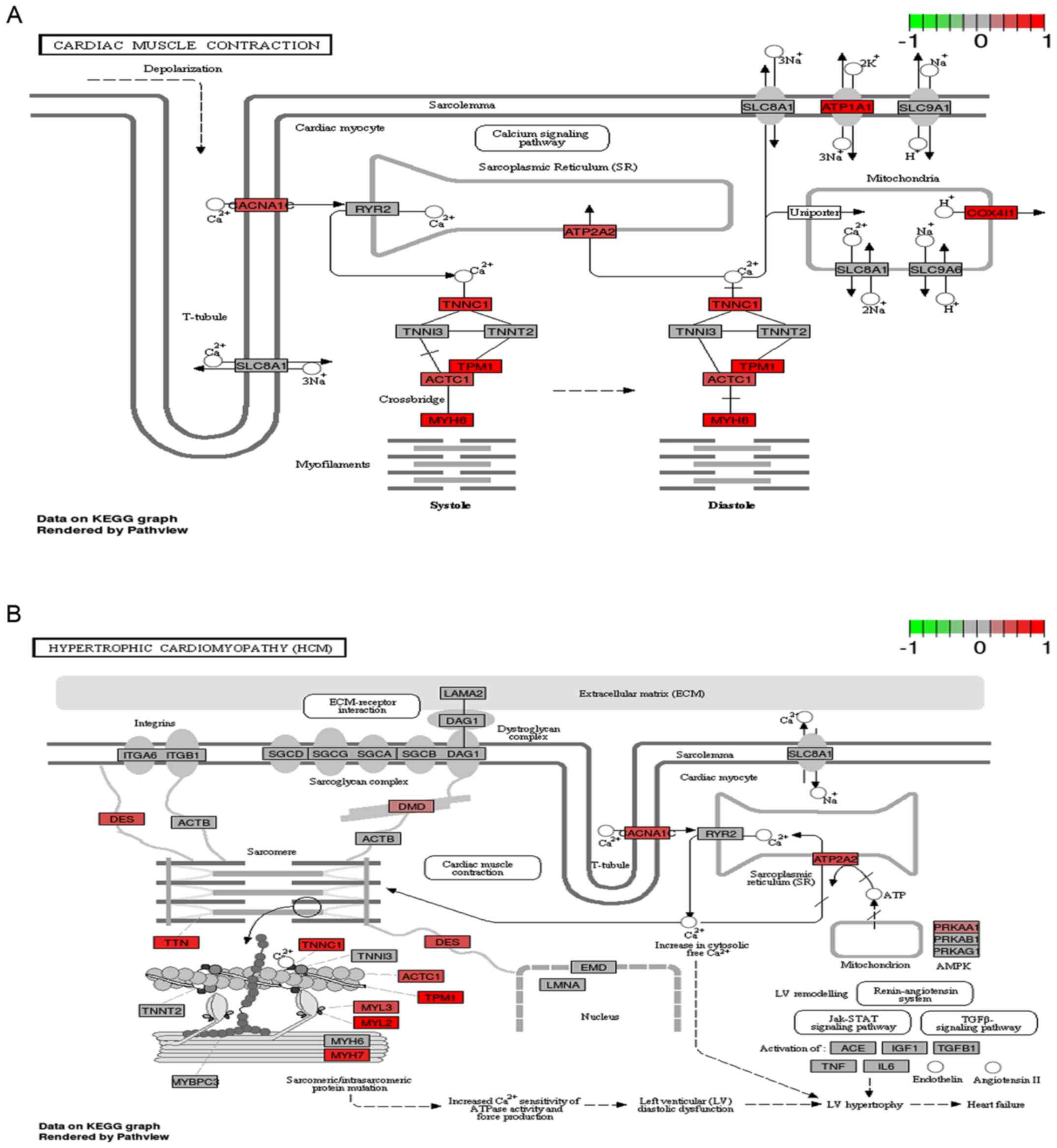
pathway and 35 were co-expressed genes. As shown in Fig. 3, the regulatory relationship of the
pathway gene participating in the differential pathway demonstrated
that 12 pathway genes and 13 pathway genes were upregulated,
respectively, in pathways of cardiac muscle contraction and
hypertrophic cardiomyopathy.
Identification of hub genes
In order to explore the potential key genes relative
to PMOP, hub genes in the differential pathway were selected via
Gibbs sampling. Based on the alfa-adj >0.7, three hub genes
including TNNC1, MYL2, and TTN were selected, as shown in
Fig. 4. Moreover, their expression
status in the normal and PMOP condition were indicated in a box
scatter diagram (Fig. 5).
Discussion
PMOP, defined as a systemic skeletal disorder, is a
silent disease without recognition in patients until fractures
emerge. Concomitantly, novel diagnostics and therapeutics of PMOP
are under investigation. Currently, the diagnostic potential of
crucial pathways or key genes for disorders has been studied and
several pathways and genes were identified as underlying biomarkers
(18,19). In this study, we presented PMOP
correlatively pivotal pathways and key genes, which may be applied
to diagnose and monitor PMOP. Gibbs sampling analysis indicated
that 2 differential pathways including cardiac muscle contraction
and hypertrophic cardiomyopathy were selected. Significantly, three
hub genes associated with PMOP were identified containing TNNC1,
MYL2, and TTN, which could be good candidates for
biomarkers in the diagnosis of PMOP in future clinical
applications.
Previous findings showed that cardiovascular disease
is a main reason of death among postmenopausal women (20,21).
Many treatments administered are in the form of drug therapy,
including, raloxifene (22),
drospirenone/17β-estradiol (23).
However, few studies focus on the examination and treatment of PMOP
associated with cardiac disease or prevention of cardiac disorder
in postmenopausal women while there are rare investigations on the
underlying molecular mechanisms involved. Concomitantly, research
has shown that low bone mineral density is associated with
increased cardiovascular mortality (24) and emerging findings have demonstrated
that vascular calcification and bone mineralization share various
anatomical and pathophysiological common properties (25). In the current study, by using Gibbs
sampling combined with KEGG pathway analysis to investigate gene
expression profile of PMOP, we favorably selected crucial pathways
including cardiac muscle contraction and hypertrophic
cardiomyopathy. The above facts indicated that cardiac muscle
dysfunctions are likely to be associated with the occurrence of
PMOP.
Furthermore, we identified key genes TNNC1,
MYL2, and TTN by Gibbs sampling analysis of differential
pathways. TNNC1, encoded Cardiac troponin C (cTnC), was
reported as involved in modulating cardiomyopathy (26). Troponin is composed of three
subunits, the troponin C, troponin I and troponin T. Previous
research demonstrated that elevated serum cardiac troponin I was
associated with hip fracture in older patients including
postmenopausal women (27). Another
emerging report studied that highly sensitive cardiac troponin T
relates to mortality in perimenopausal women (28). It is common knowledge that
MYL2 is also known as MLC-2 and encoded myosin light
chain-2. It is reported that myosin II is functionally important in
bone resorption, besides, myosin activity favors the
osteoclast-differentiated activity of bone resorption (29). Recent findings have shown that a
decreased expression of Runx2 is accompanied by a lower expression
of myosin in ovariectomized rats, simultaneously, have revealed
that myosin-dependent nuclear-cytoplasmic shuttling of Runx2 is
likely to be crucial for supplying interconnection between myosin
and signal transduction cascades, accordingly it commands
transcription of downstream factors in osteoblastic cells (30). Titin, encoded by TTN, is a
large sarcomere protein, and an elastic protein. Eccentric
exercise, rehabilitation and athletic training, is commonly
prescribed for treatment of various types of conditions such as
sarcopenia, osteoporosis, and tendinosis, which is mediated by
titin-actin and titin-myosin interactions through the study of
sports experts (31). In light of
the evidence, it is speculated that TNNC1, MYL2, TTN as hub
genes are good for predicting and diagnosis of PMOP. In spite of
this, there are limitations of this study. This work is only a
slight improvement of probing early-discriminating clues during
disease progression as well as this algorithm is anticipated to
meliorate or combine with more complicated predicted measures, thus
enhancing efficiency and accuracy for predicting disorders with
might be the main ones. The effectiveness of the identified
differential pathway and hub genes still need further support from
animal experiments or clinical investigations. In this work, we
presented a bioinformatics analysis of genetic chip databases based
on Gibbs sampling to identify biomarkers including crucial pathways
and key genes, which had been shown to be effective by real
datasets.
Collectively, the findings of the present study
demonstrated that the pathways of cardiac muscle contraction and
hypertrophic cardiomyopathy as well as hub genes TNNC1,
MYL2, and TTN may exert significant effects in the
development of PMOP, which may provide a non-invasive methodology
for the prediction, diagnosis, and even personalized treatment of
clinical osteoporosis, furthermore it may be helpful for
comprehending molecular pathogenesis of PMOP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL conceived the study and drafted the manuscript.
YW, YMJ and HZ acquired the data. YPZ, SA and YL analyzed the data
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pavel OR, Popescu M, Novac L, Mogoantă L,
Pavel LP, Vicaş RM and Trăistaru MR: Postmenopausal osteoporosis -
clinical, biological and histopathological aspects. Rom J Morphol
Embryol. 57:121–130. 2016.PubMed/NCBI
|
|
2
|
Bijelic R, Milicevic S and Balaban J: Risk
factors for osteoporosis in postmenopausal women. Medical archives
Med Arch. 71:25–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bandeira L and Bilezikian JP: Novel
therapies for postmenopausal osteoporosis. Endocrinol Metab Clin
North Am. 46:207–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Q, Tang T, Zhang P, Liu C, Pu Y, Zhang
Y, Song H, Wang Y, Song Y, Su M, et al: Correlation of IL-31 gene
polymorphisms with susceptibility and clinical recurrence of
bladder cancer. Fam Cancer. Nov 8–2017.(Epub ahead of print). doi:
10.1007/s10689-017-0060-4. PubMed/NCBI
|
|
5
|
Fan H, Ji F, Lin Y, Zhang M, Qin W, Zhou Q
and Wu Q: Electroacupuncture stimulation at CV4 prevents
ovariectomy-induced osteoporosis in rats via Wnt-β-catenin
signaling. Mol Med Rep. 13:2485–2491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolski H, Drews K, Bogacz A, Kamiński A,
Barlik M, Bartkowiak-Wieczorek J, Klejewski A, Ożarowski M,
Majchrzycki M and Seremak-Mrozikiewicz A: The RANKL/RANK/OPG signal
trail: Significance of genetic polymorphisms in the etiology of
postmenopausal osteoporosis. Ginekol Pol. 87:347–352. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taguchi Y, Jin G and Inoue JI: NF-κB
signaling in osteoclastogenesis. In: Protein Modifications in
Pathogenic Dysregulation of Signaling. Springer Japan.
2015.https://doi.org/10.1007/978-4-431-55561-2_13.
|
|
8
|
Lim J and Hwang S: Identification of
osteoporosis-associated protein biomarkers from ovariectomized rat
urine. Curr Proteomics. 14:130–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Liu C and Wang H: Screening for
specific biomarkers in the serum of postmenopausal osteoporosis
patients using proteomic fingerprint techniques. Biomed Rep.
1:129–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Wang Y, Yang N, Wu S, Lv Y and Xu
L: In silico analysis of the molecular mechanism of
postmenopausal osteoporosis. Mol Med Rep. 12:6584–6590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma M, Luo S, Zhou W, Lu L, Cai J, Yuan F
and Yin F: Bioinformatics analysis of gene expression profiles in B
cells of postmenopausal osteoporosis patients. Taiwan J Obstet
Gynecol. 56:165–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma M, Chen X, Lu L, Yuan F, Zeng W, Luo S,
Yin F and Cai J: Identification of crucial genes related to
postmenopausal osteoporosis using gene expression profiling. Aging
Clin Exp Res. 28:1067–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gelfand AE: Gibbs sampling. J Am Stat
Assoc. 95:1300–1304. 2000. View Article : Google Scholar
|
|
14
|
Asyali MH, Colak D, Demirkaya O and Inan
MS: Gene expression profile classification: A review. Curr
Bioinform. 1:55–73. 2006. View Article : Google Scholar
|
|
15
|
Chen P, Guo LH, Guo YK, Qu ZJ, Gao Y and
Qiu H: Identification of disturbed pathways in heart failure based
on Gibbs sampling and pathway enrichment analysis. Genet Mol Res.
15:gmr79562016.
|
|
16
|
Reppe S, Refvem H, Gautvik VT, Olstad OK,
Høvring PI, Reinholt FP, Holden M, Frigessi A, Jemtland R and
Gautvik KM: Eight genes are highly associated with BMD variation in
postmenopausal Caucasian women. Bone. 46:604–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogata H, Goto S, Sato K, Fujibuchi W, Bono
H and Kanehisa M: KEGG: Kyoto encyclopedia of genes and genomes.
Nucleic Acids Res. 27:29–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helbig KJ and Beard MR: The interferon
signaling pathway genes as biomarkers of hepatitis C virus disease
progression and response to treatment. Biomarkers Med. 6:141–150.
2012. View
Article : Google Scholar
|
|
19
|
Zhou J, Hang D, Jiang Y, Chen J, Han J,
Zhou W, Jin G, Ma H and Dai J: Evaluation of genetic variants in
autophagy pathway genes as prognostic biomarkers for breast cancer.
Gene. 627:549–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tankó LB, Christiansen C, Cox DA, Geiger
MJ, McNabb MA and Cummings SR: Relationship between osteoporosis
and cardiovascular disease in postmenopausal women. J Bone Miner
Res. 20:1912–1920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bagger YZ, Rasmussen HB, Alexandersen P,
Werge T, Christiansen C and Tankó LB; PERF study group, : Links
between cardiovascular disease and osteoporosis in postmenopausal
women: Serum lipids or atherosclerosis per se? Osteoporos Int.
18:505–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liew R, Stagg MA, MacLeod KT and Collins
P: Raloxifene acutely suppresses ventricular myocyte contractility
through inhibition of the L-type calcium current. Br J Pharmacol.
142:89–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosano GM, Vitale C, Marazzi G and
Volterrani M: Menopause and cardiovascular disease: The evidence.
Climacteric. 10 (Suppl 1):19–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim YH, Shin J, Lee JU, Lim HK, Hong S,
Kim MK, Choi BY and Kim YM: Bone mineral density is an independent
determinant of left ventricular mass index in the general female
population. Korean Circ J. 40:573–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sprini D, Rini GB, Di Stefano L,
Cianferotti L and Napoli N: Correlation between osteoporosis and
cardiovascular disease. Clin Cases Miner Bone Metab. 11:117–119.
2014.PubMed/NCBI
|
|
26
|
Pinto JR, Parvatiyar MS, Jones MA, Liang
J, Ackerman MJ and Potter JD: A functional and structural study of
troponin C mutations related to hypertrophic cardiomyopathy. J Biol
Chem. 284:19090–19100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher AA, Southcott EN, Goh SL,
Srikusalanukul W, Hickman PE, Davis MW, Potter JM, Budge MM and
Smith PN: Elevated serum cardiac troponin I in older patients with
hip fracture: Incidence and prognostic significance. Arch Orthop
Trauma Surg. 128:1073–1079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cramer GE, Brouwer MA, Vader HL, de Boer
MJ, Pop GA, Pop VJ and Verheugt FW: Highly sensitive cardiac
troponin T and long-term mortality in a population of
community-derived perimenopausal women: Nested case-control study.
Heart. 99:528–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato M and Grasser W: Myosin II antibodies
inhibit the resorption activity of isolated rat osteoclasts. Cell
Motil Cytoskeleton. 17:250–263. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schreckenberg R, Wenzel S, da Costa Rebelo
RM, Röthig A, Meyer R and Schlüter K-D: Cell-specific effects of
nitric oxide deficiency on parathyroid hormone-related peptide
(PTHrP) responsiveness and PTH1 receptor expression in
cardiovascular cells. Endocrinology. 150:3735–3741. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hessel AL, Lindstedt SL and Nishikawa KC:
Physiological mechanisms of eccentric contraction and its
applications: A role for the giant titin protein. Front Physiol.
8:702017. View Article : Google Scholar : PubMed/NCBI
|