Introduction
Acute pancreatitis (AP) is a common emergency. AP
patients suffer from inflammatory responses such as bleeding, edema
and necrosis caused by pancreatic tissue digestion due to
abnormally activated pancreatic trypsin resulting from various
factors such as drinking, overeating, hyperlipidemia and
cholelithiasis (1,2). The clinical manifestations of AP
patients are acute and persistent abdominal pains, accompanied with
clinical symptoms including nausea, vomiting, fever and stop of
flatus and defecation in most patients (3). Severe acute pancreatitis (SAP) is a
severe systemic inflammatory response (SIR) disorder initiated by
pancreatic autodigestion. Inflammatory cells are activated and
release massive cytokines, and the resulting cytokine-level chain
reaction is an important reason for the aggravation of SAP
(4). These inflammatory mediators
cause systemic inflammatory response syndrome (SIRS) that is a
major cause of complications and deaths (5). Based on the notions of SIRS and
multiple organ dysfunction syndrome (MODS) raised in the 1990s,
people now have understood the relationship between SIRS and MODS.
In other words, SIRS is the foundation, and MODS is the most
serious consequence during the development of SIRS and also the
most common cause of death in severe pancreatitis (6). Blocking the development of SIRS into
MODS can significantly reduce the mortality rate (7). Therefore, hemofiltration (HF) begins to
be considered as a method to remove inflammatory mediators as much
as possible in clinical practice (8). HF technique is able to evidently
improve the roles of monocytes in SAP patients, rebuild the
internal stability of the body's immune system, and non-selectively
eliminate a variety of pro-inflammatory factors at the same time
(9). The technique may be able to
control SIRS, thus maintaining hemodynamic stability and preventing
MODS.
Continuous veno-venous hemofiltration (CVVH) in the
treatment of early SAP mainly targets pro-inflammatory cytokines
causing SIR, which is conducive to downregulating SIR and restoring
the balance between pro-inflammatory response and anti-inflammatory
response (10). Some studies have
shown that application of short-term HF in early SAP is proved to
be effective in blocking SIR, reducing pancreatic necrosis and
protecting organ function, and have put forward at the same time
that for fulminant acute pancreatitis (FAP), a good measure further
improving the prognosis is to perform continuous HF after emergency
operative drainage (11,12). Through the mechanisms of convection
and adsorption, HF can not only directly remove amylase and urea
nitrogen in the blood, but also selectively eliminate molecules
with a diameter less than filter pore size in the plasma using a
certain pore size filter with features of large filter area and
high permeability. Previous sepsis studies have also confirmed that
HF can filter out cytokines to effectively reduce the level of
cytokines in the plasma, thus preventing the pancreatic
autodigestion caused by the over-activation of amylase, which plays
a role in the protection of pancreatic cells, solves the excessive
inflammatory reaction due to pro-inflammatory cytokines in early
SAP, and is capable of preventing the progression of the disease
(13–15). In addition, continuous blood
purification technique can significantly improve the roles of
mononuclear cells in patients and rebuild the homeostasis of the
body's immune system.
Patients and methods
A total of 60 patients with SAP treated in
Affiliated Dongtai Hospital of Nantong University (Dongtai, China)
from October 2015 to October 2017 were collected, including 32
patients treated with CVVH and routine internal medicine therapy
(CVVH group), and 28 patients treated with routine internal
medicine therapy (control group). This study was approved by the
Ethics Committee of Affiliated Dongtai Hospital of Nantong
University. Signed informed consents were obtained from the
participants before the study. The general clinical data of
patients are shown in Table I.
Diagnostic criteria for AP: patients met any two of the following
criteria: i) symptoms such as acute and sudden onset of upper
abdominal pain, which was persistent, severe, and often accompanied
with back radiating pain, were observed, ii) serum amylase value
was >3× upper limit of normal, and/or serum lipase activity was
>3× upper limit of normal, and iii) imaging manifestations of AP
such as pancreatic inflammation, pancreatic necrosis and
extra-pancreatic complications were observed on computed tomography
(CT) and/or magnetic resonance imaging (MRI). Diagnostic criteria
for SAP: patients met the following three conditions at the same
time: i) the symptoms, signs, and blood and imaging findings met
diagnostic criteria for AP, ii) persistent organ failure [single
organ (respiratory organ, circulatory organ and kidney) failure or
MODS] lasted for >48 h, and iii) improved Marshall score
(16) was ≥2 points.
 | Table I.General data of patients with severe
acute pancreatitis (mean ± standard deviation). |
Table I.
General data of patients with severe
acute pancreatitis (mean ± standard deviation).
| Groups | HEMO group
(n=32) | Control group
(n=28) | P-value |
|---|
| Male | 20 | 18 | 0.8861 |
| Female | 12 | 10 |
|
| Age (years) | 54.32±10.65 | 58.94±9.02 | 0.0772 |
| Onset time (h) | 21.03±6.36 | 24.01±8.20 | 0.1189 |
| Fever | 30 | 27 | 0.6348 |
| Nausea | 28 | 27 | 0.2119 |
| Emesis | 26 | 26 | 0.1870 |
| Abdominal pain | 29 | 27 | 0.3686 |
| Abdominal
distension | 29 | 27 | 0.3686 |
| Jaundice | 10 | 8 | 0.8213 |
Inclusion criteria were: i) patients meeting
diagnostic criteria for SAP, ii) patients with time from onset to
admission <72 h, iii) patients aged over 18 years and below 70
years, with no sex limitation, and iv) patients suffer from the
disease for the first time and never received treatment for AP.
Exclusion criteria were: i) patients died within 24
h after admission, ii) SAP patients with surgical indications such
as pancreatic pseudocysts, iii) SAP patients after surgery, iv)
patients with malignant tumor or complicated with severe
circulatory, respiratory and renal function diseases, v) patients
with continuous renal replacement contraindications, vi) patients
with mental illness or poor compliance, and vii) patients with
other acute abdominal diseases such as acute cholecystitis, acute
appendicitis and acute gastrointestinal perforation as well as
diseases including chronic pancreatitis and mild acute pancreatitis
(AP).
Methods
Routine internal medicine therapy
Vital signs of patients were closely monitored, and
patients were forbidden from drinking and eating and inhaled oxygen
(oxygen saturation was >95%). Gastrointestinal decompression was
performed according to the condition of patients. In the early
stage, controlled liquid resuscitation was adopted, omeprazole
sodium for injection was intravenously injected to protect gastric
mucosa and indirectly inhibit pancreatic enzyme secretion. Patients
received parenteral nutrition firstly, and then enteral nutrition
after 48–72 h by placing a nasointestinal tube, and the dose of
enteral nutrition was adjusted according to the result feedback of
examinations. Then, 0.3 mg octreotide acetate injection +250 ml
normal saline was injected at a rate of 0.025 mg/h to inhibit
pancreatic enzyme secretion. After admission, 200,000 U ulinastatin
+250 ml normal saline was intravenously injected in the early stage
to inhibit the activity of pancreatic enzymes relating to the
progression of SAP. Symptomatic and supportive treatment was
carried out according to the condition of patients.
CVVH
After definitive diagnosis, patients received CVVH
treatment every day in the early stage using a multifiltrate blood
purifier (Fresenius SE & Co., Bad Homburg, Germany) and an
Ultraflux AV600S hemodialysis filter (Fresenius SE & Co.). The
effective membrane area was 1.4 mm2. A total of 1,500 ml
normal saline + 37,500 U unfractionated heparin was used for
pre-rinse for 30 min, and hemorrhage treatment was conducted after
completion of pre-rinse.
Determination of biochemical indexes
and severity of SAP
Venous blood was collected from patients before
treatment and at 7 day during treatment to detect inflammatory
factors such as tumor necrosis factor-α (TNF-α), interleukin-1β
(IL-1β) and IL-6, blood routine indexes, liver and renal function
as well as amylase in the blood and urine. Acute Physiology and
Chronic Health Evaluation (APACHE)-II grade was used to assess the
severity of SAP.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 software (IBM Corp., Armonk, NY, USA) was used for statistical
analysis of data. Homogeneity test for variance was applied for
data meeting normal distribution, using independent-sample or
paired-sample t-test. Comparison between multiple groups was done
using one-way ANOVA test followed by post hoc test (Least
Significant Difference). Rank sum test was used for data not
meeting normal distribution. Quantitative data were expressed as
rate or percentage, using χ2 test, and α=0.05 was used
as the test level. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inflammatory factors are significantly
decreased in both CVVH and control groups after treatment
After analyzing the levels of TNF-α, IL-1β and IL-6
between the CVVH and control groups after treatment, it was found
that these inflammatory factors were remarkably lower in the CVVH
and control groups. Besides, comparison of expression of
inflammatory factors between the two groups showed that HF and
routine internal medicine therapy could eliminate more factors than
the control group (Fig. 1).
Blood biochemical indexes after
treatment in control group
Biochemical indexes such as alanine aminotransferase
(ALT), aspartate aminotransferase (AST), total bilirubin (TBIL),
albumin (ALB), white blood cell (WBC), serum amylase, urine
amylase, blood urea nitrogen (BUN) and creatinine (Scr) were
significantly lower after treatment compared with those before
treatment, while red blood cells (RBC) were overtly higher than
that before treatment, showing statistically significant
differences (P<0.05). There was no statistically significant
difference in platelets (PLT) before and after treatment
(P>0.05) (Fig. 2).
 | Figure 2.ALT, AST, TBIL, ALB, WBC, serum
amylase, urine amylase, BUN and Scr are significantly lower after
treatment compared with the level before treatment in control
group. *P<0.05, compared with before treatment. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; TBIL, total
bilirubin; ALB, albumin; WBC, white blood cell; RBC, red blood
cell; PLT, platelets; BUN, blood urea nitrogen; Scr,
creatinine. |
Blood biochemical indexes alter with
HF and routine internal medicine therapy and CVVH group achieves
better results than the control group
After CVVH treatment, ALT, AST, TBIL, ALB, WBC,
serum amylase, urine amylase, BUN and Scr were significantly
decreased (Fig. 3). Moreover, its
clearance was better than that in the control group (Table II).
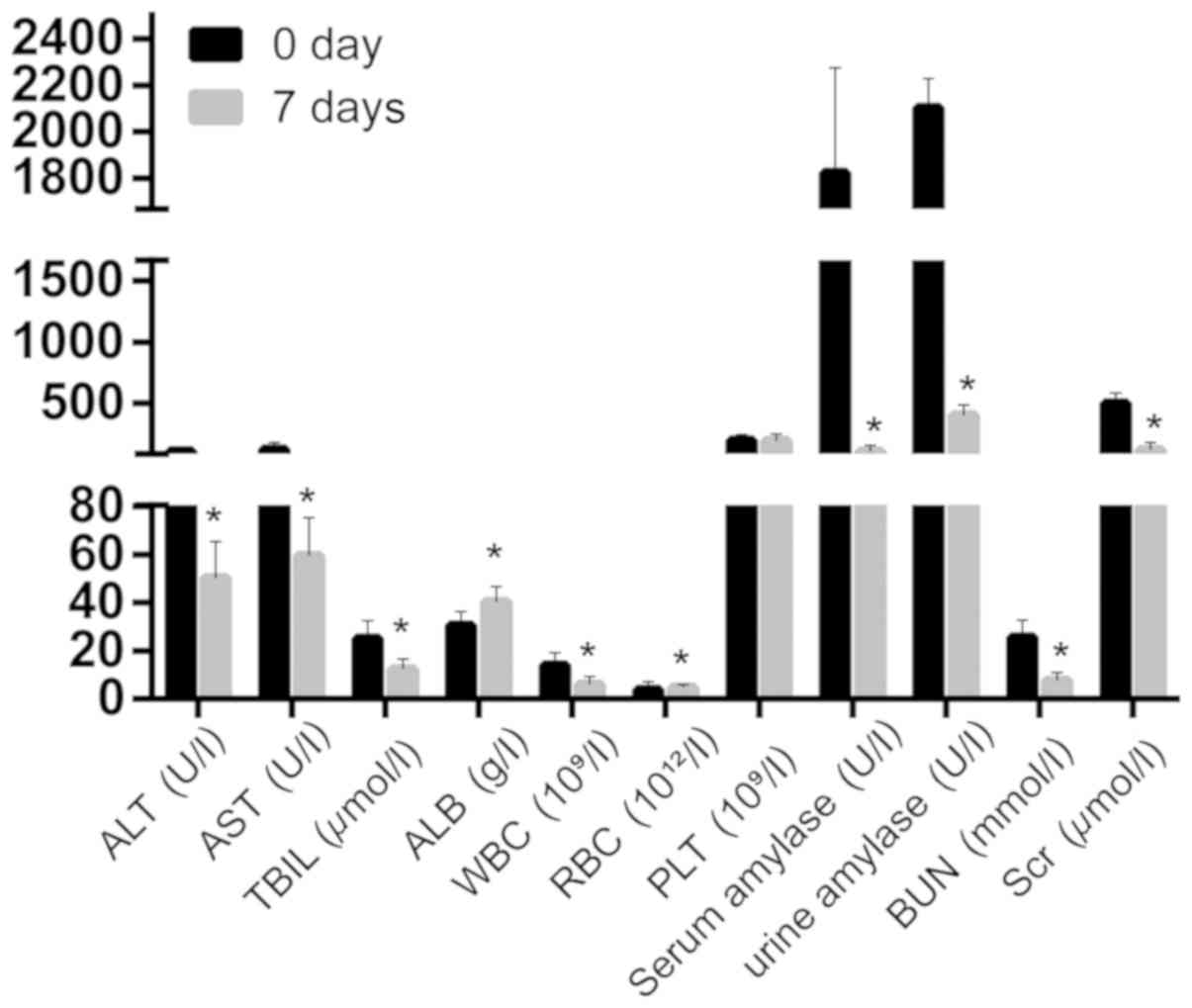 | Figure 3.ALT, AST, TBIL, ALB, WBC, serum
amylase, urine amylase, BUN and Scr are significantly decreased
after CVVH treatment. *P<0.05, compared with before treatment.
ALT, alanine aminotransferase; AST, aspartate aminotransferase;
TBIL, total bilirubin; ALB, albumin; WBC, white blood cell; RBC,
red blood cell; PLT, platelets; BUN, blood urea nitrogen; Scr,
creatinine; CVVH, continuous veno-venous hemofiltration. |
 | Table II.Comparison of biochemical index (mean
± SD) between the two groups after 7-day treatment. |
Table II.
Comparison of biochemical index (mean
± SD) between the two groups after 7-day treatment.
| Biochemical
index | Control group | HEMO group | t value | P-value |
|---|
| ALT | 63.32±16.36 | 50.22±15.22 | 3.196 | 0.0023 |
| AST | 70.56±16.39 | 59.36±15.88 | 2.679 | 0.0096 |
| TBIL | 20.69±5.78 | 12.36±4.25 | 6.281 | <0.0001 |
| ALB | 36.58±7.55 | 40.35±6.36 | 2.075 | 0.0424 |
| WBC | 8.46±5.11 | 6.02±3.21 | 2.177 | 0.0335 |
| RBC | 3.98±2.12 | 4.88±1.09 | 2.203 | 0.0477 |
| PLT | 178.66±50.63 | 190.88±56.21 | 0.886 | 0.3793 |
| Serum amylase | 256.98±45.32 | 102.23±55.36 | 11.90 | <0.0001 |
| Urine amylase | 626.36±69.89 | 400.56±85.96 | 11.22 | <0.0001 |
| BUN | 12.88±4.21 | 7.65±3.26 | 5.322 | <0.0001 |
| Scr | 165.23±56.44 | 120.36±57.01 | 3.058 | 0.0034 |
APACHE-II grade significantly
decreases in the CVVH group compared with that in the control group
after 7 days
There were no significant differences in APACHE-II
grade between the two groups on the 1st day. APACHE-II was used to
evaluate the severity of SAP at 3 and 7 days after treatment. The
data suggested that there were no remarkable differences after 3
days of treatment. However, significant differences were detected
after 7 days of treatment (Fig.
4).
Discussion
AP refers to pancreatic enzyme activation due to a
variety of causes, with the main feature of local inflammatory
response of the pancreas (17).
Patients in severe cases may have SIRS and may be complicated with
diseases of organ dysfunction. A large number of inflammatory
cytokines produced during SAP can lead to damage of vascular
endothelial cells and the activation of tissue factor-mediated
abnormal coagulation, resulting in coagulation disorders,
clinically manifested as thrombosis and secondary hemorrhage
(18). Pathophysiological changes
including micro-thrombosis formation and vascular permeability
increase can lead to microcirculation disturbance, tissue edema and
ischemia-hypoxia, which is one of the important mechanisms leading
to MODS. Approximately 70% of SAP patients are complicated with
acute respiratory distress syndrome ARDS), which is one of the
major causes of death in SAP patients. For the treatment of SAP,
the principle of ‘early treatment and operation expansion’ was
followed in the early stage. However, in recent years, more
attention has been paid to the coexistence of ‘surgical treatment’
and ‘non-surgical’ treatment in the patient-oriented personalized
treatment program. Moreover, some researchers have proposed to
determine the treatment plan according to the stage of the disease.
At present, comprehensive treatment methods combining multiple ways
have been formed. In the early stage of SAP (19), the therapeutic goal is to control
further response of inflammation because of the possible occurrence
of abdominal compartment syndrome, and the main treatment portions
are the observation in the Intensive Care Unit (ICU) and the
application of professional life-sustaining equipment in ICU for
humoral recovery (20). In addition,
vital signs and metabolic changes were observed in real time, and
timely interventions, symptomatic treatment and strict control of
complications were carried out. In ICU treatment for SAP, combined
therapy should be performed based on the specific symptoms of
different patients. Commonly used comprehensive treatment methods
include nutritional support, blood purification, artificial
respirator assisted ventilation and operative drainage.
CVVH is a blood purification technique that removes
solutes by using convection (21).
It was first used clinically in 1988 for acute and chronic renal
failure patients who could not receive hemodialysis because they
were complicated with hemodynamic instability or other
complications, or used as an emergency renal replacement therapy.
CVVH does not require complicated and expensive equipment. As a
safe and effective method for blood purification, CVVH has been
widely used in the rescue of critically ill patients. Since the
filter has a molecular weight cut-off of 50 ku and is mainly used
for the removal of serum molecular weight substances, various major
inflammatory cytokines can be removed via CVVH. The results of this
study showed that there were obvious differences in clearance of
inflammatory cytokines and improvement of blood biochemical markers
and clinical symptoms between the CVVH and control groups
(P<0.05).
In conclusion, our results clarified that CVVH
therapy can decrease the expression of inflammatory factors,
improve biochemical and physiological indicators in SAP patients,
and thus needs to be promoted extensively.
Acknowledgements
Not applicable.
Funding
This work was supported by the Medical and Health
Science and Technology Program of Yancheng city (YK2015082).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XC and WS designed the study and performed the
experiments. XC and MS collected the data. XM and XL analyzed the
data. XC and WS prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Affiliated Dongtai Hospital of Nantong University (Dongtai, China).
Signed informed consents were obtained from the patients or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Norman J: The role of cytokines in the
pathogenesis of acute pancreatitis. Am J Surg. 175:76–83. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin Z, Xu L, Wang X and Yang D: Risk
factors for worsening of acute pancreatitis in patients admitted
with mild acute pancreatitis. Med Sci Monit. 23:1026–1032. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hackert T, Hartwig W, Fritz S, Schneider
L, Strobel O and Werner J: Ischemic acute pancreatitis: Clinical
features of 11 patients and review of the literature. Am J Surg.
197:450–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kashyap AS, Anand KP and Kashyap S: Severe
acute pancreatitis. JAMA. 292:13052004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kellum JA, Johnson JP, Kramer D, Palevsky
P, Brady JJ and Pinsky MR: Diffusive vs. convective therapy:
Effects on mediators of inflammation in patient with severe
systemic inflammatory response syndrome. Crit Care Med.
26:1995–2000. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beal AL and Cerra FB: Multiple organ
failure syndrome in the 1990s. Systemic inflammatory response and
organ dysfunction. JAMA. 271:226–233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marshall JC: SIRS and MODS: What is their
relevance to the science and practice of intensive care? Shock.
14:586–589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teraoka S, Mineshima M, Hoshino T,
Ishimori I, Kaneko I, Sato Y, Haruguchi H and Agishi T: Can
cytokines be removed by hemofiltration or hemoadsorption? ASAIO J.
46:448–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong D, Zhang P, Ji D, Chen Z, Li W, Li J,
Li L and Liu Z: Improvement of immune dysfunction in patients with
severe acute pancreatitis by high-volume hemofiltration: A
preliminary report. Int J Artif Organs. 33:22–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Xu L, Feng X, Li S, Feng Q, Liu C,
Zhang X and Zhao Q: Is continuous venovenous hemofiltration
effective against severe acute pancreatitis? Artif Organs.
37:615–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu C, Liu ZH, Chen ZH, Gong DH, Ji DX and
Li LS: Improvement of monocyte function and immune homeostasis by
high volume continuous venovenous hemofiltration in patients with
severe acute pancreatitis. Int J Artif Organs. 31:882–890. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu J, Tian X, Zhang C, Wang M and Li Y:
Management of abdominal compartment syndrome in severe acute
pancreatitis patients with early continuous veno-venous
hemofiltration. Hepatogastroenterology. 60:1749–1752.
2013.PubMed/NCBI
|
|
13
|
Tang Y, Zhang L, Fu P, Kang Y and Liu F:
Hemoperfusion plus continuous veno-venous hemofiltration in a
pregnant woman with severe acute pancreatitis: A case report. Int
Urol Nephrol. 44:987–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang HL, Xue WJ, Li DQ, Yin AP, Xin X, Li
CM and Gao JL: Influence of continuous veno-venous hemofiltration
on the course of acute pancreatitis. World J Gastroenterol.
11:4815–4821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bellomo R, Tipping P and Boyce N:
Continuous veno-venous hemofiltration with dialysis removes
cytokines from the circulation of septic patients. Crit Care Med.
21:522–526. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS;: Acute
Pancreatitis Classification Working Group: Classification of acute
pancreatitis - 2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heath DI, Cruickshank A, Gudgeon AM,
Jehanli A, Shenkin A and Imrie CW: The relationship between
pancreatic enzyme release and activation and the acute-phase
protein response in patients with acute pancreatitis. Pancreas.
10:347–353. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang R, Tenhunen J and Tonnessen TI: HMGB1
and histones play a significant role in inducing systemic
inflammation and multiple organ dysfunctions in severe acute
pancreatitis. Int J Inflamm. 2017:18175642017. View Article : Google Scholar
|
|
19
|
Li HG, Zhou ZG, Li Y, Zheng XL, Lei S, Zhu
L and Wang Y: Alterations of Toll-like receptor 4 expression on
peripheral blood monocytes during the early stage of human acute
pancreatitis. Dig Dis Sci. 52:1973–1978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bumbasirevic V, Radenkovic D, Jankovic Z,
Karamarkovic A, Jovanovic B, Milic N, Palibrk I and Ivancevic N:
Severe acute pancreatitis: Overall and early versus late mortality
in intensive care units. Pancreas. 38:122–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan HK, Baldwin I and Bellomo R:
Continuous veno-venous hemofiltration without anticoagulation in
high-risk patients. Intensive Care Med. 26:1652–1657. 2000.
View Article : Google Scholar : PubMed/NCBI
|


















