Introduction
Hypertension is a common chronic disease (1), and its morbidity increases year by year
with the development of society aging, the change of dietary
structure and the gradual improvement of living standards, which
threaten the life and health of the elderly (2). The morbidity of hypertensive
nephropathy has also increased, and it is the most common and most
serious complication of hypertension (3). If the glomeruluses are in a permanent
state of high pressure, high infusion, and hyperfiltration caused
by hypertension, the renal tubules and glomerular filtration
membranes will be damaged and then result in hypertensive
nephropathy (4,5). The early clinical manifestation of
hypertensive renal injury generally is nocturia, but it is very
difficult to attract patients' attention, and it usually shows
normal during the routine examinations. However, generally
speaking, the kidneys are already severely damaged when renal
function shows abnormalities (6,7). Thus,
the normality in the renal function indicators and urine protein of
the patients with hypertension do not indicate that the kidneys are
not pathologically damaged (8). So
it is particularly important to seek the sensitive indicators for
the diagnosis of renal injury of the patients with
hypertension.
In recent years, research which uses
β2-microglobulin (β2-MG) and transforming growth factor-β (TGF-β)
on the diagnosis of hypertensive nephropathy has gradually
increased (9–11). It is reported in the literature that
β2-MG is a good indicator in the diagnosis of early hypertensive
renal injury (12). It is a small
molecular globulin that is reabsorbed by renal proximal convoluted
tubules after being freely filtered through the glomerulus, and
finally is degraded into amino acids by renal tubular endothelial
cells. The increase of the level of β2-MG in the serum can reflect
the impaired filtration function or increased filtration load of
the glomerulus (13). Studies have
shown that hypertensive nephropathy is closely related to TGF-β,
which is highly expressed in hypertensive nephropathy and affects
the development course of hypertensive nephropathy and the
prognosis of patients (6). It is
also reported in the literature that the main pathological changes
of hypertensive nephropathy are renal interstitial fibrosis and
glomerulosclerosis, and the main factor that regulates
extracellular matrix deposition and stimulates the production of
collagen is TGF-β (14,15). At the same time, TGF-β is a
hematological indicator that can be detected conveniently and
operated repeatedly.
Relative study of the combined application of β2-MG
and TGF-β in the diagnosis of elderly hypertensive nephropathy has
not been established, therefore, the aim of this study was to
investigate the diagnostic value of β2-MG and TGF-β in elderly
hypertensive nephropathy, and to provide some theoretical basises
and references for the early clinical diagnosis, treatment and
prognosis.
Materials and methods
General data
The clinical data of 56 patients, who were more than
60 years old, with hypertensive nephropathy, and admitted to
Affiliated Hospital of Chengde Medical College (Chengde, China)
from December 2015 to December 2017, were retrospectively analyzed
and the clinical data were used as the study group, including 34
males and 22 females. The systolic pressure of all the patients was
140 mmHg or more and the diastolic pressure of them was 90 mmHg or
more on average, and the 24 h urine protein of the patients in the
study group was more than 30 mg/l or any urine protein was more
than 37 mg/l, moreover, the clinical data of 50 patients, who were
more than 60 years old, with hypertension, but did not have
nephropathy, were selected as the control group, including 29 males
and 21 females. There were no significant difference in each
indicator of the general data of the patients in the two groups
(P>0.05). Some physiological conditions of the patients were
excluded, including the insufficiency of the cardiac and hepatic
functions, the incompletion of clinical data, the existence of
cancer, coagulopathy and severe metabolic diseases as well as other
renal organic lesions.
This study was approved by the Εthics Committee of
Affiliated Hospital of Chengde Medical College. Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians. The general data of the selected persons are shown in
Table I.
 | Table I.Comparison of general data between two
groups of patients [n(%)]. |
Table I.
Comparison of general data between two
groups of patients [n(%)].
| Factors | Observation group
(n=56) | Control group
(n=50) | t/χ2 | P-value |
|---|
| Sex |
|
| 0.081 | 0.844 |
| Male | 34 (60.71) | 29 (58.00) |
|
|
|
Female | 22 (39.29) | 21 (42.00) |
|
|
| Average age,
year | 65.68±4.27 | 65.13±4.68 | 0.633 | 0.528 |
| The course of
hypertension, year | 10.68±2.23 | 11.05±1.95 | 0.904 | 0.368 |
| BMI,
kg/m2 | 23.86±1.64 | 23.75±1.52 | 0.357 | 0.722 |
| Smoking |
|
| 0.968 | 0.339 |
| Yes | 26 (46.43) | 28 (56.00) |
|
|
| No | 30 (53.57) | 22 (44.00) |
|
|
| Drinking |
|
| 1.772 | 0.242 |
| Yes | 23 (41.07) | 27 (54.00) |
|
|
| No | 33 (58.93) | 23 (46.00) |
|
|
| The place of
residence |
|
| 0.089 | 0.844 |
| City | 32 (57.14) | 30 (60.00) |
|
|
|
Country | 24 (42.86) | 20 (40.00) |
|
|
Detection methods
The fasting venous blood of the patients was taken
in the morning (33 ml in a vacuum blood collection tube). The blood
was naturally agglutinated for 10 to 20 min at indoor temperature
and centrifuged at 3,000 × g for 5 min at 4°C and the supernatant
was taken. The processes of sample loading were as follows:
Firstly, 100 µl of the sample diluent was added into the blank
well, and then 100 µl of the sample to be tested was respectively
added into the standard well and the sample well, and 100 µl of the
test solution was added into each well after the sample to be
tested was drained and dried, next, the test solution was incubated
for 1 h at 37°C, then it was drained and dried again, and rinsed 3
times using PBS, after being dried, 100 µl of another test solution
was added, then the mixed solution was incubated for 1 h at 37°C
and was drained and dried, and rinsed 3 times using PBS, then 90 µl
of the substrate solution was added, and the color was developed in
the dark at 37°C, finally 50 µl of the stop solution was added to
terminate the translation. ELISA and Multiskan Spectrum Microplate
Spectrophotometer (SPECTROstar® Omega; Boqi
Biotechnology Co., Ltd.) were used to detect β2-MG (QY-MB12035;
Shanghai Qiaoyu Biotechnology Co., Ltd., Shanghai, China) and TGF-β
(YM-E3369W; Shanghai Yunmai Biotechnology Co., Ltd., Shanghai,
China) and the serum of the patients was measured, as OD value at
450 nm wavelength. All the operations were strictly carried out
according to the specifications.
Statistical analysis
The analysis was performed by using SPSS 19.0 (SPSS,
Inc., Chicago, IL, USA) statistical software. Chi-square test was
used for enumeration data and t-test was used for measurement data.
Pearsons analysis was used for correlation analysis. The diagnostic
value of β2-MG, TGF-β and their combined application were analyzed,
using ROC curve. P<0.05 was considered to indicate a
statistically significant difference.
Results
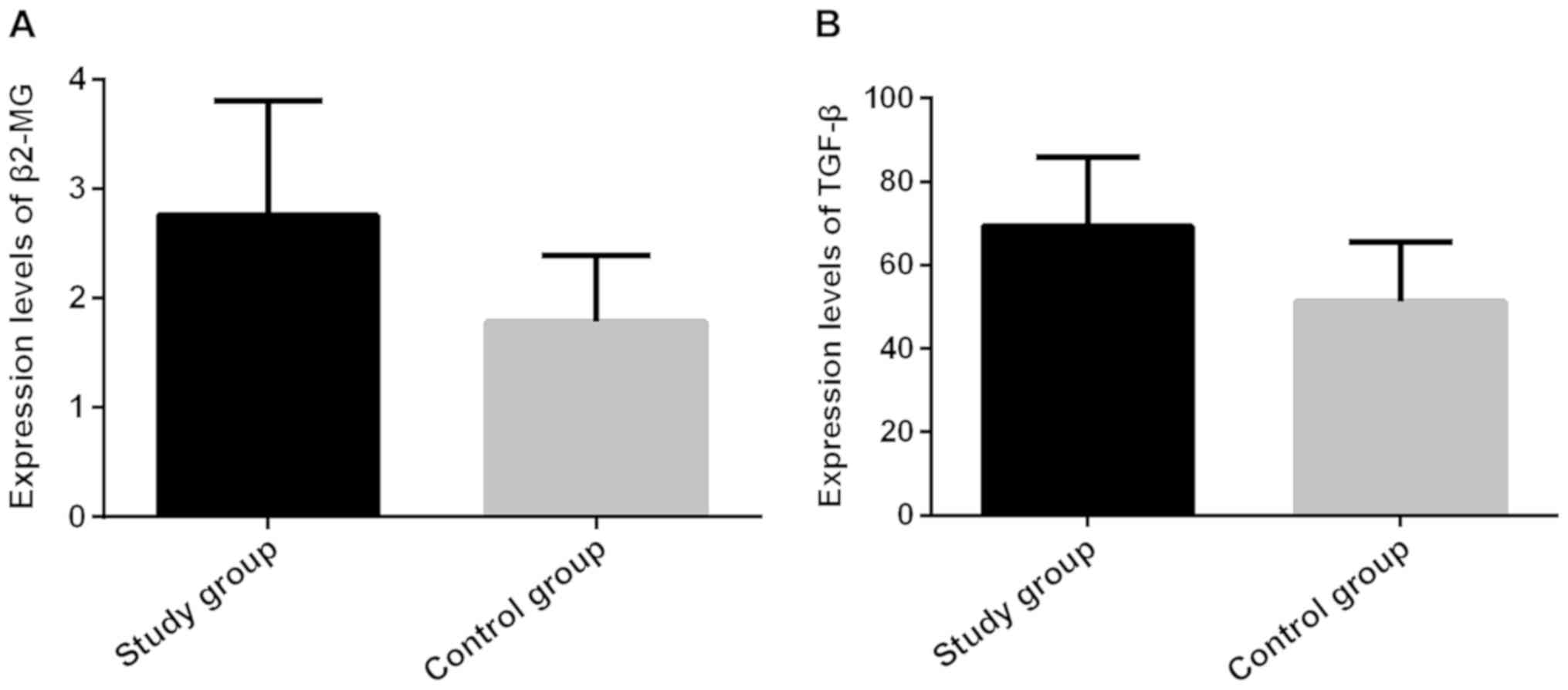
The expression levels of β2-MG and
TGF-β in the serum of the patients in the two groups
The expression levels of β2-MG and TGF-β in the
serum of the patients in the study group (2.86±1.18 mg/l and
73.46±15.63 µg/l) were significantly higher than those in the
control group (1.87±0.65 mg/l and 52.89±13.58 µg/l), the difference
was statistically significant (P<0.001; Fig. 1 and Table
II).
 | Table II.The expression levels of β2-MG and
TGF-β in the serum of the patients in the two groups. |
Table II.
The expression levels of β2-MG and
TGF-β in the serum of the patients in the two groups.
| Groups | Case | β2-MG (mg/l) | TGF-β (µg/l) |
|---|
| Study | 56 | 2.86±1.18 | 73.46±15.63 |
| Control | 50 | 1.87±0.65 | 52.89±13.58 |
| t |
|
5.261 |
7.192 |
| P-value |
| <0.001 | <0.001 |
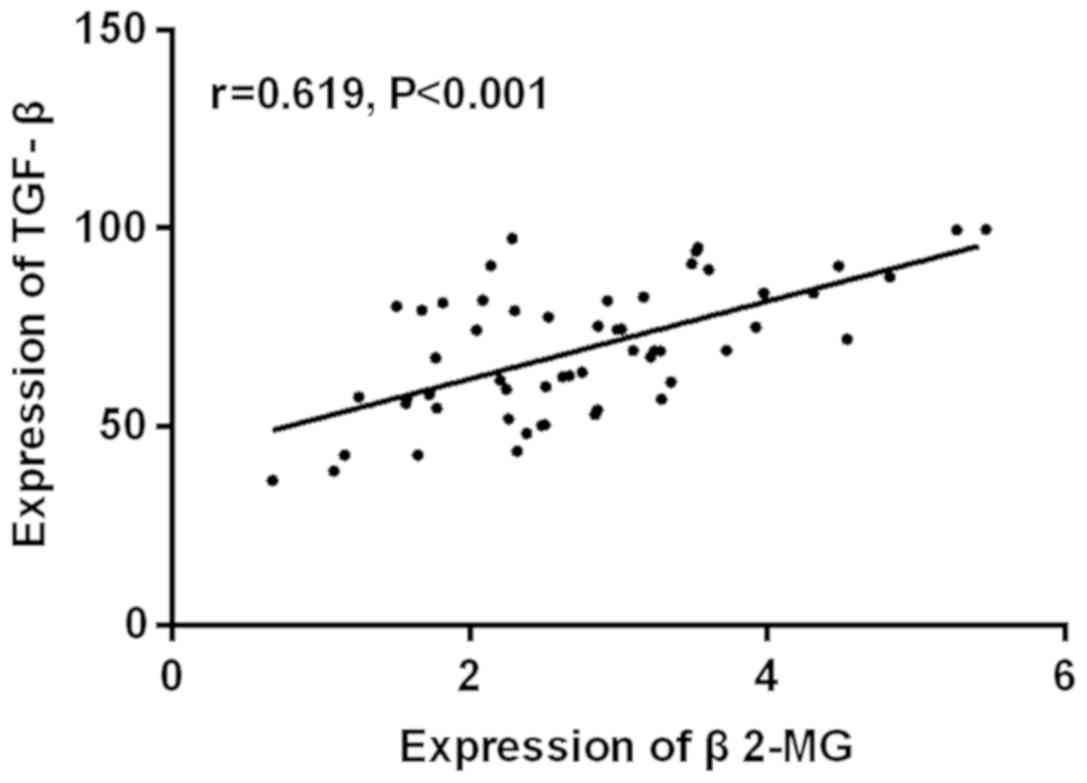
There was a positive correlation between the
expression levels of β2-MG and TGF-β in the serum of the patients
who had hypertensive nephropathy (r=0.619, P<0.001; Fig. 2).
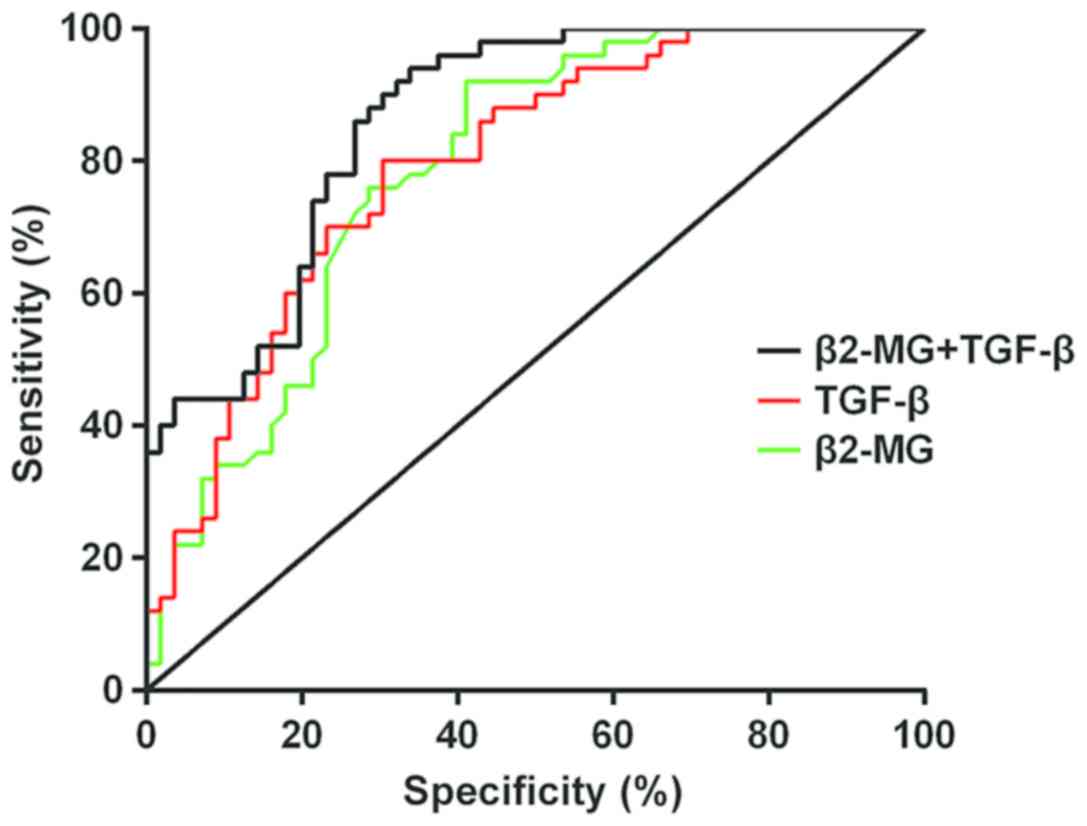
The sensitivity and specificity of
β2-MG and TGF-β in the diagnosis of hypertensive nephropathy
The analysis result of the ROC curve of β2-MG in the
diagnosis of hypertensive nephropathy showed that the AUC of β2-MG
in the diagnosis of hypertensive nephropathy was 0.786; the 95%
confidence interval was from 0.699 to 0.871, and the cut-off value
was 2.480; there were 33 positive cases in the study group and 4 in
the control group; the sensitivity was 58.93%, and the specificity
was 92.00%.
The analysis of the ROC curve of TGF-β in the
diagnosis of hypertensive nephropathy showed that the AUC of TGF-β
in the diagnosis of hypertensive nephropathy was 0.793; the 95%
confidence interval was from 0.709 to 0.877, and the cut-off value
was 59.330; there were 39 positive cases in the study group and 10
in the control group; the sensitivity was 69.64%, and the
specificity was 80.00%.
The analysis result of the ROC curve of the combined
application of β2-MG and TGF-β in the diagnosis of hypertensive
nephropathy showed that the AUC of the combined application of
β2-MG and TGF-β in the diagnosis of hypertensive nephropathy was
0.860; the 95% confidence interval was from 0.791 to 0.928, and the
cut-off value was 0.303; there were 53 positive cases in the study
group and 17 in the control group; the sensitivity was 94.64%, and
the specificity was 66.00% (Fig. 3
and Table III).
 | Table III.The sensitivity and specificity of
β2-MG, TGF-β in the diagnosis of hypertensive nephropathy. |
Table III.
The sensitivity and specificity of
β2-MG, TGF-β in the diagnosis of hypertensive nephropathy.
| Indicator | n | The positive
case | Sensitivity | Specificity |
|---|
| β2-MG | 56 | 33 | 58.93 | 92.00 |
| TGF-β | 56 | 39 | 69.64 | 80.00 |
| β2-MG+TGF-β | 56 | 53 | 94.64 | 66.00 |
Discussion
Hypertension is a disease that is affected by
environmental and genetic factors (16). According to the statistics, there are
approximately 1.1 billion adult patients with hypertension in the
world, among which approximately 700 million adult patients with
hypertension are in the developing countries and approximately 400
million adult patients with hypertension are in the developed
countries (17). In the state of
persistent hypertension, the organism will suffer from
arteriolosclerosis, however, the impairment of the kidney is
particularly obvious when compared with all the visceral organs.
The regulatory functions of the kidney will be weaken by a state of
high pressure, high filtration, and high perfusion, which will lead
to the disequilibrium of hemodynamics and the abnormity of
functions in the kidney (18,19).
According to the reports in the literature, generally β2-MG is
rarely found in the blood and urine of healthy people, and the
resultant rate is stable, and there is no significant difference in
its changes between the morning and evening. Therefore, the
evaluation of the functions of renal tubular and glomerulus will be
more stable and accurate when using β2-MG as indicator (20). It is also found in other studies that
TGF-β is overexpressed in diabetic hypertensive nephropathy and is
involved in the pathogenesis of diabetic hypertensive nephropathy
(21).
This study showed that the expression levels of
β2-MG and TGF-β in the serum of the patients in the study group
were significantly higher than those in the control group, and the
difference was statistically significant (P<0.001). Study has
shown that the rise of β2-MG in the serum indicates that the
filtration function of glomerulus is damaged or is overloaded
(22). TGF-β plays a major role in
many renal diseases and the formation of hypertensive renal scars,
and is an early indicator of hypertensive benign renal disease
(23). The study of Rouse et
al showed that the expression level of β2-MG in the serum of
the patients who had hypertensive nephropathy was significantly
higher than that of patients with only hypertension (6). The study of Kurts et al showed
that the expression level of TGF-β in the serum of the patients
with hypertensive nephropathy was significantly higher than that of
patients who had hypertension, but did not have renal disease
(24). The results of these studies
were consistent with our findings. There was a positive correlation
between the expression levels of β2-MG and TGF-β in the serum of
the patients with hypertensive nephropathy (r=0.619, P<0.001).
At present, no similar literature has been found to support the
findings, but the results of our study demonstrated that if the
expression levels of β2-MG and TGF-β in the serum of the patients
increased, the impairment of renal functions is most likely to
occur. The combined application of the two in the diagnosis could
help to detect the occurrence of hypertensive nephropathy.
The analysis of the ROC curve of β2-MG in the
diagnosis of hypertensive nephropathy showed that the AUC of β2-MG
in the diagnosis of hypertensive nephropathy was 0.786; the 95%
confidence interval was from 0.699 to 0.871, and the cut-off value
was 2.480; there were 33 positive cases in the study group, the
sensitivity was 58.93%, and the specificity was 92.00%. The result
of the ROC curve of TGF-β in the diagnosis of hypertensive
nephropathy showed that the AUC of TGF-β in the diagnosis of
hypertensive nephropathy was 0.793; the 95% confidence interval was
from 0.709 to 0.877, and the cut-off value was 59.330; there were
39 positive cases in the study group, the sensitivity was 69.64%,
and the specificity was 80.00%. The result of the ROC curve of the
combined application of β2-MG and TGF-β in the diagnosis of
hypertensive nephropathy showed that the AUC of the combined
application of β2-MG and TGF-β in the diagnosis of hypertensive
nephropathy was 0.860; the 95% confidence interval was from 0.791
to 0.928, and the cut-off value was 0.303; there were 53 positive
cases in the study group, the sensitivity was 94.64%, and the
specificity was 66.00%. This indicated that TGF-β was not a
specific indicator in the diagnosis of hypertensive nephropathy,
but it still had screening value. The sensitivity of β2-MG in the
diagnosis of hypertensive nephropathy was low, but the specificity
was good. The sensitivity of the combined application of β2-MG and
TGF-β in the diagnosis of hypertensive nephropathy was high, but
the specificity was poor, which provided a more effective and
comprehensive diagnostic basis for hypertensive nephropathy.
In summary, β2-MG and TGF-β were highly expressed in
the patients with hypertensive nephropathy, and the expression
levels of β2-MG and TGF-β were positively correlated (r=0.619,
P<0.001). The sensitivity of the combined application of β2-MG
and TGF-β was highest in the diagnosis of hypertensive nephropathy,
but the specificity was worst. Therefore, the combined application
of β2-MG and TGF-β in the diagnosis of hypertensive nephropathy
could reduce or even avoid the missed diagnosis caused by single
detection. The two indicators complemented and confirmed each
other, which had a great significance for improving the positive
diagnosis rate of hypertensive nephropathy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC wrote the manuscript. JC, RH and CZ performed
ELISA. JL and KZ collected and analyzed the general data of
patients. HJ, YF and YW were responsible for statistical analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Εthics Committee of
Affiliated Hospital of Chengde Medical College (Chengde, China).
Patients who participated in this research had complete clinical
data. The signed informed consents were obtained from the patients
or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
James PA, Oparil S, Carter BL, Cushman WC,
Dennison -Himmelfarb C, Handler J, Lackland DT, LeFevre ML,
MacKenzie TD, Ogedegbe O, et al: 2014 evidence-based guideline for
the management of high blood pressure in adults: Report from the
panel members appointed to the Eighth Joint National Committee (JNC
8). JAMA. 311:507–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kearney PM, Whelton M, Reynolds K, Muntner
P, Whelton PK and He J: Global burden of hypertension: Analysis of
worldwide data. Lancet. 365:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart PD and Bakris GL: Hypertensive
nephropathy: Prevention and treatment recommendations. Expert Opin
Pharmacother. 11:2675–2686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christiansen RE, Tenstad O, Leh S and
Iversen BM: Glomerular charge selectivity is impaired in
hypertensive nephropathy. Nephrol Dial Transplant. 19:1083–1091.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fauvel JP and Laville M: Hypertensive
nephropathy: A growing cause of renal insufficiency. Presse Med.
30:81–86. 2001.(In French). PubMed/NCBI
|
|
6
|
Rouse RL, Stewart SR, Thompson KL and
Zhang J: Kidney injury biomarkers in hypertensive, diabetic, and
nephropathy rat models treated with contrast media. Toxicol Pathol.
41:662–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahajan A, Simoni J, Sheather SJ, Broglio
KR, Rajab MH and Wesson DE: Daily oral sodium bicarbonate preserves
glomerular filtration rate by slowing its decline in early
hypertensive nephropathy. Kidney Int. 78:303–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tylicki L and Rutkowski B: Hypertensive
nephropathy: pathogenesis, diagnosis and treatment. Pol Merkuriusz
Lek. 14:168–173. 2003.(In Polish).
|
|
9
|
Aksun SA, Ozmen D, Ozmen B, Parildar Z,
Mutaf I, Turgan N, Habif S, Kumanlioğluc K and Bayindir O:
Beta2-microglobulin and cystatin C in type 2 diabetes: Assessment
of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 112:195–200.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H and Li H: Clinical implication of
cystatin C and β2-microglobulin in early detection of diabetic
nephropathy. Clin Lab. 63:241–247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nozue T, Michishita I and Mizuguchi I:
Predictive value of serum cystatin C, β2-microglobulin, and urinary
liver-type fatty acid-binding protein on the development of
contrast-induced nephropathy. Cardiovasc Interv Ther. 25:85–90.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurashige T, Takahashi T, Yamazaki Y,
Nagano Y, Kondo K, Nakamura T, Yamawaki T, Tsuburaya R, Hayashi YK,
Nonaka I, et al: Elevated urinary β2 microglobulin in the first
identified Japanese family afflicted by X-linked myopathy with
excessive autophagy. Neuromuscul Disord. 23:911–916. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stakhova TIu, Shcherbak AV, Kozlovskaia
LV, Taranova MV and Balkarov IM: Clinical value of the
determination of markers for endothelial dysfunction (endothelin-1,
microalbuminuria) and tubulointerstitial tissue lesion
(β2-microglobulin, monocyte chemotactic protein-1) in hypertensive
patients with uric acid metabolic disorders. Ter Arkh. 86:45–51.
2014.(In Russian). PubMed/NCBI
|
|
14
|
Liu GX, Li YQ, Huang XR, Wei LH, Zhang Y,
Feng M, Meng XM, Chen HY, Shi YJ and Lan HY: Smad7 inhibits
AngII-mediated hypertensive nephropathy in a mouse model of
hypertension. Clin Sci. 127:195–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu GX, Li YQ, Huang XR, Wei L, Chen HY,
Shi YJ, Heuchel RL and Lan HY: Disruption of Smad7 promotes ANG
II-mediated renal inflammation and fibrosis via
Sp1-TGF-β/Smad3-NFκB-dependent mechanisms in mice. PLoS One.
8:e535732013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klingbeil AU, Schneider M, Martus P,
Messerli FH and Schmieder RE: A meta-analysis of the effects of
treatment on left ventricular mass in essential hypertension. Am J
Med. 115:41–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staessen JA, Wang J, Bianchi G and
Birkenhäger WH: Essential hypertension. Lancet. 361:1629–1641.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schillaci G, Pirro M, Mannarino MR, Pucci
G, Savarese G, Franklin SS and Mannarino E: Relation between renal
function within the normal range and central and peripheral
arterial stiffness in hypertension. Hypertension. 48:616–621. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leoncini G, Viazzi F, Conti N, Baratto E,
Tomolillo C, Bezante GP, Deferrari G and Pontremoli R: Renal and
cardiac abnormalities in primary hypertension. J Hypertens.
27:1064–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin JR, Kim SM, Yoo JS, Park JY, Kim SK,
Cho JH, Jeong KH, Lee TW and Ihm CG: Urinary excretion of
β2-microglobulin as a prognostic marker in immunoglobulin A
nephropathy. Korean J Intern Med. 29:334–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohamed RH, Abdel-Aziz HR, Abd El Motteleb
DM and Abd El-Aziz TA: Effect of RAS inhibition on TGF-β, renal
function and structure in experimentally induced diabetic
hypertensive nephropathy rats. Biomed Pharmacother. 67:209–214.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dieterle F, Perentes E, Cordier A, Roth
DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, et al:
Urinary clusterin, cystatin C, beta2-microglobulin and total
protein as markers to detect drug-induced kidney injury. Nat
Biotechnol. 28:463–469. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bicik Z, Gönen S, Bahçebasi T, Reis K,
Arinsoy T and Sindel S: Role of transforming growth factor-β2 in,
and apossible transforming growth factor-β 2 gene polymorphism as a
marker of, renal dysfunction in essential hypertension: A study in
Turkish patients. Curr Ther Res Clin Exp. 66:266–278. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurts C, Panzer U, Anders HJ and Rees AJ:
The immune system and kidney disease: Basic concepts and clinical
implications. Nat Rev Immunol. 13:738–753. 2013. View Article : Google Scholar : PubMed/NCBI
|