Introduction
Anatomical and physiological functions of children
change rapidly, and is different from adults. Therefore,
physiological characteristics of children should be carefully
considered for the determination of anaesthesia dosage, methods and
equipment. In addition, drug metabolism should also be considered
(1,2). Sevoflurane as a volatile colorless
halide is commonly used for the induction and maintenance of
general anaesthesia through inhalation (3). Sevoflurane is a commonly used drug for
pediatric anesthesia. Sevoflurane has the advantages of quick
recovery, low distribution coefficient and less damage to
respiratory system (4). However,
sevoflurane also causes restless reaction and unstable mood
(5), leading to the increased
difficulties in post-operation care (6). Propofol (7) is a new type of short-acting intravenous
anesthetic. It is commonly used in the induction and maintenance of
general anesthesia. Propofol has a sedative effect and is often
used with other narcotic drugs (8).
In this study, the application of propofol in the prevention of
agitation after sevoflurane anesthesia in children was evaluated.
Our study provided guidance for the use of propofol in the
prevention of agitation in children.
Patients and methods
Clinical data of children
Pediatric patients who received treatment in
Children's Hospital of Xuzhou Medical University (Xuzhou, China)
from December 2016 to March 2018 were included. Clinical data,
agitation index, anaesthesia recovery index, extubation time and
PACU time were analyzed. Among those patients, 120 patients who
received inhaled sevoflurane for pediatric anesthesia and
intravenous infusion of propofol (2 mg/kg) were included in
observation group. The remaining 80 patients who were directly
anesthetized by sevoflurane alone were included in the control
group. Pediatric Anesthesia Emergence Delirium (PAED), Aldrete
scores, extubation time, PACU time and adverse reaction
(gastrointestinal tract reaction) were compared between observation
and control group, and PAED scores and Aldrete scores were
recorded.
The present study was approved by the Ethics
Committee of Children's Hospital of Xuzhou Medical University.
Signed written informed consents were obtained from the parents of
the child patients.
Inclusion and exclusion criteria
Inclusion criteria were: Patients who signed
informed consent forms; who treated with ligation of inguinal
hernia; without other severe diseases and with normal height, and
weight. All patients were in American Society of Anesthesiologists
(ASA) rate class I–II.
Exclusion criteria were: Patients combined with
serious heart disease; with liver and kidney dysfunction; with
dementia or mental disorders; allergic to propofol or sevoflurane
and other drugs used in this study.
Materials and drugs
Atropine (national medicine permission no.
H50020044, Southwest Pharmaceutical Co., Ltd., Chongqing, China);
sevoflurane (national medicine permission no. H20080681, Nonan Bate
Pharmaceuticals Co., Ltd., Linyi, China); dexamethasone 0.3 mg/kg
(national medicine permission no. H41020056, Zhengzhou Zhuo Feng
Pharmaceutical Co., Ltd., Zhengzhou, China); sodium chloride 0.9%
(national medicine permission no. H37021756, Shandong Lu Chen Xin
Pharmaceutical Co., Ltd., Shandong, China); propofol (national
medicine permission no. H20051842, Guangdong Gabor Pharmaceutical
Co., Ltd.); anesthesia monitor (General Electric Company, Boston,
MA, USA).
Anaesthesia for different groups
Both groups of patients were not allowed to drink
water 6 h before operation, and were not allowed to eat 4 h before
operation. Intramuscular injection of atropine (0.01 mg/kg) was
performed 30 min before operation. By setting a venous trocar, 4 ml
dexamethasone (0.3 mg/kg) sodium chloride (0.9%) solution were
injected through the indwelling needle. An anaesthesia monitor was
used to monitor the vital signs of the children.
Intraoperative anesthesia: General anesthesia was
performed with mask inhalation. Sevoflurane (8%) was first used to
induce anaesthesia and the reactions of children's eyelashes and
spontaneous breathing were observed. When there was no reaction of
eyelashes but spontaneous breathing was maintained, the
concentration of sevoflurane was reduced to 3–4% (adjusted
according to the depth of the anaesthesia). Oxygen inhalation was
kept at a speed of 2 l/min, and anesthesia was maintained until 5
min before the end of operation.
Post-operative treatment: Oxygen inhalation speed
was raised to 6 l/min. Patients in observation group were
intravenously injected with 2 mg/kg propofol, while patients in
control group received intravenous drip of 0.1 ml/kg of normal
saline. Monitoring blood pressure and other indexes of children was
performed. After vital signs returned to normal, children were sent
back to the ward.
PAED scores
PAED (9) mainly
evaluates the conditions through 3 aspects: i) Whether they have
eye contact with other people, ii) whether they can perceive
changes in surrounding environment, and iii) whether they can
dominate their own activities. The 3 items are reversely rated
according to the severity, 4 points for none, 3 points for poor
activity, 2 points for fair activity, 1 point for good activity and
0 point for excellent activity. The total scores is 20 points, the
higher the scores is, the more serious the restlessness is; 11–15
points are evaluated as restlessness, and more than 15 points are
evaluated as severe agitation.
Modified Aldrete scores
Modified Aldrete scoring (10) were performed according to the
standard shown in Table I. The total
score is 10. Higher scores indicate better awakening situation.
Patients with scores >9 points were extubated. Patients were
monitored in recovery room for 15 min and were sent back to ICU if
no abnormal conditions were observed.
 | Table I.The standard for modified Aldrete
scores. |
Table I.
The standard for modified Aldrete
scores.
| Items | Standards | Scores |
|---|
| Movement | Moving arms and legs
and head spontaneously or by request | 2 |
|
| Moving arms or legs
spontaneously or by request; restrictedly raising head
spontaneously or by request | 1 |
|
| Not able to move
limbs or raise head | 0 |
| Breathing | Deep breathing and
effective coughing, normal respiratory rate and amplitude | 2 |
|
| Breathing is
difficult or restricted, but spontaneous breathing is shallow and
slow, and it is possible to breath through oropharyngeal
airway | 1 |
|
| Breathing is paused
or weak, it requires respirator therapy or assisted breathing | 0 |
| Blood pressure | Within ±20% before
anesthesia | 2 |
|
| ±20–49% before
anesthesia | 1 |
|
| Above ±50% before
anesthesia | 0 |
| Consciousness | Completely awakening,
answer questions accurately | 2 |
|
| Able to wake up,
drowsiness | 1 |
|
| No reaction | 0 |
| SpO2 | Air breathing
SpO2 >92% | 2 |
|
| Oxygen breathing
SpO2 >92% | 1 |
|
| Oxygen breathing
SpO2 <92% | 0 |
Other observation indexes
Extubation time, time period from the end of the
operation to the extubation; PACU time, from the time of the
operation to the time of stay in the recovery room. Incidence of
adverse reactions: Adverse reactions include mental stress, nausea
and vomiting, gastrointestinal reactions, hypotension and
arrhythmia.
Statistic analysis
The scoring results, extubation time, PACU time and
other measurement data were recorded as mean ± standard deviation
and compared by t-test. Incidence of adverse reactions was tested
by χ2. Correlation between PAED scores/modified Aldrete
scores and extubation time/PACU time/incidence of adverse reactions
was analyzed by Spearman's correlation coefficient. The significant
level of the test was α=0.05.
Results
Comparison of general data between two
groups
There was no significant difference in age, weight,
sex, operation time and classification of ASA between observation
and control group (P>0.05) (Table
II).
 | Table II.General information on patients. |
Table II.
General information on patients.
| Clinical
features | Observation group
(n=120) | Control group
(n=80) | χ2/t | P-value |
|---|
| Age (years) | 5.86±1.87 | 5.78±1.72 | 0.31 | 0.76 |
| Operation time
(h) | 1.76±0.46 | 1.82±0.51 | 0.85 | 0.40 |
| Weight (kg) | 15.52±1.65 | 15.78±1.74 | 1.06 | 0.29 |
| Sex (n, %) |
|
| 0.65 | 0.42 |
| Male | 64 (53.33) | 38 (47.50) |
|
|
|
Female | 56 (46.67) | 42 (52.50) |
|
|
| ASA
classification |
|
| 0.16 | 0.69 |
| I | 62 (51.67) | 39 (48.75) |
|
|
| II | 58 (48.33) | 41 (51.25) |
|
|
| HR (times/min) | 107.14±7.25 | 105.21±8.93 | 1.61 | 0.11 |
| SBP (mmHg) | 98.24±6.24 | 96.88±7.45 | 1.35 | 0.18 |
| DBP (mmHg) | 76.24±6.68 | 77.42±5.67 | 1.34 | 0.18 |
Comparison of PAED scores between
observation and control group
Mean PAED score of the control group was 9.87±3.15,
and mean PAED score of the observation group was 5.66±1.74. PAED
score of the observation group were significantly lower in
observation group than those in control group (P<0.01; Fig. 1).
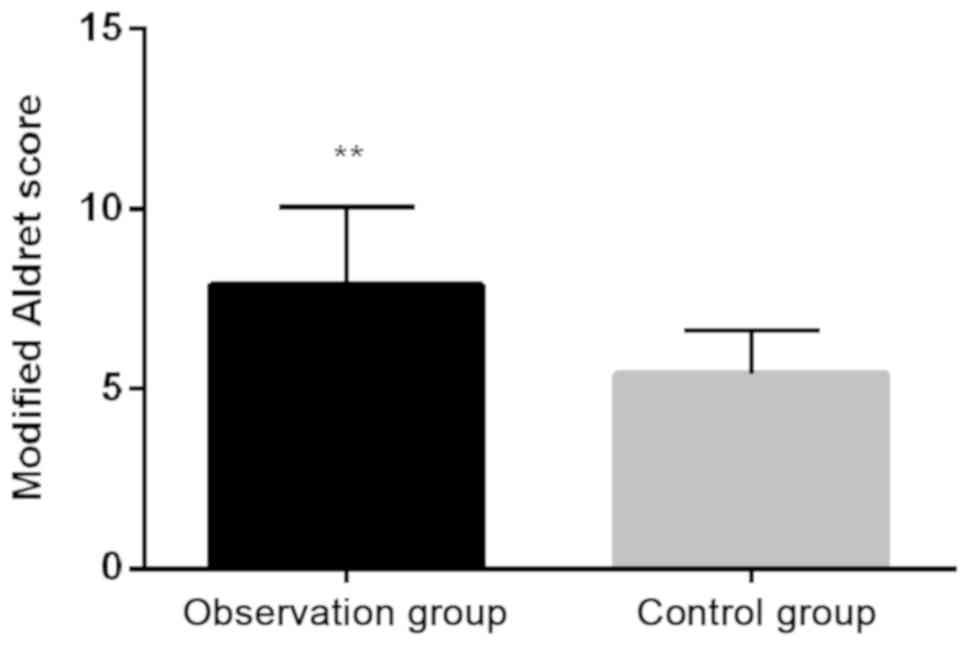
Comparison of modified Aldrete scores
between observation group and control group
Mean modified Aldrete scores of the observation
group was 7.91±2.14, which was significantly higher than that in
control group (5.41±1.22) (P<0.01; Fig. 2).
Comparison of extubation time, PACU
time and incidence of adverse reactions in each group
Mean extubation time of observation group was
11.35±3.17 min, which was significantly lower than that of control
group (21.41±4.62 min). PACU time of observation group was
46.57±7.43 min, which was significantly lower than that in control
group (49.23±8.22 min). Incidence of adverse reactions in
observation group was 5.00%, which was also significantly lower
than that in control group (13.75%; P<05) (Table III).
 | Table III.Comparison of extubation time, PACU
time and incidence of adverse reactions among patients. |
Table III.
Comparison of extubation time, PACU
time and incidence of adverse reactions among patients.
| Items | Observation group
(n=120) | Control group
(n=80) | χ2/t | P-value |
|---|
| Extubation time
(min) | 11.35±3.17 | 21.41±4.62 | 16.99 | <0.001 |
| PACU time (min) | 46.57±7.43 | 49.23±8.22 | 2.33 | 0.02 |
| The incidence of
adverse reactions (n, %) | 6 (5.00) | 11 (13.75) | 6.52 | 0.03 |
Spearman correlation analysis
results
PAED scores were positively correlated with
extubation time, PACU time and incidence of adverse reactions
(r=0.774, 0.689, 0.433). Modified Aldret scores were negatively
correlated with PACU time and incidence of adverse reactions (r= −
0.821, − 0.769, − 0.524) (all P<0.05) (Table IV).
 | Table IV.Value r for analysis of each
correlation. |
Table IV.
Value r for analysis of each
correlation.
| Items | PAED scores | Modified Aldrete
scores |
|---|
| Extubation
time | 0.774 | −0.821 |
| PACU time | 0.689 | −0.769 |
| Incidence of
adverse reactions | 0.433 | −0.524 |
Discussion
The physiological conditions and premature immune
system should be taken into consideration in the selection of
narcotic drugs, dosages and methods for pediatric anesthesia
(10,11). Dosage of sevoflurane used for
pediatric anesthesia is low, the damage to respiratory system is
small and the recovery is rapid. However, the use of sevoflurane
also causes restless reaction. Restless reaction will affect
surgical operations and threaten children's life (12,13).
Propofol has a sedative effect and can potentially alleviate the
restlessness of children after sevoflurane anesthesia (1,14). In
this study, the application of propofol in the prevention of
agitation after sevoflurane anesthesia in children was evaluated to
provide guidance for the use of propofol for the prevention of
agitation.
PAED scores, extubation time, PACU time and
incidence of adverse reactions in observation group were
significantly lower than those in control group, and modified
Aldrete scores of observation group were higher than those in
control group. PAED scores was positively correlated with
extubation time, PACU time and incidence of adverse reactions.
Aldrete scores were negatively correlated with extubation time,
PACU time and incidence of adverse reactions (P<0.05). It
indicates that propofol can reduce the agitation reaction of
children after sevoflurane anesthesia, shorten the extubation time,
PACU time and reduce the incidence of adverse reactions. Liang
et al (12) reported that
propofol combined with general anesthesia reduced the intubation
time and incidence of adverse reactions, which was consistent with
the findings in our study. Meta analysis by Kanaya et al
(15) revealed that propofol can
indeed reduce the incidence of restlessness after general
anesthesia in children. Picard et al (16) showed that the use of propofol in
sevoflurane anaesthesia decreased the PAED scores and reduced the
reaction of agitation. Rosen et al (17) studied the prophylactic effect of
propofol on children's restlessness after sevoflurane anesthesia.
It was found that PAED scores were significantly reduced after
using propofol. Propofol at 1 mg/kg delayed recovery and prolonged
extubation time, which was different from the findings in our
study. In the case of propofol administration, intravenous
inhalation of propofol can be performed after sevoflurane or
intravenous infusion of propofol can be performed during
sevoflurane inhalation anesthesia (12,16).
Similar to our study, intravenous infusion of propofol was given
after sevoflurane inhalation anesthesia in other studies (18). Costi et al (19) believe that intravenous infusion of
propofol at the end of sevoflurane anesthesia is not as effective
in reducing the rate of inflammatory response as propofol is
infused throughout the anesthesia maintenance process, but
administration is more complicated. Therefore, the administration
time of propofol and sevoflurane, and the mode of administration
(whether the two are administered simultaneously or continuously)
may affect the anesthetic effect and recovery. Our future studies
will explore the potential effects. PAED score and the Aldrete
score are dynamic. The PAED score and the Aldrete score may change
over time. Therefore, it is recommended to perform the scoring at
different time-points. In conclusion, propofol can present
agitation reaction of children after sevoflurane anesthesia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW drafted the manuscript. XW and JunhuaC were
mainly devoted to collecting and interpreting the general data of
patients. CS and BP recorded and analyzed PAED scores. RZ, JunliC
and FZ were responsible for observation index analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Children's Hospital of Xuzhou Medical University (Xuzhou, China).
Signed written informed consents were obtained from the parents of
the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vanis-Vatrenjak S, Mesic A, Abdagic I,
Mujezinovic D and Zvizdic Z: Quality and safety of general
anesthesia with propofol and sevoflurane in children aged 1–14
based on laboratory parameters. Med Arh. 69:218–221. 2015.
View Article : Google Scholar
|
|
2
|
Abdel-Ma'boud MA: Effect of
dexemeditomedine and propofol on the prevention of emergence
agitation following sevoflurane anesthesia in Egyptian children. J
Egypt Soc Parasitol. 44:687–694. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erturk E, Topaloglu S, Dohman D, Kutanis
D, Beşir A, Demirci Y, Kayir S and Mentese A: The comparison of the
effects of sevoflurane inhalation anesthesia and intravenous
propofol anesthesia on oxidative stress in one lung ventilation.
BioMed Res Int. 2014:3609362014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson RE, Barr G, Assareh H and
Jakobsson J: The AAI index, the BIS index and end-tidal
concentration during wash in and wash out of sevoflurane.
Anaesthesia. 58:531–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen XR, Fu YY, Liu HZ, Wu J, Shao XP,
Zhang XB, Tang M, Shi Y, Ma K, Zhang F, et al: Neuroprotection of
sevoflurane against ischemia/reperfusion-induced brain injury
through inhibiting JNK3/caspase-3 by enhancing Akt signaling
pathway. Mol Neurobiol. 53:1661–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottschalk A, Berkow LC, Stevens RD,
Mirski M, Thompson RE, White ED, Weingart JD, Long DM and Yaster M:
Prospective evaluation of pain and analgesic use following major
elective intracranial surgery. J Neurosurg. 106:210–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henderson F, Absalom AR and Kenny GN:
Patient-maintained propofol sedation: A follow up safety study
using a modified system in volunteers. Anaesthesia. 57:387–390.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathy-Hartert M, Mouithys-Mickalad A,
Kohnen S, Deby-Dupont G, Lamy M and Hans P: Effects of propofol on
endothelial cells subjected to a peroxynitrite donor (SIN-1).
Anaesthesia. 55:1066–1071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hubbard RC, Naumann TM, Traylor L and
Dhadda S: Parecoxib sodium has opioid-sparing effects in patients
undergoing total knee arthroplasty under spinal anaesthesia. Br J
Anaesth. 90:166–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aldrete JA: Postanesthesia recovery scores
and postoperative hypoxemia. Anesth Analg. 67:1016–1017. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ortiz J, Chang LC, Tolpin DA, Minard CG,
Scott BG and Rivers JM: Randomized, controlled trial comparing the
effects of anesthesia with propofol, isoflurane, desflurane and
sevoflurane on pain after laparoscopic cholecystectomy. Braz J
Anesthesiol. 64:145–151. 2014. View Article : Google Scholar
|
|
12
|
Liang C, Ding M, Du F, Cang J and Xue Z:
Sevoflurane/propofol coadministration provides better recovery than
sevoflurane in combined general/epidural anesthesia: A randomized
clinical trial. J Anesth. 28:721–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuenszberg E, Loeckinger A, Kleinsasser A,
Lindner KH, Puehringer F and Hoermann C: Sevoflurane progressively
prolongs the QT interval in unpremedicated female adults. Eur J
Anaesthesiol. 17:662–664. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aggarwal S, Goyal VK, Chaturvedi SK,
Mathur V, Baj B and Kumar A: A comparative study between propofol
and etomidate in patients under general anesthesia. Braz J
Anesthesiol. 66:237–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanaya A, Kuratani N, Satoh D and Kurosawa
S: Lower incidence of emergence agitation in children after
propofol anesthesia compared with sevoflurane: A meta-analysis of
randomized controlled trials. J Anesth. 28:4–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Picard V, Dumont L and Pellegrini M:
Quality of recovery in children: Sevoflurane versus propofol. Acta
Anaesthesiol Scand. 44:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosen HD, Mervitz D and Cravero JP:
Pediatric emergence delirium: Canadian Pediatric Anesthesiologists'
experience. Paediatr Anaesth. 26:207–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dennhardt N, Boethig D, Beck C, Heiderich
S, Boehne M, Leffler A, Schultz B and Sümpelmann R: Optimization of
initial propofol bolus dose for EEG Narcotrend Index-guided
transition from sevoflurane induction to intravenous anesthesia in
children. Paediatr Anaesth. 27:425–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costi D, Ellwood J, Wallace A, Ahmed S,
Waring L and Cyna A: Transition to propofol after sevoflurane
anesthesia to prevent emergence agitation: A randomized controlled
trial. Paediatr Anaesth. 25:517–523. 2015. View Article : Google Scholar : PubMed/NCBI
|