Introduction
There has been an increase in the use of
sialendoscopy for the treatment and diagnosis of obstructive
salivary gland infection (1). In
cases where ductal stenosis has occurred, salivary duct stent
placement appears to be one of the main treatment options (2,3). A
previous study demonstrated that salivary duct stent placement
using hypospadias silastic and pediatric feeding tubes (size 5-Fr)
was successfully achieved and maintained for approximately two
weeks before displacement (4).
Although stenting is routinely used in clinical practice, a common
limitation associated with ductal stents is the relatively short
duration of stent implantation following surgery. Typically ductal
stents are expected to remain in the salivary duct for only 1–2
weeks until they are removed during follow-up visits, or earlier,
if the securing sutures break prematurely (4). Even with a short duration of stent
implantation, complications including iatrogenic stenosis can occur
due to the undesired mechanical contact between the ductal wall and
stent edge (5). Furthermore, there
is no recommended duration of stent implantation for salivary
ductal stenosis. By contrast, intravascular stents are often placed
for months, even when using bioresorbable vascular scaffolds
(6). Beilvert et al (7) demonstrated the use of a new resorbable
starch-based shape-memory salivary duct stent in an animal model,
for the treatment of salivary ducts under sialendoscopic surgery.
However, the resorbable starch-based stents were rapidly hydrolyzed
in simulated saliva, resulting in undesired stent placement.
The aim of the current study was to establish a
methodology for the fabrication of a PLLA salivary duct stent and
to examine its function in a porcine head model.
Materials and methods
PLLA stent fabrication
PLLA (Poly L-lactide; molecular weight, 140 kDa;
glass transition temperature, 60–62°C; Biotech One Inc., Taipei
City, Taiwan). was dissolved in dichloromethane (Burdick &
Jackson™; Honeywell Research Chemicals, NJ, United States) and
stirred for 30 min at 25°C with a final concentration of 15% (w/v).
For stent fabrication, a 15 cm stainless steel rod with a 1.1 mm
outer diameter was immersed in the PLLA solution and then quickly
withdrawn. The PLLA-coated rod was placed on a custom-made rotor at
20 rpm to ensure even distribution of the PLLA solution on the
surface of the rod. The PLLA-coated rods were left to dry overnight
at room temperature. The PLLA tube was easily removed from the
stainless rod and cut into different lengths depending on its
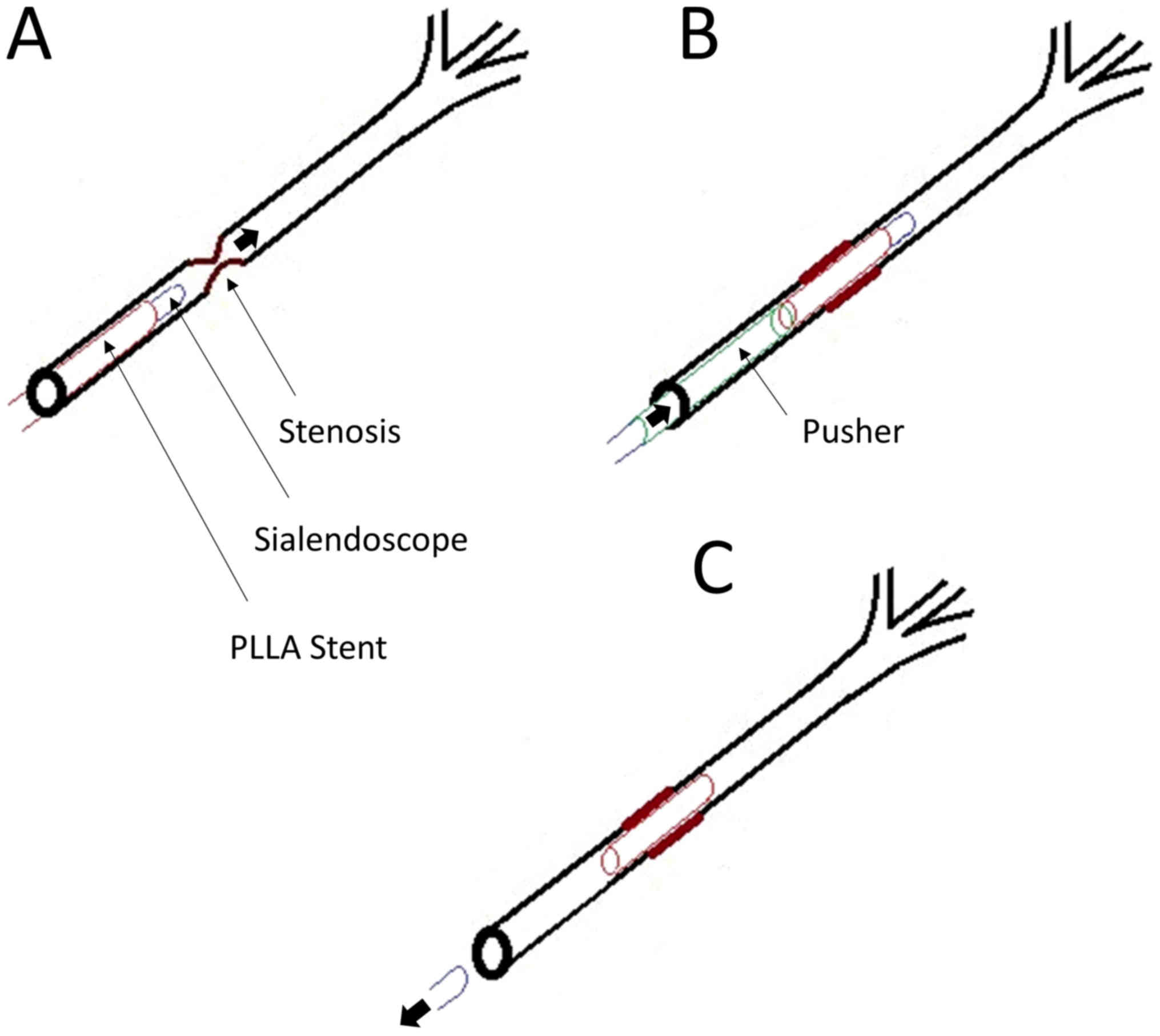
clinical application (Fig. 1A).
Using a sialendoscope, a silicon tube with an inner diameter of 1.2
mm and an outer diameter of 1.4 mm was pre-inserted proximally to
the stent and used as a stent ‘pusher’ during application (Fig. 1B).
Mechanical properties
The fabricated PLLA salivary duct stent was compared
with three commercially available non-biodegradable stents: 14G
needle cannula (B. Braun Melsungen AG, Hessen, Germany), feeding
tube (Symphon Medical Technology Co., Ltd., New Taipei City,
Taiwan; size 5-Fr) and hypospadias silastic tube (Symphon Medical
Technology Co., Ltd.; Fig. 2). The
mechanical characteristics were measured using a universal LFPlus
digital testing machine (Lloyd Instruments; AMETEK, Inc., Berwyn,
PA, USA) to measure stretch and elongation. The length of the
tested stents was set to 4 cm. Between two gripper arms, the tube
axis was aligned with the stretching axis. In addition, the Young's
modulus (GPa) was calculated from the linear slope of a
stress-strain curve for each tested stent. Testing was performed in
triplicate.
Animal model
In this study, the porcine head model was used. The
oral-maxillofacial region in pigs is most similar to humans with
regard to anatomy, development, physiology and pathophysiology
(8). Pig heads were obtained from a
local pork supplier. Following the removal of the upper jaw and
buccal mucosa, the tongue was retracted providing full exposure of
Stensen's and Wharton's ductal papillae.
Sialendoscopy set-up
Sialendoscopy was performed using a semi rigid 0°
Miniature Straight Forward Telescope (11573A; Karl Storz,
Tuttlingen, Germany), outer diameter 1.1 mm, working length 12 cm,
working channel diameter 0.45 mm and irrigation channel diameter
0.25 mm.
Sialendoscopy
Following identification of the papillae, the
stent-sheathed sialendoscope was inserted into the opening of the
salivary gland duct. The scope was directed to the site of stenosis
(Fig. 3A). At the site of stenosis,
the silicon tube stent ‘pusher’ was used to facilitate stent
placement (Fig. 3B). The scope with
the silicon ‘pusher’ attached, was gently withdrawn, leaving the
salivary duct stent in place (Fig.
3C).
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± standard deviation. A one-way
analysis of variance followed by Tukey's post hoc test was used to
analyze the differences in mechanical properties with SPSS v.16
software (SPSS, INC., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Mechanical analysis
The mechanical properties of the PLLA stent,
hypospadias silastic tube, feeding tube (size 5-Fr) and 14 G needle
cannula are shown in Table I. The
elongation stress-strain curves indicate that the PLLA stent is
considerably stiffer than commercially available tubes, resulting
in fewer dynamic shape changes under similar loads (Fig. 4). The tensile strength of the PLLA
stent was similar to that of the commercially available stents. The
Loading (N) for the PLLA stent was lower compared with commercially
available stents. The Young's modulus, which was calculated from
the linear slope of the stress-strain curve, was markedly higher
for the PLLA stent compared with the commercially available stents
(P<0.01; Fig. 5).
 | Table I.Mechanical properties of PLLA and
commercial non-biodegradable stents. |
Table I.
Mechanical properties of PLLA and
commercial non-biodegradable stents.
| Material | Outer diameter
(mm) | Inner diameter
(mm) | Wall thickness
(mm) | Area
(mm2) | Loading (N) | Tensile strength
(MPa) | Young's modulus
(GPa) |
|---|
| PLLA | 1.391 | 1.136 | 0.177 | 0.6755 | 19.5±4.24 | 28.8±6.27 | 40.12 |
| Hypospadias silastic
tube | 2.081 | 1.363 | 0.359 | 1.9421 | 26.2±5.05 | 13.5±2.60 | 0.13 |
| Feeding tube (size
5-Fr) | 2.128 | 1.209 | 0.459 | 2.4081 | 54.9±6.65 | 22.8±2.76 | 1.30 |
| 14G needle
cannula | 1.527 | 1.163 | 0.182 | 0.7678 | 24.0±3.61 | 31.3±4.71 | 15.00 |
Functional evaluation of PLLA salivary
duct stent placement
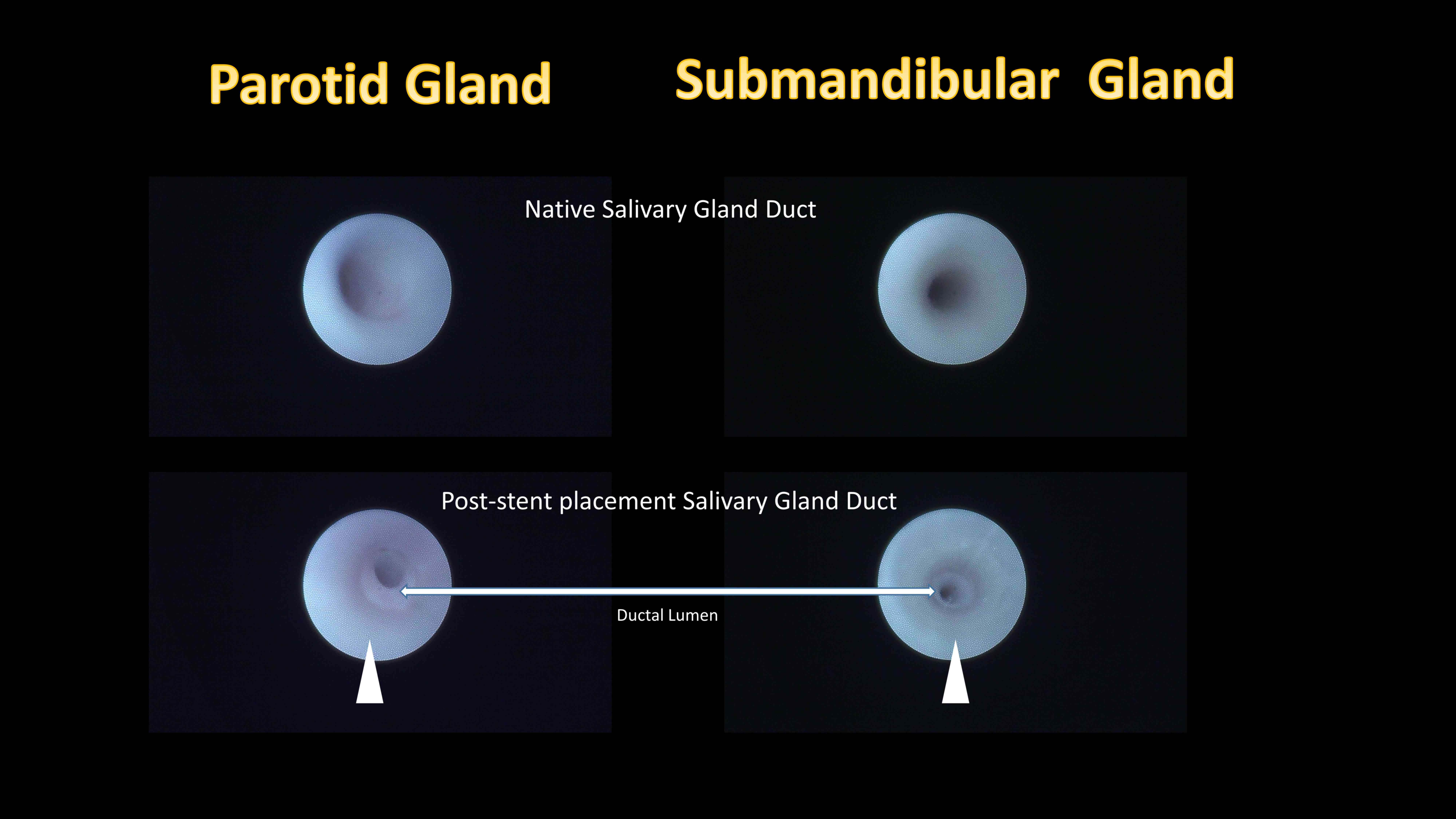
Following identification of the papilla, the main
salivary gland duct could be easily identified. Post-placement
sialendoscopic evaluations revealed that the stent placement inside
the salivary gland ductal lumen was secured in place and allowed
continuous irrigation (Fig. 6, arrow
head).
Discussion
In the current study, fabrication of a PLLA-based
biodegradable salivary duct stent and its functional application
using mechanical tests was established in a porcine head model. The
PLLA stent had a significantly higher Young's modulus compared with
commercially available non-biodegradable stents, which suggest that
it has a greater collapse-resisting strength. In addition, the
current study demonstrated that the PLLA salivary duct stent was
easily used with current sialendoscopy techniques, allowing
accurate stent placement in an animal model with a relatively small
ductal lumen.
Salivary duct stenosis is a well-known condition in
which the duct of a major salivary gland responsible for saliva
flow out of the salivary gland parenchyma is narrowed, leading to a
decreased lumen, which compromises the secretory function of the
gland (9). Previous studies revealed
that salivary duct obstruction can occur as a result of trauma,
calculi, autoimmune disorders, radiation, stenosis or fibro
mucinous plugs, which result in salivary stasis a predisposing
factor to bacterial infection (10,11).
Once a ductal stenosis has formed, it is initially managed through
ductal dilatations using probes or balloons (12). Unsatisfactory dilatation of the
salivary duct stenosis can sometimes result in total gland removal
(13). For the management and
treatment of salivary duct stenosis, new treatment options have
emerged as a result of the introduction of an endoscopic approach
to salivary gland ductal pathologies, known as sialendoscopy.
Since its introduction more than a decade ago by
Professor Francis Marchal, interventional sialendoscopy has become
the predominant therapeutic approach for the management of
obstructive salivary disorders (14,15).
During this time, the procedure has evolved from its initial use
for simple diagnostic observation to the integration of various
treatment modalities. In 2009, Koch et al (16) demonstrated that sialendoscopy-based
diagnosis is a first choice diagnostic method, which enabled the
direct classification of parotid duct stenosis, which provided
additional information and a more effective treatment. Once
stenoses are located, balloon sialoplasty is commonly used to
dilate the stenotic site (15).
Following balloon sialoplasty, surgeons have the option to leave
the site alone or to stent it with a tube for a certain period of
time which prevents the site from collapsing again. In 2012, Lempe
et al (2) demonstrated the
successful use of a stent for the repair of an injured parotid duct
in a thoroughbred colt horse. In addition, Kopeć et al
(17) revealed the use of vascular
radiologic examination catheters as salivary duct stents, whilst
Koch et al (18) reported the
use of polyurethane stents with different diameters (Fr. 4.5–6.0)
based on the size of the scope. A previous study demonstrated the
use of commercially available custom-made stents for sialendoscopy
(19). Furthermore, Kopeć et
al (17) reported the treatment
of 69 stenoses in 51 patients with stent insertion following
stenosis dilatation. The stent was placed for 14–21 days before
removal, and in total seven patients remained partially symptomatic
and four patients had no improvement. A previous study demonstrated
the use of hypospadias silastic tubes and pediatric feeding tubes
(size 5-Fr) as post-sialendoscopy stents (4), which were maintained for approximately
2 weeks. Early dislocation was observed following the premature
breaking of the securing sutures. It would therefore be beneficial
to have a salivary duct stent that could be placed exactly at the
site of stenosis without the need for removal, and which could last
for a longer period of time to allow stabilization at the dilated
stenosis site. Beilvert et al (7) developed a resorbable shape-memory
starch-based stent for the treatment of salivary ducts under
sialendoscopic surgery. Potato starch and high-amylose-content
maize starch (Eurylon 7) were used as materials to fabricate the
stents used in the study. Beilvert et al (7) demonstrated that the stents made from
plasticized starch had the required shape-memory properties to be
used as self-deploying stents, however, the stents were rapidly
hydrolyzed in simulated saliva. In this previous study, starch was
used to fabricate the salivary duct stents as this would allow
salivary amylase to naturally degrade the stent over time, however,
the integrity of the stents was lost during the first 24–48 h of
the study. A previous study demonstrated the use of PLLA
biodegradable stents for the use of resorbable endovascular
stenting (20). A PLLA stent has the
potential to last for a few months or longer, but PLLA degradation
may activate local inflammatory responses (21). However, the normal flow of saliva may
remove any molecules released during PLLA degradation, thereby
reducing the impact to the salivary ductal wall (7).
In the current study, the elongation stress-strain
curves demonstrated that the PLLA stent appears to be stiffer than
the commercially available non-biodegradable tubes, resulting in
fewer dynamic shape changes under similar loads. A harder stent may
increase the degree of discomfort for patients. However, a previous
study revealed that the feeding tube, which is harder than the
hypospadias silastic tube, has a lower rate of irritation and
obstructive complications (4). These
results suggest that the material used for the fabrication of the
salivary duct stent may not need to be soft; it may be that a
harder stent has a greater resistance to the collapsing force.
Furthermore, the diameter of the PLLA stent was the smallest of all
the stents tested. This was designed to fit the outer diameter of
the sialendoscope to allow a smooth installation and passage to the
site of stenosis. The advantage of having a larger diameter stent
would be an increase in the rate of saliva flow and more secure
stent localization after placement. However, a larger diameter
stent increases the difficulty of stent placement. In addition, a
smaller stent may be used for the initial passage through to the
ductal stenosis site, which could be replaced by a larger stent if
required.
This preliminary study has several limitations,
which need to be addressed. The current study used an in
vitro porcine head model; however, the PLLA stent needs to be
examined using an in vivo model. In addition, the
composition of the PLLA stent may require further modification and
should be investigated further. Furthermore, the degradation time
of the PLLA stent was estimated to last for months. Salivary gland
ducts secrete enzymes and the constant flow of saliva may have an
effect on the degradation of the implanted stent. Therefore,
further in vitro analysis examining the degradation rate as
well as other physical properties associated with the stent is
required. Although there is a general consensus that a stent is
used to prevent re-stenosis and for maintaining the opening of the
salivary duct after complete sialendoscopy, there is no recommended
duration of stent implantation for salivary ductal stenosis.
Several studies have demonstrated that the duration of stent
placement is usually a minimum of 2–8 weeks (17–19).
However, with a biodegradable stent the optimal time could be
weeks, months or longer and therefore further investigation is
required.
In conclusion, the current study demonstrated that
the PLLA biodegradable salivary duct stent had a greater resistance
to the collapsing force, compared with commercially available
non-biodegradable stents. In addition, the PLLA stent was easily
used with current sialendoscopy techniques, allowing accurate stent
placement in an animal model with a relatively small ductal
lumen.
Acknowledgements
The authors would like to thank Professor M. Koch
for their assistance with the current study.
Funding
This present study was supported by a grant from the
Taipei Medical University Wan-Fang Hospital Research Fund (grant
no. 105TMU-WFH-17).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD wrote the manuscript and performed the
experiments. HT and CS interpreted the data and performed the
experiments. CT, YW, CH and CL performed the experiments and
recorded the data. SH designed the study, interpreted the data,
supervised the experiments and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maresh A, Kutler DI and Kacker A:
Sialoendoscopy in the diagnosis and management of obstructive
sialadenitis. Laryngoscope. 121:495–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lempe A, Brehm W and Scharner D: Stent
reconstruction of an injured parotid duct in a thoroughbred colt.
Vet Surg. 41:536–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eckel HE and Bootz F: Preventive salivary
stent use. Laryngorhinootologie. 92:374–375. 2013.(In German).
PubMed/NCBI
|
|
4
|
Su CH, Lee KS, Tseng TM and Hung SH:
Post-sialendoscopy ductoplasty by salivary duct stent placements.
Eur Arch Otorhinolaryngol. 273:189–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang CH, Hung SH and Su CH: Reply: To
PMID 25216563. J Oral Maxillofac Surg. 73:799–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park SJ, Park DW, Kim YH, Kang SJ, Lee SW,
Lee CW, Han KH, Park SW, Yun SC, Lee SG, et al: Duration of dual
antiplatelet therapy after implantation of drug-eluting stents. N
Engl J Med. 362:1374–1382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beilvert A, Faure F, Meddahi-Pellé A,
Chaunier L, Guilois S, Chaubet F, Lourdin D and Bizeau A: A
resorbable shape-memory starch-based stent for the treatment of
salivary ducts under sialendoscopic surgery. Laryngoscope.
124:875–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Liu Y, Fang D and Shi S: The
miniature pig: A useful large animal model for dental and orofacial
research. Oral Dis. 13:530–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchal F, Chossegros C, Faure F, Delas B,
Bizeau A, Mortensen B, Schaitkin B, Buchwald C, Cenjor C, Yu C, et
al: Salivary stones and stenosis. A comprehensive classification.
Rev Stomatol Chir Maxillofac. 109:233–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Epker BN: Obstructive and inflammatory
diseases of the major salivary glands. Oral Surg Oral Med Oral
Pathol. 33:2–27. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ngu R, Brown J, Whaites E, Drage N, Ng S
and Makdissi J: Salivary duct strictures: nature and incidence in
benign salivary obstruction. Dentomaxillofacial Radiology.
36:63–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown AL, Shepherd D and Buckenham TM: Per
oral balloon sialoplasty: Results in the treatment of salivary duct
stenosis. Cardiovasc Intervent Radiol. 20:337–342. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nahlieli O, Shacham R, Yoffe B and Eliav
E: Diagnosis and treatment of strictures and kinks in salivary
gland ducts. J Oral Maxillofac Surg. 59:484–492. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchal F, Dulguerov P and Lehmann W:
Interventional sialendoscopy. N Engl J Med. 341:1242–1243. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marchal F, Becker M, Dulguerov P and
Lehmann W: Interventional sialendoscopy. Laryngoscope. 110:318–320.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koch M, Iro H and Zenk J:
Sialendoscopy-based diagnosis and classification of parotid duct
stenoses. Laryngoscope. 119:1696–1703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kopeć T, Szyfter W, Wierzbicka M and
Nealis J: Stenoses of the salivary ducts-sialendoscopy based
diagnosis and treatment. Br J Oral Maxillofac Surg. 51:e174–e177.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koch M, Iro H, Klintworth N, Psychogios G
and Zenk J: Results of minimally invasive gland-preserving
treatment in different types of parotid duct stenosis. Arch
Otolaryngol Head Neck Surg. 138:804–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strychowsky JE, Sommer DD, Gupta MK, Cohen
N and Nahlieli O: Sialendoscopy for the management of obstructive
salivary gland disease: A systematic review and meta-analysis. Arch
Otolaryngol Head Neck Surg. 138:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamai H, Igaki K, Kyo E, Kosuga K,
Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S and Uehata H:
Initial and 6-month results of biodegradable poly-l-lactic acid
coronary stents in humans. Circulation. 102:399–404. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferdous J, Kolachalama VB and Shazly T:
Impact of polymer structure and composition on fully resorbable
endovascular scaffold performance. Acta Biomater. 9:6052–6061.
2013. View Article : Google Scholar : PubMed/NCBI
|