Introduction
Blunt chest trauma and hemorrhagic shock (T/HS)
occurs in patients with poly-trauma (possibly following vehicular
accidents) and is a leading risk factor in the development of acute
lung injury (ALI) and acute respiratory distress syndrome (ARDS)
(1,2). T/HS exhibits high morbidity and
mortality rates (3,4). Furthermore, it is well known that
apoptosis is involved in distinct types of ALI. Experimentally,
Messer et al (5) and Thakkar
et al (6) demonstrated that
the activation of apoptosis via the Fas/Fas ligand (FasL) signaling
pathway was important for the development of ALI caused by trauma
or hemorrhagic shock. Clinically, Glavan et al (7) revealed that the content of soluble Fas
and FasL was increased in pulmonary edema fluid, which was strongly
associated with increased morbidity and mortality in patients with
ALI. Additionally, Herrero et al (8) determined that the inhibition of the
Fas/FasL signaling pathway alleviated ALI/ARDS damage.
Penehyclidine hydrochloride (PHC) is a novel
anti-cholinergic drug that was first developed by the Beijing
Institute of Pharmacology and Toxicology, Academy of Military
Medical Sciences (Beijing, China), which can be applied in
anti-apoptosis and anti-inflammation treatment (9,10).
Recently, an increasing number of studies have indicated that PHC
may alleviate lung injuries by inhibiting ALI-induced apoptosis and
inflammation (11–13). Furthermore, Wang et al
(14) revealed that PHC mitigates
lung injury by regulating the expression of Bcl-2-associated X
(bax) and B-cell lymphoma-2 (Bcl-2) in a rat model of ALI following
blunt chest trauma. Cui et al (15) also demonstrated that PHC
pre-treatment reduced the expression of bax and caspase-3,
decreased the indices of apoptosis and pulmonary vascular
resistance, and improved PaO2/FiO2 and
Bcl-2/bax ratios in pigs with dichlorvos-induced ALI. However, the
underlying mechanism of PHC in ALI requires further elucidation.
The current study aimed to determine the effect of PHC on ALI as
well as associated signaling pathways.
Materials and methods
Animals
A total of 40 Male Sprague-Dawley rats (age, 8–10
weeks) with a body weight of 245–275 g were obtained from the Hunan
Institute for Biologic Sciences (Hunan, China; certificate no. SCXX
2009-0004) under specific pathogen-free conditions. The current
study was approved by Medical Ethics Committee of Renmin Hospital
of Wuhan University (Wuhan, China) and was performed in accordance
with the National Institutes of Health Guidelines for the Care and
Use of Laboratory Animals. Animals were housed under a 12 h
light/dark cycle at a temperature of 22°C and a humidity of 50–60%
with free access to food and water.
T/HS model
Rats were anesthetized with intraperitoneal (IP)
injections of sodium pentobarbital (30 mg/kg). To establish a rat
model of blunt chest trauma, the current study generated an
isolated bilateral lung contusion as described previously (16). Following anesthesia induction, a
hollow cylinder (weight, 300 g) was dropped from a defined height
(83.3 cm). The cylinder was encased in a vertical stainless steel
tube, which was positioned on a platform. The precordial shield
directed the impact force bilaterally to the lungs to prevent
cardiac trauma (impact energy, 2.45 J). This experiment was
performed by our laboratory according to a previous study by
Raghavendran et al (17). A
rat model of nonlethal hemorrhagic shock was then established based
on a previous study (12). The
femoral artery and vein were cannulated with polyethylene (PE-50)
tubing, and blood flowed via tubing, which was attached to the
monitor (IntelliVue MP40; Phillips Medical Systems B.V., Eindhoven,
The Netherlands), until the average arterial blood pressure reached
35±5 mm Hg. This pressure was then maintained for 60 min. Following
a hypotensive period of 60 min, rats were resuscitated via the
transfusion of removed blood with Ringer's lactate solution [Baxter
Healthcare (Tanjin) Company, Ltd., Tianjin, China; Composition, 6.0
g/l Na+, 6.0; 0.3 g/l K+; 0.2 g/l
Ca2+, 2H2O and Cl] twice over a period of 60
min. ALI was defined as a PaO2/FiO2 of
<300 mm Hg.
Experimental protocols
A total of 40 rats were randomly divided into 4
equal groups (each, n=10): A sham group, a T/HS group, a PHC1 group
and a PHC2 group. According to previous studies (12,16),
rats of the PHC1 group were infused with 2 mg/kg PHC 30 min prior
to blunt chest trauma. PHC2 group rats were infused with 2 mg/kg
PHC 60 min following hemorrhagic shock. The Sham and T/HS groups
received the same volume (0.5 ml) of 0.9% normal saline solution.
Prior to blunt chest trauma, rats were anesthetized and the femoral
artery and vein were cannulated with polyethylene (PE-50) tubing
for continuous invasive pressure monitoring and to establish venous
access. Rats in the sham group were subjected to the same
experimental procedures, including the cannulation of femoral
artery and vein, but without T/HS. All animals were sacrificed
under deep anesthesia (50 mg/kg IP pentobarbital) and exsanguinated
from the right carotid artery 6 h following T/HS. Blood and lung
samples were then collected.
Arterial blood gas analysis
Following the induction of T/HS for 6 h, rats were
anesthetized with an IP injection of pentobarbital (50 mg/kg) and
arterial blood was obtained from the right carotid artery (1 ml
each). Samples were immediately analyzed using a blood gas analyzer
(Abbott Point of Care Inc., Princeton, NJ, USA) for the
determination of PaO2 and
PaO2/FiO2.
Polymorphonuclear neutrophils (PMNs)
and protein in BALF
Following rat sacrifice, the trachea was cannulated
and lavaged. Bronchoalveolar lavage fluid (BALF) was prepared by
washing the lungs three times with 2 ml PBS. BALF was centrifuged
at 400 × g for 10 min at 4°C to pellet cells. BALF supernatant was
then used for protein analysis. The cell pellet was resuspended in
PBS and then the BALF cell counts were performed following trypan
blue exclusion. An aliquot of pooled BALF (50 µl) from each rat was
diluted 1:1 with trypan blue dye (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the total number of cells was counted using a
standard hemocytometer. To analyze differential cell counts, 100 µl
of BALF from each rat was centrifuged 1,000 × g for 10 min at 4°C
using a Cytospin (Thermo Fisher Scientific, Inc.). After slides
were dried, cells were fixed with 3% glutaraldehyde in PBS for 15
min at room temperature, and stained using Wright Stain solution
(cat. no. 32857; Sigma-Aldrich, USA; incubated for 30 sec at room
temperature) according to the manufacturer's instructions, and then
differential cell counts were obtained by manually counting 200
cells per rat as previously described (18). BALF protein content was determined
via a bicinchoninic acid (BCA) assay and absorbance was measured at
595 nm.
Hematoxylin and eosin (H&E)
staining and acute lung injury score
Following animal sacrifice through bleeding from the
right carotid artery at 6 h following T/HS, lung tissue samples
were harvested immediately. Right middle-lung specimens were fixed
using 10% formalin at room temperature for 24 h, sectioned (5 µm)
and stained with H&E (hematoxylin staining for 10 min and eosin
staining for 2 min; each at room temperature). Sections were
observed under a light microscope with a magnification of ×100.
Sections were then evaluated and graded for the presence of
interstitial neutrophillic infiltrate, intra-alveolar hemorrhage
and pulmonary edema with a light microscope (BX51; Olympus
Corporation, Tokyo, Japan).
Western blotting
Western blot analysis was performed to determine
Fas, FasL, caspase-3 and caspase-8 protein levels in rat lung
tissues. Lung tissues were homogenized and lysed in lysis buffer
[25 mM Tris-HCl (pH 7.6), 1% NP-40, 0.5% sodium deoxycholate and
0.1% sodium dodecyl sulfate). Protein concentration was determined
using a BCA protein assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and equal quantities (50 µg) of protein were
loaded per lane on a 14% SDS-PAGE gel. Samples were separated
electrophoretically and then transferred to polyvinylidene
difluoride membranes. Subsequently, membranes were blocked with 5%
skimmed milk (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
Tris-buffered saline containing 0.1% Tween-20 (TBS-T) at room
temperature for 2 h on a rotary shaker (60 rpm). Samples were then
incubated with the following primary antibodies overnight at 4°C:
Rabbit anti-Fas (1:1,000; cat. no. sc-21730; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-FasL (1:1,000; cat. no.
sc-834; Santa Cruz Biotechnology, Inc.), anti-caspase-3 (1:500;
cat. no. 9662), anti-caspase-8 (1:500; cat. no. 9662; both Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-β-actin
(1:1,000; cat. no. ab8227; Abcam, Cambridge, UK). Subsequently,
membranes were washed with TBS-T three times and incubated with
peroxidase-conjugated secondary antibodies (1:2,000; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Membranes were then washed three times with TBST
solution and the blotted protein bands were observed using enhanced
chemiluminescence (Amersham; GE Healthcare Life Sciences, Little
Chalfont, UK) and exposed to Kodak X-ray film (Kodak Biomax; cat.
no. Z350400, Sigma-Aldrich; Merck KGaA). Proteins bands were
quantified using Quantity One software (Bio-Rad, Hercules, CA,
USA).
Immunohistochemistry
The expression of Fas and FasL were determined via
immunohistochemistry. Lung tissues were fixed in 4%
paraformaldehyde for 24 h at 4°C, dehydrated, embedded in paraffin
and subsequently cut into 5 mm slices. Sections were incubated with
rabbit polyclonal anti-Fas (1:100; cat. no. sc-21730) and FasL
(1:100; cat. no. sc-834; both Santa Cruz Biotechnology, Inc.)
antibodies at 4°C for 15 h. Samples were then incubated with
horseradish peroxidase conjugated goat anti-rabbit immunoglobulin G
(1:250; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 30
min at room temperature and diluted in blocking solution (5% bovine
serum albumin; cat. no. B2064; Sigma-Aldrich; Merck KGaA) for 30
min at room temperature. Biotin-peroxide and diaminobenzidine were
used as substrates for the color reaction. The mean optical density
of Fas and FasL positive cells from each section were analyzed
using image cytometry with HIPAS-2000 image analysis software
(Wuhan Qianli Technical Imaging Co. Ltd., Wuhan, China). The number
of positive microvessels in each section was counted in 10
microscopic fields (at magnification, ×400) under a light
microscope (BX51; Olympus Corporation, Tokyo, Japan). The
specificity of immunohistochemical staining was tested using PBS at
the same dilution. Tissue sections in the sham group were used as
negative controls.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
Apoptotic cells were stained via the TUNEL technique
using an apoptosis detection kit (cat. no. APOAF-50TST;
Sigma-Aldrich; Merck KGaA) following the manufacturer's protocol.
The sections were added onto a coverslip with DPX mounting medium
(cat. no. 06522; Sigma-Aldrich; Merck KGaA). The total number of
cells and the number of positive cells were counted in two sections
from each animal (at magnification, ×400) in at least 10 fields of
view in each section. The apoptosis index (AI) was calculated using
the following formula: AI (%)=number of apoptotic cells/number of
total cells ×100%.
Cytokine measurement
The concentration of tumor necrosis factor-α (TNF-α;
cat. no. RK00027), interleukin (IL)-6 (cat. no. RK00008) and IL-1β
(cat. no. RK00006) in lung tissue homogenate were measured using
commercially available ELISA kits obtained from Abclonal Biotech
Co., Ltd., Woburn, MA, USA) according to the manufacturer's
protocol. The absorbance of each well was detected at 450 nm with a
microplate reader. Each average value represents the values of
triplicate experiments.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
utilized to perform statistical analysis. Statistical comparisons
between multiple groups were performed using one-way analysis of
variance followed by a bonferroni post-hoc. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of PHC on the PaO2
and PaO2/FiO2 of T/HS rats
As an evaluation index of gas exchange,
PaO2/FiO2 was measured to estimate the degree
of lung injury. Significant decreases in PaO2 (Fig. 1A) and the
PaO2/FiO2 ratio (Fig. 1B) were observed in T/HS rats compared
with the sham group. However, when compared with the T/HS group,
pre-treatment or treatment with PHC efficiently increased the
PaO2 and PaO2/FiO2 ratios in ALI
rats (P<0.05).
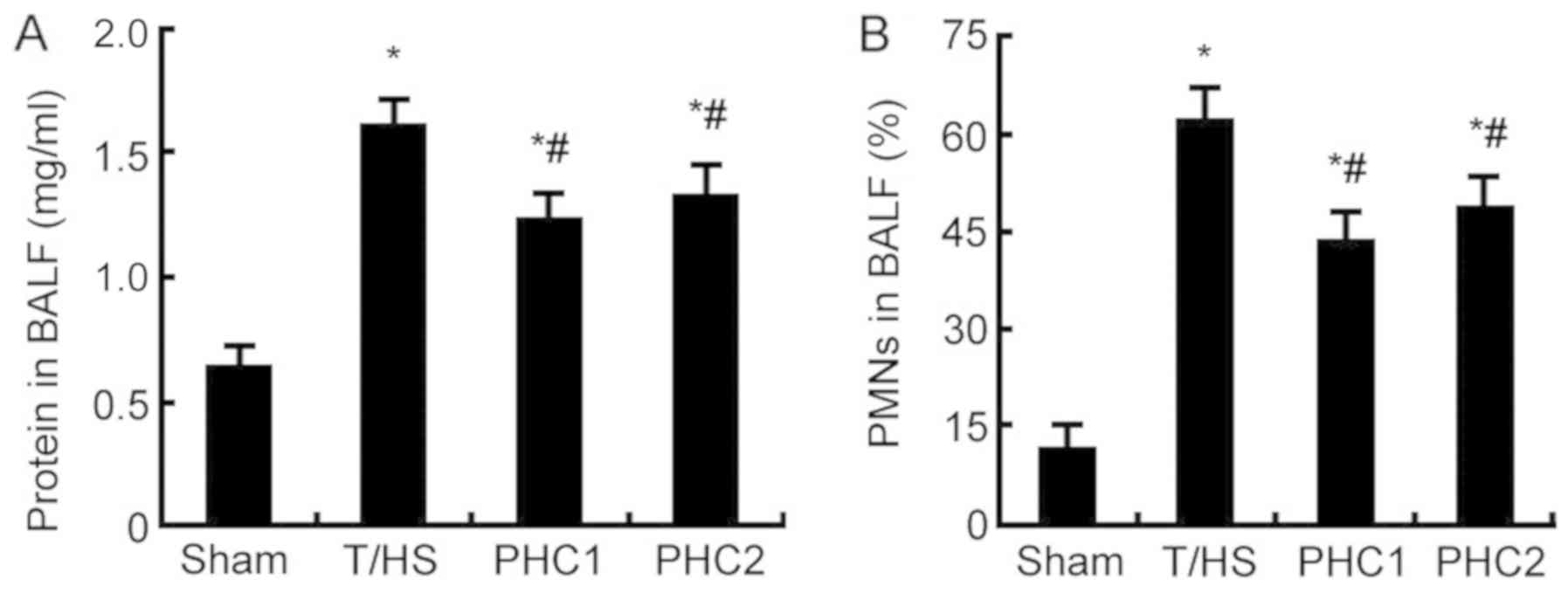
Effect of PHC on PMNs and BALF protein
concentration in T/HS rats
PMNs are the primary inflammatory cells present in
many diseases of the lung, including ALI/ARDS (19). Furthermore, the concentration of
protein in BALF is an indicator commonly used to detect pulmonary
vascular permeability, which is an important characteristic of
ALI/ARDS (20). When compared with
the sham group, the concentration of protein (Fig. 2A) and the number of PMNs (Fig. 2B) in BALF were significantly
increased 6 h following T/HS. However, PHC treatment prior to or
following T/HS significantly reduced PMN infiltration and protein
concentration compared with the T/HS group.
Effect of PHC on the histopathological
changes observed in T/HS rats
To assess the effect of PHC on the histopathological
changes observed in ALI rats, lung tissues from each group were
subjected to H&E staining. As presented in Fig. 3, the sham group exhibited normal
pulmonary histology with intact structures and clear pulmonary
alveoli. By contrast, the tissue of the T/HS group exhibited
serious damage, including severe hemorrhage and congestion,
alveolar wall thickening, alveolar collapse and inflammatory cell
infiltration into alveoli. However, the administration of PHC
ameliorated pulmonary histopathological changes in ALI rats.
 | Figure 3.Effect of PHC on the lung
histopathological changes observed in ALI rats. Hematoxylin and
eosin staining revealed that severe hemorrhage and congestion,
thickening of the alveolar wall, alveolar collapse and infiltration
of alveoli with inflammatory cells was observed in the lung tissues
of T/HS, PHC1 and PHC2 treated rats, compared with the sham group.
However, compared with the T/HS group, lung injury was
significantly ameliorated in the PHC1 and PHC2 groups. Original
magnification, ×100. PHC, penehyclidine hydrochloride; ALI, acute
lung injury; T/HS, blunt chest trauma and hemorrhagic shock. |
Effect of PHC on the expression of
Fas, FasL, caspase-3 and caspase-8
Western blotting was performed to detect the level
of Fas, FasL, caspase-3 and caspase-8 proteins. The results
revealed that, in response to T/HS, the protein expression of Fas,
FasL, caspase-3 and caspase-8 were significantly increased; while
PHC treatment administered prior to or following T/HS induction
significantly inhibited this increase (Fig. 4).
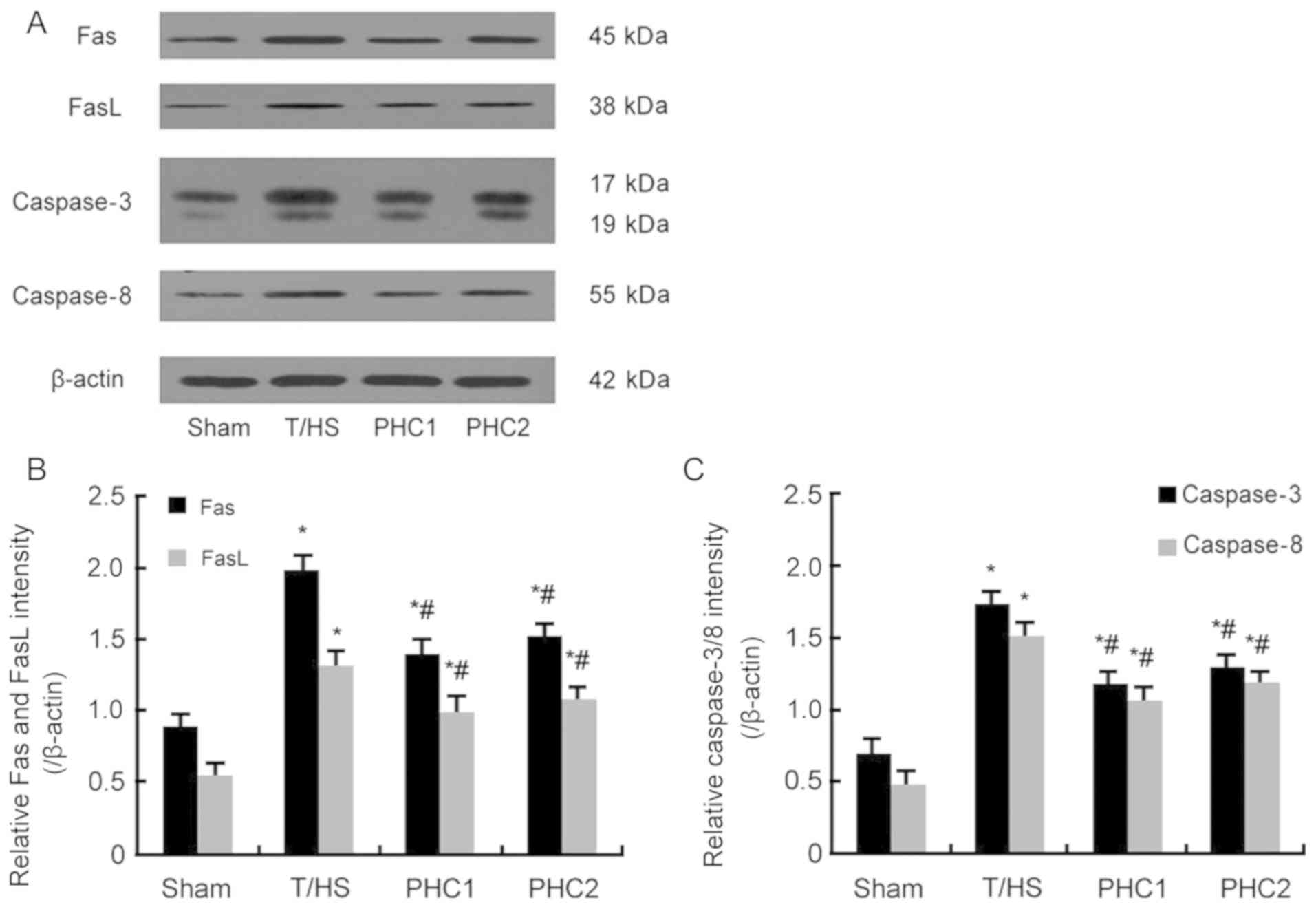 | Figure 4.Effect of PHC on the expression of
Fas, FasL, caspase-3 and caspase-8. (A) Western blotting was
performed to assess protein levels of Fas, FasL, caspase-3 and
caspase-8. (B) Statistical analysis of (B) Fas and FasL, and (C)
caspase-3 and caspase-8. Increased levels of all proteins were
observed in the lung tissue of T/HS, PHC1 and PHC2 treated rats
when compared with the Sham group. However, protein levels were
decreased in the PHC1 and PHC2 groups compared with the T/HS group.
Data are expressed as mean ± standard error of the mean. *P<0.05
vs. the Sham group; #P<0.05 vs. the T/HS group. PHC,
penehyclidine hydrochloride; FasL, Fas ligand; T/HS, blunt chest
trauma and hemorrhagic shock. |
Effect of PHC on Fas and FasL protein
expression in ALI lung tissue
Immunohistochemistry assays were performed to detect
the protein expression of Fas and FasL. The results revealed that,
when compared with the sham group, T/HS induction markedly
increased the protein levels of Fas (Fig. 5) and FasL (Fig. 6) in the alveolar area of the lung.
Furthermore, the administration of PHC markedly reduced Fas and
FasL levels compared with the T/HS group.
Effect of PHC on the degree of
apoptosis in ALI rat lung tissue
As presented in Fig.
7, a small number of apoptotic cells were observed in the sham
group (Fig. 7A), but apoptotic cells
were significantly increased following T/HS induction (Fig. 7B, C and D). Compared with the T/HS
group, the number of apoptotic cells in the PHC1 and PHC2 groups
was decreased (Fig. 7C and D).
Concomitantly, the apoptosis index of T/HS rat lungs was
significantly increased compared with the sham group and the
apoptosis index was statistically decreased following the
administration of PHC (Fig. 7E).
Effect of PHC on TNF-α, IL-6 and IL-1β
levels
Lung tissues were collected 6 h following T/HS
induction and the levels of cytokines within were measured using
ELISA. The results demonstrated that the levels of TNF-α, IL-1β and
IL-6 were significantly increased in T/HS rats compared with the
sham group (Fig. 8). However, PHC
treatment prior to or following T/HS induction significantly
reduced the levels of TNF-α, IL-1β and IL-6 compared with the T/HS
group.
 | Figure 8.Effect of PHC on TNF-α, IL-6 and
IL-1β levels in ALI rat lung tissue. Compared with the sham group,
the levels of TNF-α, IL-1β and IL-6 in lung tissues were
significantly increased in the T/HS, PHC1 and PHC2 groups. Compared
with the T/HS group, the levels of thee cytokines were
significantly decreased in the PHC1 and PHC2 groups. Data are
expressed as mean ± standard error of the mean. *P<0.05 vs. the
Sham group; #P<0.05 vs. the T/HS group. PHC,
penehyclidine hydrochloride; TNF-α, tumor necrosis factor-α; IL,
interleukin; ALI, acute lung injury; T/HS, blunt chest trauma and
hemorrhagic shock. |
Discussion
ALI and ARDS induce a continuum of lung changes
arising from a wide variety of lung injuries, causing high
morbidity and mortality rates in intensive care units (21). An increasing number of studies have
demonstrated the critical role of Fas/FasL activation in pulmonary
epithelial cells (22,23). However, little is known about the
exact mechanism that underlies this. The present study established
a novel double-hit rat model of ALI (blunt trauma followed by
hemorrhage shock), induced by T/HS, and determined the effect of
PHC on the Fas/FasL signaling pathway by assessing pulmonary
apoptosis, inflammation and lung damage. The results indicated that
the T/HS-constructed ALI rat model significantly increased arterial
hypoxemia, alveolar edema, leucocytosis in the interstitial
capillaries and alveolar hemorrhage in histological assessments,
which is consistent with a previous study (12). Additionally, it was revealed that
PaO2/FiO2 in T/HS rats was <300.
It has been demonstrated that a multitude of
pathways are involved in the regulation of apoptosis, including
Fas/FasL (24). Gil et al
(25) indicated that the Fas
signaling pathway was associated with ALI severity in mice. In the
present study, to determine the involvement of the Fas/FasL
signaling pathway in the mediation of lung tissue cell apoptosis,
the lungs of rats with ALI induced by T/HS were assessed via TUNEL
staining 6 h following T/HS. The results demonstrated that
following T/HS challenge, the expression of Fas, FasL caspase-3 and
caspase-8 was significantly increased, and the BALF PMN count and
protein concentration were markedly increased. The current study
therefore hypothesized that Fas/FasL activation resulted in an
increase of proinflammatory cytokines (TNF-α, IL-6 and IL-1β) and
apoptotic cells in the lungs during T/HS. In this regard, the
activation of the Fas/FasL signaling pathway may not only induce
apoptosis, but may also lead to the production and secretion of
cytokines. Consistent with the findings of Weckbach et al
(26), the combination of T/HS
induced lung cell apoptosis, lung inflammation, subsequent PMN
recruitment and disruption of the alveolocapillary barrier.
Additionally, Serrao et al (27) demonstrated that PMNs induce the
apoptosis of lung epithelial cells by upregulating FasL. Perl et
al (28) also indicated that in
the absence of PMNs, blunt chest trauma-induced ALI was mitigated.
Therefore, lung cell apoptosis and lung inflammation have been
identified as pathophysiologically relevant mechanisms in the
development of ALI.
Multiple findings have revealed that PHC is
effective in the treatment of ALI (29,30).
Additional studies further indicate that PHC selectively blocks
muscarinic acetylcholine (M) receptor M1, M3 and nicotinic
acetylcholine receptors, with fewer M2 receptor-associated
cardiovascular side effects than hyoscyamine (31). An increasing number of studies have
indicated that PHC exhibits anti-apoptotic, anti-inflammatory and
anti-oxidative stress effects under organ dysfunction (16,32,33). A
previous study demonstrated that PHC exerted anti-inflammatory
properties and protective effects during ALI via the inhibition of
the toll-like receptor 4 signaling pathway (34). Furthermore, Cao et al
(35) confirmed that PHC alleviated
cerebral injury by inhibiting the p38 mitogen-activated protein
kinase (MAPK) and caspase-3. Wang et al (36) also revealed that the administration
of PHC in renal ischemia-reperfusion injured rats decreased the
level of malondialdehyde and the expression of p38 MAPK, nuclear
factor-κB and caspase-3 expression, and attenuated the reduction in
superoxide dismutase activity. Wu et al (34) clarified that PHC significantly
increased PaO2, pH, PaO2/FiO2 and
PaCO2, reduced IL-6 and IL-1β levels, and reduced
pulmonary myeloperoxidase activity in an ALI model induced by
lipopolysaccharide.
The results of the current study revealed that PHC
treatment prior to or following T/HS induction attenuated lung
damage by significantly improving pulmonary oxygenation and by
decreasing BALF PMN count and protein concentration. Furthermore,
the Fas/FasL signaling pathway, levels of cell apoptosis and the
expression of proinflammatory cytokines, including TNF-α, IL-6 and
IL-1β, were inhibited following PHC treatment. Whether PHC improves
the overall survival of rats with ALI was not assessed in the
present study and as such, should be a subject of future
experiments. Additionally, the exact mechanism of how PHC
ameliorates ALI remains poorly understood, which warrants future
investigation.
In conclusion, the present study indicates that the
Fas/FasL signaling pathway may serve a pivotal role in the
pathogenesis of lung injury following T/HS. PHC may also serve a
protective role in ALI by inhibiting the Fas/FasL signaling
pathway. Therefore, PHC may be a potential agent for future
treatment of inflammatory diseases, including ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81571941) and the
Natural Science Foundation of Hubei Province of China (grant no.
2016CFB251).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and XS designed the experiments. XW, QK and WD
performed the experiments. WD and LZ analyzed experimental results.
XW, QK and XS wrote the manuscript and critically revised it for
important intellectual content. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval was provided by the Medical Ethics
Committee of Renmin Hospital of Wuhan University. All surgical
procedures were performed in accordance with Wuhan University
Animal Care and Use Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
PHC
|
penehyclidine hydrochloride
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
T/HS
|
blunt chest trauma and hemorrhagic
shock
|
References
|
1
|
Liu Y, Du DY, Hu X, Xia DK, Xiang XY,
Huang C, Zhou JH and Jiang JX: Prevalence and mortality of severe
chest trauma in three gorges area of China. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 34:567–572. 2012.(In Chinese). PubMed/NCBI
|
|
2
|
Erickson SE, Martin GS, Davis JL, Matthay
MA and Eisner MD; NIH NHLBI ARDS Network, : Recent trends in acute
lung injury mortality: 1996–2005. Crit Care Med. 37:1574–1579.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallagher JJ: Management of blunt
pulmonary injury. AACN Adv Crit Care. 25:375–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown LM, Kallet RH, Matthay MA and Dicker
RA: The influence of race on the development of acute lung injury
in trauma patients. Am J Surg. 201:486–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Messer MP, Kellermann P, Weber SJ, Hohmann
C, Denk S, Klohs B, Schultze A, Braumüller S, Huber-Lang MS and
Perl M: Silencing of fas, fas-associated via death domain, or
caspase 3 differentially affects lung inflammation, apoptosis, and
development of trauma-induced septic acute lung injury. Shock.
39:19–27. 2013.PubMed/NCBI
|
|
6
|
Thakkar RK, Chung CS, Chen Y, Monaghan SF,
Lomas-Neira J, Heffernan DS, Cioffi WG and Ayala A: Local tissue
expression of the cell death ligand, fas ligand, plays a central
role in the development of extrapulmonary acute lung injury. Shock.
36:138–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glavan BJ, Holden TD, Goss CH, Black RA,
Neff MJ, Nathens AB, Martin TR and Wurfel MM; ARDSnet
Investigators, : Genetic variation in the FAS gene and associations
with acute lung injury. Am J Respir Crit Care Med. 183:356–363.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herrero R, Tanino M, Smith LS, Kajikawa O,
Wong VA, Mongovin S, Matute-Bello G and Martin TR: The Fas/FasL
pathway impairs the alveolar fluid clearance in mouse lungs. Am J
Physiol Lung Cell Mol Physiol. 305:L377–L388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Lin D, Zhang L, Liu W, Tan H and
Ma J: Penehyclidine hydrochloride prevents anoxia/reoxygenation
injury and induces H9c2 cardiomyocyte apoptosis via a mitochondrial
pathway. Eur J Pharmacol. 797:115–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Zhao L and Ma J: Penehyclidine
hydrochloride preconditioning provides cardiac protection in a rat
model of myocardial ischemia/reperfusion injury via the mechanism
of mitochondrial dynamics mechanism. Eur J Pharmacol. 813:130–139.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu R, Zhao Y, Li X, Bai T, Wang S, Wang W
and Sun Y: Effects of penehyclidine hydrochloride on severe acute
pancreatitis-associated acute lung injury in rats. Biomed
Pharmacother. 97:1689–1693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu XJ, Liu HM, Song XM, Zhao B, Leng Y,
Wang EY, Zhan LY, Meng QT and Xia ZY: Penehyclidine hydrochloride
inhibits TLR4 signaling and inflammation, and attenuates blunt
chest trauma and hemorrhagic shock-induced acute lung injury in
rats. Mol Med Rep. 17:6327–6336. 2018.PubMed/NCBI
|
|
13
|
Zheng F, Xiao F, Yuan QH, Liu QS, Zhang
ZZ, Wang YL and Zhan J: Penehyclidine hydrochloride decreases
pulmonary microvascular endothelial inflammatory injury through a
beta-arrestin-1-dependent mechanism. Inflammation. 41:1610–1620.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang LL, Zhan LY, Wu XJ and Xia ZY:
Effects of penehyclidine hydrochloride on apoptosis of lung tissues
in rats with traumatic acute lung injury. Chin J Traumatol.
13:15–19. 2010.PubMed/NCBI
|
|
15
|
Cui J, Li CS, He XH and Song YG:
Protective effects of penehyclidine hydrochloride on acute lung
injury caused by severe dichlorvos poisoning in swine. Chin Med J
(Engl). 126:4764–4770. 2013.PubMed/NCBI
|
|
16
|
Wu XJ, Xia ZY, Wang LL, Luo T, Zhan LY,
Meng QT and Song XM: Effects of penehyclidine hydrochloride on
pulmonary contusion from blunt chest trauma in rats. Injury.
43:232–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raghavendran K, Davidson BA, Helinski JD,
Marschke CJ, Manderscheid P, Woytash JA, Notter RH and Knight PR: A
rat model for isolated bilateral lung contusion from blunt chest
trauma. Anesth Analg. 101:1482–1489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Song X, Li N, Zhan L, Meng Q and Xia
Z: Protective effects of dexmedetomidine on blunt chest
trauma-induced pulmonary contusion in rats. J Trauma Acute Care
Surg. 74:524–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kellner M, Noonepalle S, Lu Q, Srivastava
A, Zemskov E and Black SM: ROS signaling in the pathogenesis of
Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome
(ARDS). Adv Exp Med Biol. 967:105–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu DQ, Wu HB, Zhang M and Wang JA: Effects
of zinc finger protein A20 on Lipopolysaccharide (LPS)-induced
pulmonary inflammation/anti-inflammatory mediators in an acute lung
injury/acute respiratory distress syndrome rat model. Med Sci
Monit. 23:3536–3545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agarwal R, Handa A, Aggarwal AN, Gupta D
and Behera D: Outcomes of noninvasive ventilation in acute
hypoxemic respiratory failure in a respiratory intensive care unit
in north India. Respir Care. 54:1679–1887. 2009.PubMed/NCBI
|
|
22
|
Han F, Luo Y, Li Y, Liu Z, Xu D, Jin F and
Li Z: Seawater induces apoptosis in alveolar epithelial cells via
the Fas/FasL-mediated pathway. Respir Physiol Neurobiol. 182:71–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seitz DH, Palmer A, Niesler U, Braumüller
ST, Bauknecht S, Gebhard F and Knöferl MW: Altered expression of
Fas receptor on alveolar macrophages and inflammatory effects of
soluble Fas ligand following blunt chest trauma. Shock. 35:610–617.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobata T, Takasaki K, Asahara H, Hong NM,
Masuko-Hongo K, Kato T, Hirose S, Shirai T, Kayagaki N, Yagita H,
et al: Apoptosis with FasL+ cell infiltration in the periphery and
thymus of corrected autoimmune mice. Immunology. 92:206–213. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gil S, Farnand AW, Altemeier WA, Gill SE,
Kurdowska A, Krupa A, Florence JM and Matute-Bello G: Fas-deficient
mice have impaired alveolar neutrophil recruitment and decreased
expression of anti-KC autoantibody: KC complexes in a model of
acute lung injury. Respir Res. 13:912012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weckbach S, Hohmann C, Braumueller S, Denk
S, Klohs B, Stahel PF, Gebhard F, Huber-Lang MS and Perl M:
Inflammatory and apoptotic alterations in serum and injured tissue
after experimental polytrauma in mice: Distinct early response
compared with single trauma or ‘double-hit’ injury. J Trauma Acute
Care Surg. 74:489–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Serrao KL, Fortenberry JD, Owens ML,
Harris FL and Brown LA: Neutrophils induce apoptosis of lung
epithelial cells via release of soluble Fas ligand. Am J Physiol
Lung Cell Mol Physiol. 280:L298–L305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perl M, Hohmann C, Denk S, Kellermann P,
Lu D, Braumüller S, Bachem MG, Thomas J, Knöferl MW, Ayala A, et
al: Role of activated neutrophils in chest trauma-induced septic
acute lung injury. Shock. 38:98–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Qian Z, Li J, Han X and Liu M:
Effects of early administration of a novel anticholinergic drug on
acute respiratory distress syndrome induced by sepsis. Med Sci
Monit. 11:BR319–BR325. 2011.
|
|
30
|
Shen W, Gan J, Xu S, Jiang G and Wu H:
Penehyclidine hydrochloride attenuates LPS-induced acute lung
injury involvement of NF-kappaB pathway. Pharmacol Res. 60:296–302.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han XY, Liu H, Liu CH, Wu B, Chen LF,
Zhong BH and Liu KL: Synthesis of the optical isomers of a new
anticholinergic drug, penehyclidine hydrochloride (8018). Bioorg
Med Chem Lett. 15:1979–1982. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan H, Chen L and Ma J: Penehyclidine
hydrochloride post-conditioning reduces
ischemia/reperfusion-induced cardiomyocyte apoptosis in rats. Exp
Ther Med. 14:4272–4278. 2017.PubMed/NCBI
|
|
33
|
Wang D, Jiang Q and Du X: Protective
effects of scopolamine and penehyclidine hydrochloride on acute
cerebral ischemia-reperfusion injury after cardiopulmonary
resuscitation and effects on cytokines. Exp Ther Med. 15:2027–2031.
2018.PubMed/NCBI
|
|
34
|
Wu GM, Mou M, Mo LQ, Liu L, Ren CH, Chen Y
and Zhou J: Penehyclidine hydrochloride postconditioning on
lipopolysaccharide-induced acute lung injury by inhibition of
inflammatory factors in a rodent model. J Surg Res. 195:219–227.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao HJ, Sun YJ, Zhang TZ, Zhou J and Diao
YG: Penehyclidine hydrochloride attenuates the cerebral injury in a
rat model of cardiopulmonary bypass. Can J Physiol Pharmacol.
91:521–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang YP, Li G, Ma LL, Zheng Y, Zhang SD,
Zhang HX, Qiu M and Ma X: Penehyclidine hydrochloride ameliorates
renal ischemia-reperfusion injury in rats. J Surg Res. 186:390–397.
2014. View Article : Google Scholar : PubMed/NCBI
|