Introduction
Transformers comprise the main equipment in
converter stations, particularly in urban areas; they are also one
of the main sources of noise from these locations (1). Major electric power research institutes
worldwide are committed to the reasonable control of transformer
noise in order to reduce public resistance to substation
installation. However, complaints regarding transformer noise have
not reduced in recent years (2).
Transformer noise may cause discomfort as many individuals exhibit
a low degree of tolerance for low-frequency noise at night
(3). The A-weighted sound level used
for fundamental sound measurement sometimes does not reflect the
actual experience of individuals, particularly in relation to
low-frequency noise (4–6). Therefore, environmental noise,
particularly transformer noise, remains a prominent complaint
despite extensive efforts to reduce and optimize transformer noise
in converter stations. These complaints usually relate to the
impact of noise on neurophysiological characteristics, including
endocrine, mental or emotional conditions and sleep (7).
Transformer noise falls in the category of
low-intensity and low-frequency noise (8,9). The
frequency of sound generated by transformers under normal operation
is primarily concentrated in the 50–800 Hz range, while the
intensity of the sound transmitted to surroundings is typically
<70 dB (10,11). To date, studies on noise have focused
on short exposure time (generally no more than 8 weeks) and high
intensity noise, including 120 dB (12), 118.9 dB (13), 100 dB (14), 126 dB (15) and 75 dB (16). In addition, Liu et al
(17) demonstrated that a short-term
period (35 days) of exposure to transformer noise with 60 and 65 dB
had no effect on neurotransmitters and nervous tissue in the
hippocampus of Sprague-Dawley (SD) rats. To the best of our
knowledge, the effects of noise at <70 dB (as applicable to
transformer noise), over a relatively long-term period (>8
weeks) have not yet been reported.
In order to determine whether exposure to a
relatively long-term period (8 weeks) and low intensity (60/65 dB)
of transformer noise has an impact on behavior and
neurophysiological functions in experimental animals, SD rats were
utilized in the current study. Rats were exposed to transformer
noise recorded from residential areas and the resulting behavioral
and neurophysiological effects were assessed.
Materials and methods
Establishment of sound insulation
environment
To simulate the transformer noise environment, an
experimental apparatus was designed in the current study with good
sound insulation (Fig. 1). The
apparatus included a 2×2×2 m acoustic absorption cube with
soundproof walls, in which the background noise was not higher than
35 dB after closing the door. The relative position of the sound
source and sound insulation was adjusted so that noise distribution
inside the device was uniform. The measurement of sound level meter
(model, AWA 6291; Hangzhou Aihua Instruments Co., Ltd., China;
www.hzaihua.com) revealed that noise intensity
inside the device was no more than 3 dB.
Animals and test groups
Ninety (45 male and 45 female) 6-week-old healthy
adult SD rats (weight, 120–180 g) with similar behavioral index
scores, good auricle reflex sensitivity and no middle ear infection
were obtained from the Experimental Animal Research Center of Hubei
Province (Wuhan, China). Animals were fed in an air-conditioned
room (constant temperature, 22±2°C; humidity, 50–60%) with
background noise <35 dB. Rats were housed in an artificial
constant environment (12 h light/dark cycle, 08:00-20:00) with free
access to food and water. Rats were then randomly divided into two
experimental groups (65 and 60 dB group) and a control group (group
C; each, n=30 per group; 1:1 male to female ratio in each group).
The experimental groups were exposed to recorded transformer noise
(sound level limits: 65 or 60 dB). The control group was subjected
to the same feeding conditions, but did not receive noise
stimulation. The feeding and associated experiments were performed
at the Center for Animal Experiments of Renmin Hospital of Wuhan
University. All experiments were approved by the Committee on
Ethics of Animal Experiments of Renmin Hospital of Wuhan University
(Wuhan, China) and performed in compliance with the Guide for the
Care and Use of Laboratory Animals from the National Institutes of
Health (NIH publication no. 85-23, revised 1996).
Recording and exposure of transformer
noise
The noise sample for the current study was obtained
from a substation transformer (model DFPS-1000000/1000; rated
capacity, 1,000 MVA; rated frequency, 50 Hz; cooling method, oil
forced air forced; TBEA Co., Ltd., Xinjiang, China). The sound
sample collection point was 1.5 m above the ground and 1 m away
from the transformer tank wall beside the side of the fan and the
recording was taken when the transformer was working normally. An
artificial head with binaural signal acquisition systems was used
for collecting sounds and the recordings were analyzed using the
ArtemiS 10.0 noise signal analysis software (Head Acoustics GmbH,
Herzongenrath, Germany). The upper boundary of the recordings was
~76 dB(A), with a dominant frequency of 100–800 Hz. Prior to the
experiment, recordings were treated using a power amplifier (Model,
SWA100; BSWA Technology Co., Ltd., China, www.bswa.com.cn) to adjust the upper boundary to 65
dB(A) or 60 dB(A). The playback apparatus used was the dodecahedron
speaker (Model, OS003; Beijing Sound Reputation Technology Co.,
Ltd., China). The sound environment distribution status in the
sound arrester was detected using a simple sound level meter
(Model, AWA 6291; Hangzhou Aihua Instruments Co., Ltd., Hangzhou,
China) prior to playing the sound, in order to lower the difference
due to the rats' activity to 3 dB by adjusting the relative
position of the sound device. The sound level varied from 65±3 or
60±3 dB. Experimental groups were continuously exposed to the
recorded noises for 10 h/day (from 22:00 to 8:00) for 8 weeks
(12,18). Each group had free access to food and
water. The water bottles were filled twice daily. The drinking,
diets and activities of the rats were observed daily, and the rats
were weighed once per week.
Tail suspension test
After exposure to noise for 56 days, 10 randomly
selected rats from each group were used to conduct the tail
suspension test. The rat-tail suspension test device was made
according to internationally established methods (16). Each rat was observed for 6 min and
indices including struggle indicators (struggle amplitude of mouse
head), total immobility time and longest immobility time within 6
min, were recorded.
Open field test
After exposure to noise for 56 days, 10 randomly
selected rats from each group were performed open field test. An
open field with a size of 120×90×35 cm was created according to
previous literature (17). The walls
and floor were black. The floor was split into 9 rectangles (each,
40×30 cm) and the middle rectangle was defined as the center. The
test was performed in a quiet, light and temperature-suitable room
(constant temperature, 22±2°C) between 8:00 and 12:00. Exploratory
behavior of the rats in the open field was recorded and analyzed
using the animal behavior tracking system (EthoVision 3.0; Noldus
Information Technology bv, Wageningen, The Netherlands). Indicators
of spontaneous motor activity, including total distance travelled,
average speed, time spent in the central cell, rearing frequency
(rat ‘stands up’ on its hind legs with the forelegs off the ground,
regardless of the standing time) and the number of fecal pellets,
were assessed over a 10 min period.
Detection of neurotransmitter content
in the hippocampus
Following exposure to the recorded noises for 10
h/day for 8 weeks, 6 randomly selected rats from each group were
anesthetized with isoflurane (Sumitomo Dainippon Pharma Co., Ltd.,
Osaka, Japan; 1 l/min O2 flow rate) at 4% for induction
and 1.5–2% for maintenance. The anesthetized rats were then
decapitated. Fresh hippocampal tissues were removed immediately.
Following homogenization, High Performance Liquid Chromatography
(HPLC) was performed for the quantitative analysis of the amino
acid neurotransmitters glutamate (Glu) and γ-aminobutyric acid
(GABA), and the monoamine neurotransmitters dopamine (DA) and
5-hydroxytryptamine (5-HT) in the hippocampus. The chromatographic
conditions were: Chromatographic column from Hypersil ODS-3 4.6×250
mm, 5 µm (GL Sciences, Inc., Tokyo, Japan); column temperature,
40°C; mobile phase, potassium dihydrogen phosphate (0.1 mol/l, pH
6.0): methanol: acetonitrile at 6:3:1; flow velocity, 1.0 ml/min;
emission wavelength, 455 nm; and excitation wavelength, 340 nm. For
the hippocampus samples, 0.5 ml samples were taken and added into
tubes. Subsequently, 1 ml of perchlorate was added after
homogenization, then centrifuged at 1,248 × g at 4°C for 15 min.
The supernatant was then transferred into another clean tube at
40°C, 100 µl mobile phase was used for constant volume of the
residue, followed by an injection of 20 µl.
Transmission electron microscopy
(TEM)
Following exposure to the recorded noises for 10
h/day for 8 weeks, 4 randomly selected rats from each group were
anesthetized with isoflurane (1 l/min O2 flow rate) at
4% for induction and 1.5–2% for maintenance, where temperature was
monitored with a rectal probe and maintained at 37.5±1°C using a
non-electrical heating pad. The remaining 60 rats were used for
further experiments not presented in the current study. SD rats
were then perfused (via a transcardial approach) with 0.9% saline
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and 2.5%
glutaraldehyde (Sinopharm Chemical Reagent Co., Ltd.). Following
euthanasia, hippocampal tissues were removed. Tissues were fixed by
immersion in 2% glutaraldehyde at 4°C for 2 h. Following rinsing in
0.1 M PBS (Jinuo Biomedical Technology Co., Ltd., Hangzhou, China;
http://www.genom.com.cn/), the specimens were
post-fixed in 1% OsO4 (Sinopharm Chemical Reagent Co., Ltd.) at 4°C
for 1 h, dehydrated in graded concentrations of acetone (Sinopharm
Chemical Reagent Co., Ltd.) and embedded in a mixture of Epon and
Araldite (Electron Microscopy Sciences, Hatfield, PA, USA).
Semithin sections, at 1 mm thickness, were stained with toluidine
blue (Sinopharm Chemical Reagent Co., Ltd.) at 37°C for 30 sec.
Ultrathin sections were cut to a thickness of 70 nm, stained with
lead citrate and uranyl acetate (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 15 min. Cell morphology and the
apoptosis of hippocampal neurons were assessed via TEM (model,
HT7800; Hitachi Ltd., Tokyo, Japan), with focus on the
morphological changes of neuronal nuclei and synapses. The
apoptotic neurons were condensed and had clumped chromatin with
fragmentation of the nuclear membrane.
Statistical analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used
for all statistical analyses. All values were expressed as the mean
± standard deviation. Statistical analyses were performed by
one-way analysis of variance followed by the Student-Newman-Keuls
post-hoc test. Homogeneity of variance was evaluated using the
Levene's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
General condition
Under exposure to transformer noise for 10 h/day, SD
rats were generally in a healthy condition, as demonstrated by
normal eating and drinking. The body weights of the animals in each
group were recorded once a week from the first day of exposure and
the trends between the groups were subsequently compared. During
the 56 days of noise exposure, the body weight of rats in each
group reflected normal physiological growth. There were no
significant differences among the three groups (Fig. 2).
Tail suspension test
Following exposure to noise for 56 days, struggle
indicators (struggle amplitude of mouse head), total immobility
time and longest immobility time within 6 min were recorded. The
results revealed no significant differences among the three groups
(Fig. 3).
Open field test
Following exposure to noise for 56 days, the total
distance travelled, average speed, residence time in the central
cell, rearing frequency and defecation in 10 min were recorded
(Fig. 4). No significant differences
were identified among the three groups.
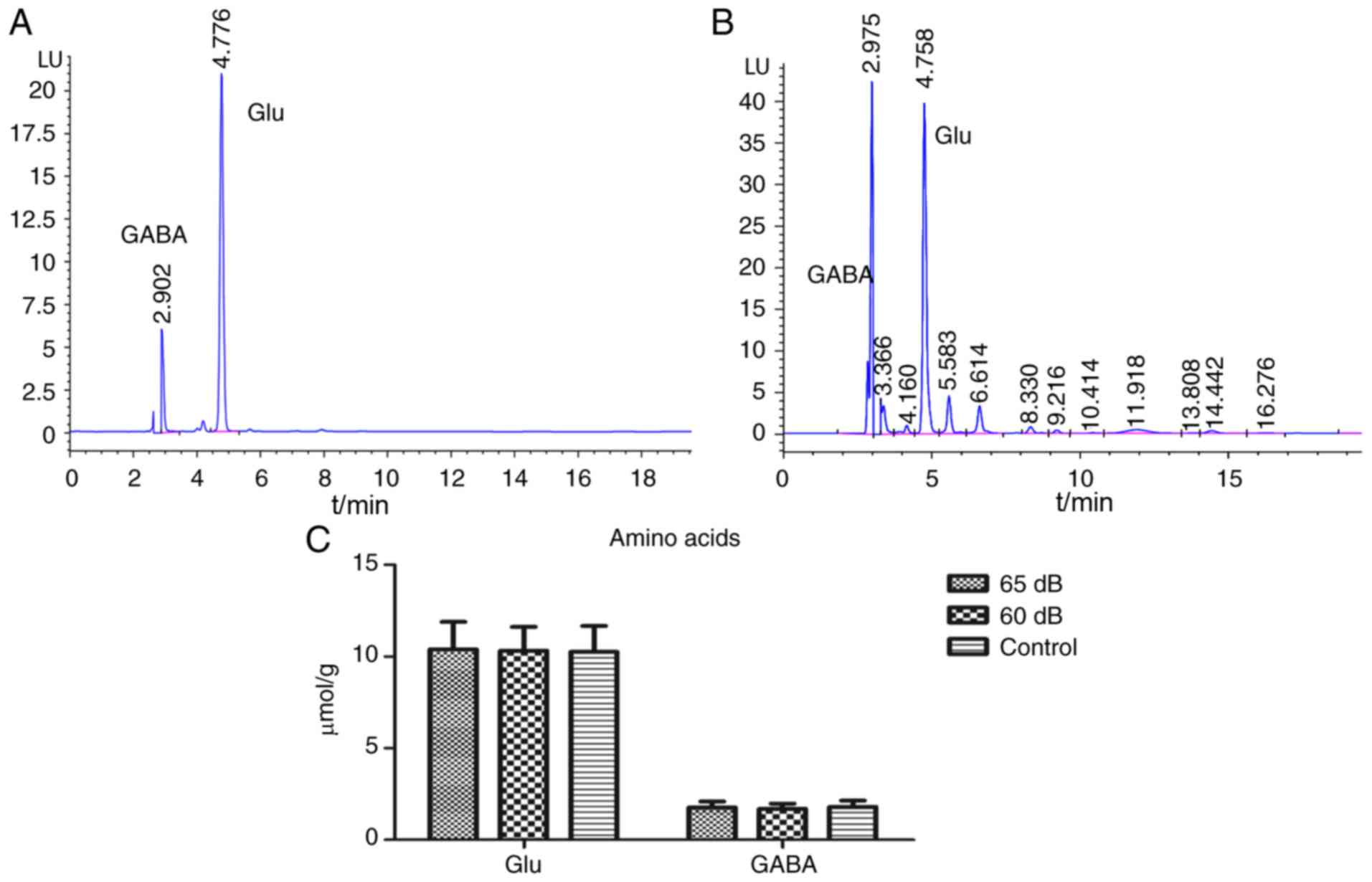
Amino acid neurotransmitters
HPLC was performed to analyze changes in Glu and
GABA in hippocampal tissue. Fig. 5
presents a standard chromatogram (Fig.
5A) and a sample chromatogram of the amino acid
neurotransmitters (Fig. 5B), as well
as measurements of Glu and GABA content (Fig. 5C). There were no significant
differences among the three groups.
Quantitative analysis of monoamine
neurotransmitters
The changes in DA and 5-HT content in hippocampal
tissue were analyzed via HPLC. Fig.
6 presents standard chromatograms (Fig. 6A and B) and a sample chromatogram
(Fig. 6C) of monoamine
neurotransmitters, as well as the content of DA and 5-HT content
(Fig. 6D). No significant
differences were identified between groups.
Morphological observation of
hippocampal neurons
TEM was performed to observe changes in the neuronal
nuclei and synapses. Following exposure to noise for 8 weeks,
hippocampal tissues from 4 randomly selected rats in each group
were extracted and the morphological structure of the hippocampal
neurons was observed using TEM. As presented in Fig. 7A-C, neuronal nuclei had a regular
oval shape, were uniformly stained and exhibited a clear nuclear
membrane structure, normal overall cell morphology and normal
mitochondrial morphology in 65, 60 dB and control group. Neuronal
morphology in all sections appeared similar. Furthermore, no marked
morphological differences were identified among the groups. As
presented in Fig. 7D-F, cells in the
65, 60 dB and control group exhibited a clear synaptic cleft and
normal synaptic vesicle aggregation without edema. There were no
marked differences among the three groups. (Fig. 7).
Discussion
Transformers are a prominent source of noise from
power supporting systems in urban areas. Transformer noise is
generated by cooling system fans and pumps, as well as mechanical
movement caused by the operation of the machine and vibrations due
to electromagnetic changes (19,20).
Generally, noise is considered a harmful physical stimulation,
which can affect the growth and development of rats, impeding
weight gain (21). Exposure to noise
pollution for a certain period of time and intensity may change the
balance of energy metabolism, which would cause weight change
(22,23). The current study observed no
significant differences in weight gain or food intake between
experimental and control groups, indicating that 65- or 60-dB sound
pressure level (SPL) transformer noise exposure for 8 weeks had no
significant influence on the growth of SD rats.
The tail suspension test is commonly employed to
assess overall the physical strength, endurance and mental
condition of animals in medical research (24). In the inverted position, the
immobility time of animals reflects a state of ‘behavioral despair’
(25,26). In the current study, no significant
differences were identified in immobility time (total immobility
and longest immobility time) among the three groups, indicating
that exposure to transformer noise did not impact the endurance and
mental condition of the rats. This may be due to the intensity of
noise or the time of exposure being insufficient to produce
measurable changes.
The open-field test is a classical behavioral
experiment used to assess locomotor activity and anxiety in
animals. It is often performed to elucidate behavioral changes more
comprehensively (27). In the
present study, the three groups exhibited similar total travel
distance and average speed, indicating that noise exposure for 8
weeks did not change the exploratory behavior and movement of the
experimental group, which was consistent with the results of the
tail suspension test. The central cell residence time of rats
increased slightly as the intensity of sound noise exposure
decreased; however, these differences were not statistically
significant. However, there were no significant differences among
the three groups, indicating that noise exposure did not affect the
cognitive ability of SD rats. Furthermore, there were no
significant differences in the upright times of the test and
control animals, indicating that noise exposure did not impact on
SD rat behavior. It was also demonstrated that the amount of feces
in the experimental group was lower than that of the control group,
indicating that transformer noise had no significant effect on the
degree of tension in SD rats.
Epidemiological and experimental studies have
revealed that noise can affect neurobehavioral function, causing a
series of effects including cognitive decline (28). The hippocampus is the primary
functional area involved in learning, memory and emotional
regulation (29). The level of amino
acids in the hippocampus is closely associated with learning and
memory (15,30–32).
Noise exposure may cause environment changes involving chemicals
including acetylcholine, amino acids, neurotransmitters, monoamine
neurotransmitters, neuro-peptides and free radicals in the central
nervous system, which are associated with cognitive abilities
(33–36).
In the current study, the amino acid and monoamine
neurotransmitter content in the hippocampus of rats exhibited no
significant increase or decrease following exposure to 65 (A) or 60
dB(A) transformer noise. The results of the amino acid (Glu and
GABA) and monoamine neurotransmitter content (5-HT and DA) were
consistent with those from a study by Liu et al (17). There was no significant difference in
5-HT content among the three groups; therefore, it may be
hypothesized that transformer noise at the described intensity and
exposure time may not result in depression (37). In addition, no marked damage or
apoptosis was observed in the hippocampal neurons of the three
groups. The results of the hippocampal neurons were also consistent
with those from the study by Liu et al (17). This may be due to rats being at the
peak of growth, with a very low chance of pathological change
(38). These results indicated that
a relatively long-term period of exposure to transformer noise did
not affect the normal function and structure of the hippocampus in
SD rats.
In summary, the results of the present study
demonstrated that a relatively long-term period of exposure to
transformer noise with a sound level limit of 65 dB SPL or 60 dB
SPL (spectral range, 100–800 Hz) for 8 weeks (10 h/day) had no
significant impact on the neurophysiology of SD rats. Therefore,
the current study hypothesizes that low-frequency and low-intensity
noise, similar to transformer noise, may have no marked influence
on the physiological function of the human body when exposed for a
relatively long-term period. The results are worthy of further
verification in a population with similar transformer noise
exposure.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Science and
Technology Project of State Grid Corporation of China (grant no.
GY71-13-057).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
SC conceived and designed the experiments of the
current study. YZ performed the experiments and wrote the
manuscript. XY performed the experiments and analyzed the data. JZ,
XL, KY, YK, BX and ZT were responsible for data acquisition,
analysis and interpretation. All the authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Committee on
Ethics of Animal Experiments of Renmin Hospital of Wuhan University
(Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di GQ, Zhou XX and Chen XW: Annoyance
response to low frequency noise with tonal components: A case study
on transformer noise. Appl Acoust. 91:40–46. 2015. View Article : Google Scholar
|
|
2
|
Teoh C, Soh K, Zhou R, Tien D and Chan V:
Active noise control of transformer noise. Proceedings of the
International Conference on Energy Management and Power Delivery.
IEEE. (Singapore). 1998.
|
|
3
|
Waye KP, Clow A, Edwards S, Hucklebridge F
and Rylander R: Effects of night-time low frequency noise on the
cortisol response to awakening and subjectiv sleep quality. Life
Sci. 72:863–875. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leventhall HG: Low frequency noise and
annoyance. Noise Health. 6:59–72. 2004.PubMed/NCBI
|
|
5
|
Kjellberg A, Tesarz M, Holmberg K and
Landstrom U: Evaluation of frequency-weighted sound level
measurements for prediction of low-frequency noise annoyance.
Environ Int. 23:519–527. 1997. View Article : Google Scholar
|
|
6
|
Holmberg K, Landström U and Kjellberg A:
Low frequency noise level variations and annoyance in working
environments. J Low Freq Noise Vibrat Active Control. 16:81–87.
1997. View Article : Google Scholar
|
|
7
|
Muzet A: Environmental noise, sleep and
health. Sleep Med Rev. 11:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z and Di G: Reduce subjective annoyance
from transformer noise by the method of sound adjustment. Acta
Acust United Acustica. 102:452–461. 2016. View Article : Google Scholar
|
|
9
|
Olsen KN and Stevens CJ: Perceptual
overestimation of rising intensity: Is stimulus continuity
necessary? Perception. 39:695–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lau S: Code for Design of Sound Insulation
of Civil Buildings GB 50118-2010. (Beijing). China Building
Industry Press. 2010.
|
|
11
|
Yong W and Ji ZY: Impact of transformer
noise on indoor residential environment and control techniques.
Environ Monit Forewarning. 2:44–45. 2009.
|
|
12
|
Van Campen LE, Murphy WJ, Franks JR,
Mathias PI and Toraason MA: Oxidative DNA damage is associated with
intense noise exposure in the rat. Hear Res. 164:29–38. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coppola CL, Enns RM and Grandin T: Noise
in the animal shelter environment: Building design and the effects
of daily noise exposure. J Appl Anim Welf Sci. 9:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demirel R, Mollaoğlu H, Yeşilyurt H, Üçok
K, Ayçiçek A, Akkaya M, Genç A, Uygur R and Doğan M: Noise induces
oxidative stress in rat. Eur J Gen Med. 6:20–24. 2009. View Article : Google Scholar
|
|
15
|
Kraus KS, Mitra S, Jimenez Z, Hinduja S,
Ding D, Jiang H, Gray L, Lobarinas E, Sun W and Salvi RJ: Noise
trauma impairs neurogenesis in the rat hippocampus. Neuroscience.
167:1216–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di G, Zhou B and Lin Q: The effects of
aircraft noise exposure on rat behavior and serum neurotransmitter
expression. Noise Control Eng J. 59:514–518. 2011. View Article : Google Scholar
|
|
17
|
Liu XF, Tang YM, Zhou B, Liu ZH, Wan BQ,
Zhang JG, Li W, Qu M and Tang LJ: Experimental research on effect
of transformer noise exposure below 65 dB on the neurotransmitter
and nervoustissue in hippocampus of SD rats. High Volt Eng.
43:2486–2495. 2017.
|
|
18
|
Yost WA, Koita N, Maslo R and Patel P:
Dosimeter measures of sound exposure experienced by university
students. J Acoust Soc America. 120:3163. 2006. View Article : Google Scholar
|
|
19
|
Moses AJ: Measurement of magnetostriction
and vibration with regard to transformer noise. IEEE Transact
Magnet. 10:154–156. 1974. View Article : Google Scholar
|
|
20
|
Berglund B, Hassmén P and Job RFS: Sources
and effects of low-frequency noise. J Acoust Soc Am. 99:2985–3002.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smiley CS and Wilbanks WA: Some effects of
noise exposure on early development in the albino rat. J Acoust Soc
America. 67 (Suppl 1):S58–S59. 1980. View Article : Google Scholar
|
|
22
|
Lasky RE and Williams AL: Noise and light
exposures for extremely low birth weight newborns during their stay
in the neonatal intensive care unit. Pediatrics. 123:540–546. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Michaud DS, Miller SM, Ferrarotto C, Keith
SE, Bowers WJ, Kumarathsan P, Marro L and Trivedi A: Exposure to
chronic noise and fractionated X-ray radiation elicits biochemical
changes and disrupts body weight gain in rat. Int J Radiat Biol.
81:299–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin C, Gou L, Liu Y, Yin X, Zhang L, Jia G
and Zhuang X: Antidepressant-like effects of L-theanine in the
forced swim and tail suspension tests in mice. Phytother Res.
25:1636–1639. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steru L, Chermat R, Thierry B and Simon P:
The tail suspension test: A new method for screening
antidepressants in mice. Psychopharmacology. 85:367–370. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barfield ET, Barry SM, Hodgin HB, Thompson
BM, Allen SS and Grisel JE: Beta-endorphin mediates behavioral
despair and the effect of ethanol on the tail suspension test in
mice. Alcohol Clin Exp Res. 34:1066–1072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brenes Sáenz JC, Villagra OR and
Fornaguera Trías J: Factor analysis of Forced swimming test,
sucrose preference test and open field test on enriched, social and
isolated reared rats. Behav Brain Res. 169:57–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Kempen E, van Kamp I, Lebret E,
Lammers J, Emmen H and Stansfeld S: Neurobehavioral effects of
transportation noise in primary schoolchildren: A cross-sectional
study. Environ Health. 9:252010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toyoda A, Iio W, Goto T, Koike H and
Tsukahara T: Differential expression of genes encoding neurotrophic
factors and their receptors along the septal-temporal axis of the
rat hippocampus. Anim Sci J. 85:986–993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim H, Lee MH, Chang HK, Lee TH, Lee HH,
Shin MC, Shin MS, Won R, Shin HS and Kim CJ: Influence of prenatal
noise and music on the spatial memory and neurogenesis in the
hippocampus of developing rats. Brain Dev. 28:109–114. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng L, Wang SH, Huang Y and Liao XM: The
hippocampus may be more susceptible to environmental noise than the
auditory cortex. Hear Res. 333:93–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiel CM, Müller CP, Huston JP and
Schwarting RK: Auditory noise can prevent increased extracellular
acetylcholine levels in the hippocampus in response to aversive
stimulation. Brain Res. 882:112–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ravindran R, Rathinasamy SD, Samson J and
Senthilvelan M: Noise-stress-induced brain neurotransmitter changes
and the effect of Ocimum sanctum (Linn) treatment in albino
rats. J Pharmacol Sci. 98:354–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui B, Wu M, She X and Liu H: Impulse
noise exposure in rats causes cognitive deficits and changes in
hippocampal neurotransmitter signaling and tau phosphorylation.
Brain Res. 1427:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gil-Loyzaga P, Vicente-Torres MA,
Fernández-Mateos P, Arce A and Esquifino A: Piribedil affects
dopamine turnover in cochleas stimulated by white noise. Hear Res.
79:178–182. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassanvand T, Balooch M, Azarnia M and
Zardooz H: Alterations in dopamine related behavior of the
offspring of pregnant Wistar rats exposed to noise pollution
stress. Physiol Pharmacol. 16:79–85. 2012.
|
|
37
|
Van Praag HM: 5-HT-related, anxiety-
and/or aggression-driven depression. Int Clin Psychopharmacol. 9
(Suppl 1):S5–S6. 1994. View Article : Google Scholar
|
|
38
|
Turner CA, Clinton SM, Thompson RC, Watson
SJ Jr and Akill H: Fibroblast growth factor-2 (FGF2) augmentation
early in life alters hippocampal development and rescues the
anxiety phenotype in vulnerable animals. Proc Natl Acad Sci USA.
108:8021–8025. 2011. View Article : Google Scholar : PubMed/NCBI
|