Introduction
Liver cirrhosis is at the end stage of the
development of chronic liver disease, arising from various
etiologies such as chronic poisoning, viral hepatitis, and
obstruction of hepatic venous return (1), with the long-term impact of the liver
cells gradually undergoing pathological changes such as
degeneration, necrosis, and liver fibrosis, and eventually
developing into regenerated nodules, diffuse fibrosis of the liver,
and pseudolobular shape (2,3). Without effective treatment, in the long
run, liver cirrhosis develops into liver decompensation and portal
hypertension, causing multiple-system and multiple-organ damage in
the body (4). Clinically,
hepatectomy and liver transplantation are common methods for the
treatment of cirrhosis (5). Fast and
short-acting, propofol is a kind of intravenous anesthetic with the
advantages of rapid anesthesia induction, quick recovery, and less
postoperative complications (6).
Remifentanil is a new type of µ opioid receptor agonist,
characterized by the fast onset and significant analgesic effect
(7). The combination of propofol and
remifentanil can reduce the dosage and achieve sedative, analgesic
and hypnotic effects (8).
Hepatectomy is an important cause of the hepatic
ischemia-reperfusion injury which is an important reason for liver
failure during the operation (9).
Mainly secreted by vascular endothelial cells, nitric oxide (NO)
and endothelin (ET-1) are an active substance capable of regulating
vasomotor function (10). The
balance of NO/ET-1 ratio and inflammatory response are important
factors in liver ischemia-reperfusion injury (11). Additionally to their sedative and
anesthetic effects, propofol and remifentanil also have protective
effects and immune regulating effects on the body when it suffers
from ischemia-reperfusion injury (12,13). In
patients with liver cirrhosis who underwent hepatectomy, this study
investigated the effects of propofol combined with remifentanil on
the NO, ET-1 and inflammatory cytokines in the plasma of patients,
with the aim of providing a reference for the anesthesia medication
for patients with liver cirrhosis.
Patients and methods
General information
A retrospective analysis of 68 patients with liver
cirrhosis who underwent hepatectomy in Hunan Provincial People's
Hospital (Changsha, China) from March 2016 to July 2018 was made.
According to different anesthesia methods, 30 patients anesthetized
with propofol were enrolled into Group A, the other 38 patients
anesthetized with propofol combined with remifentanil were enrolled
into Group B. Among the patients in Group A, 22 were male and 8
were female, aged 29–65 years with a mean age of 45.94±10.67 years,
weighing 52–91 kg with an average body weight of 62.87±10.28 kg.
Group B contained 31 males and 7 females, aged 28–64 years, with a
mean age of 47.25±9.69 years, weighing 53–86 kg with an average
body weight of 64.07±12.54 kg. This study was approved by the
Ethics Committee of the Hunan Provincial People's Hospital and by
all the research subjects after signing the informed consent.
Inclusion and exclusion criteria
Inclusion criteria were: The patients were
identified in the physical status class I or class II according to
the grading standard of the American Society of Anesthesiologists
(ASA) (14) and diagnosed as viral
liver cirrhosis, splenomegaly, portal hypertension and
hypersplenism, with a liver function grading of Child A class by
the Child-Pugh classification (15);
patients without history of radiotherapy, chemotherapy or steroids
and opioids before surgery, and patients with complete clinical
data.
Exclusion criteria were: Patients with autoimmune,
endocrine, and metabolic diseases; patients with diabetes,
hypertensive cerebrovascular disease; patients who received
antiviral or immunosuppressive therapy, and patients with mental
illness or with a family history of mental illness.
Anesthesia method
At 30 min before the anesthesia, all the patients
were given intramuscular injection of 0.1 g Phenobarbital Sodium
(Shanghai New Asia Pharmaceutical Co., Ltd., Shanghai, China, the
code number of medical products permitted by the China Food and
Drug Administration: H31020532, China), 0.5 mg Atropine (Jiangsu
Fangqiang Pharmaceutical Factory Co., Ltd., Jiangsu, China, the
code number of medical products permitted by the China Food and
Drug Administration: H32020236, China). The upper extremity venous
access was established after entering the room, and heart rate,
blood pressure, SpO2, and BIS were continuously
detected. All patients were dosed by intravenous target-controlled
infusion. Patients in Group A were anesthetized by propofol
(Beijing Fresenius Kabi Pharmaceutical Co., Ltd., Beijing, China,
the code number of medical products permitted by the China Food and
Drug Administration: J20070010, China), the procedure was to first
perform the anesthesia induction by intravenous drip using
intravenous pump with a dosage of 2.0–2.5 mg/kg and at a rate of 4
mg/sec, then to maintain the intravenous infusion with a dosage of
4–12 mg/kg at a rate of 6–8 mg/sec after the induction was
successful. In Group B, patients were first anesthetized by the
same propofol with the same method as Group A, then were
anesthetized by remifentanil (Yichang Humanwell Pharmaceutical Co.,
Ltd., Yichang, China, the code number of medical products permitted
by the China Food and Drug Administration: H20030197, China), and
the procedure was as follows: first the anesthesia induction was
performed by intravenous pump with a dosage of 0.5–1 µ/kg at a
speed of 0.5–1 µ/kg/1 min, then the endotracheal intubation was
followed by mechanical ventilation; next, the breathing parameters
were adjusted to maintain the PETCO2 at 30–40
mmHg. Then, the infusion of propofol and remifentanil was
maintained to keep the BIS value at 40–60. All anesthetics were
discontinued in the abdomen-closing, and intravenous infusion of 10
mg of Rocuronium Bromide [Fuan Pharmaceutical (Group) Co., Ltd.,
Shenzhen, China, the code number of medical products permitted by
the China Food and Drug Administration: H20123439, China] was given
to maintain muscle relaxant.
Observation indicators
The operation time, amount of bleeding during the
operation and postoperative awake time of the two groups were
recorded. Venous blood (5 ml) was separately taken at T1
(30 min before the anesthesia), T2 (after the portal
triad clamping), T3 (3 days after the operation) and was
stored in a low-temperature refrigerator at −70°C after the plasma
was separated. The aspartate transaminase (AST), alanine
transaminase (ALT) were detected by the automatic biochemical
analyzer [Beckman Coulter Commercial Enterprise (China) Co., Ltd.,
Shanghai, China] to test the liver function, according to the
instructions of the instrument and AST, ALT kit rate method
(Shanghai Jining Industrial Co., Ltd., Shanghai, China). The
enzyme-linked immunosorbent assay (ELISA) (16) was used to measure the plasma levels
of NO, ET-1, interleukin-6 (IL-6) and tumor necrosis factor alpha
(TNF-α). The NO and ET-1 ELISA kits were purchased from Shanghai
Lanpai Biotechnology Co., Ltd. (Shanghai, China), the IL-6, TNF-α
ELISA kits were purchased from Elabscience Biotechnology Co., Ltd
(Wuhan, China). The specific detection was performed referring to
the instruction manuals of the kits. The samples to be tested and
the kits were taken from the refrigerator 20 min in advance to
equilibrate with the room temperature and then the standard wells
and the sample wells were set, with 50 µl of the standard at
different concentration in each different standard well, 10 µl of
the sample and 40 µl of the sample diluent in each sample well, and
none in each blank well. Horseradish peroxidase (HRP) labeled goat
anti-mouse polyclonal antibody (dilution, 1:600; cat. no.
E-AB-1008; Elabscience Biotechnology Co., Ltd., (Wuhan, China) (100
µl) was added into the standard wells and sample wells which were
covered with a membrane and incubated at 37°C for 60 min. Next, the
liquid of each well was discarded, wells were dried and washing
liquid was added to each well for 1 min, then discarding the
washing liquid and drying the well, the washing procedure was
repeated 5 times; after that, 50 µl of working solution A and 50 µl
of working solution B were added into each well as the substrate
and incubated for 15 min at 37°C in the dark. Finally, after adding
50 µl of stopping solution into each well and incubating for 15 min
at 37°C, the automatic enzyme label analyzer (Wuxi Hiwell Diatek
Instruments Co., Ltd., Wuxi, China) was used to detect the OD value
of each well at the wavelength of 450 nm, then the NO, ET-1, IL-6
and TNF-α contents were calculated.
Statistical analysis
Statistical analysis was performed using SPSS18.0
[Yiyun (Shanghai) Information Technology Co., Ltd., Shanghai,
China]. The measurement data were expressed as the mean ± the
standard deviation (mean ± SD), and the enumeration data were
expressed as the number/percentage (n/%). Chi-square test was used
to compare the enumeration data between groups, and the t-test was
used to compare the measurement data between groups. One-way ANOVA
with Least Significant Difference post hoc test was used to compare
the means between multiple groups. The repeated measures for
variance analysis was used to compare the data of multiple
time-points. SNK test was used for comparison between two groups.
Statistical difference was set at P<0.05.
Results
General information of the two groups
of patients
No statistical difference was detected between
Groups A and B in terms of general clinical baseline data including
sex, age, blood glucose (Glu), body weight, smoking status,
drinking status, hemoglobin (Hb), red blood cell (RBC) count,
platelet (PLT) count, operative time, amount of bleeding during the
operation, awake time (all P>0.05) (Table I).
 | Table I.General information of patients in
Groups A and B [n(%)] (mean ± SD). |
Table I.
General information of patients in
Groups A and B [n(%)] (mean ± SD).
| Factors | Group A (n=30) | Group B (n=38) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.663 | 0.416 |
|
Male | 22 (73.33) | 31 (81.58) |
|
|
|
Female | 8
(26.67) | 7
(18.42) |
|
|
| Age (years) | 45.94±10.67 | 47.25±9.69 | 0.529 | 0.598 |
| Glu (mmol/l) | 5.98±0.83 | 6.03±0.57 | 0.294 | 0.770 |
| Weight (kg) | 62.87±10.28 | 64.07±12.54 | 0.424 | 0.673 |
| Smoking status |
|
| 0.056 | 0.813 |
|
Yes | 11 (36.67) | 15 (39.47) |
|
|
| No | 19 (63.33) | 23 (60.53) |
|
|
| Drinking
status |
|
| 0.242 | 0.623 |
|
Yes | 18 (60.00) | 25 (65.79) |
|
|
| No | 12 (40.00) | 13 (34.21) |
|
|
| Hb (g/l) | 119.74±8.58 | 123.68±9.56 | 1.765 | 0.082 |
| RBC
(×1012/l) | 4.73±0.38 | 4.67±0.49 | 0.552 | 0.583 |
| PLT
(×109/l) | 139.57±12.58 | 143.08±15.09 | 1.023 | 0.310 |
| Operation time
(min) | 86.63±16.63 | 88.48±15.94 | 0.466 | 0.643 |
| Amount of bleeding
during the operation (ml) | 361.52±40.96 | 371.52±42.63 | 0.977 | 0.332 |
| Awake time
(sec) | 3.2±1.1 | 3.4±0.8 | 0.868 | 0.389 |
Changes in the liver function indexes
in the two groups during the perioperative period
No significant difference was shown in the plasma
AST and ALT levels between Groups A and B at T1 and
T3 time-points (P>0.05). In both Groups A and B, the
plasma levels of AST and ALT at the T2 time-point were
significantly higher than those at the T1 and
T3 time-points (P<0.05), and the plasma AST and ALT
levels at the T2 time-point in Group A were
significantly higher than that in Group B (P<0.05) (Table II).
 | Table II.Changes in the liver function indexes
at T1, T2 and T3 time-points in
patients in Groups A and B (mean ± SD). |
Table II.
Changes in the liver function indexes
at T1, T2 and T3 time-points in
patients in Groups A and B (mean ± SD).
|
| AST (µmol/l) | ALT (µmol/l) |
|---|
|
|
|
|
|---|
| Groups | T1 | T2 | T3 | T1 | T2 | T3 |
|---|
| Group A (n=30) | 30.24±4.36 |
61.38±2.52a | 31.83±2.74 | 31.59±2.64 |
67.53±4.41a | 32.74±2.95 |
| Group B (n=38) | 30.79±2.55 |
45.63±3.96a,b | 32.08±1.55 | 31.47±2.82 |
48.6±1.77a,b | 32.21±2.48 |
| t value | 0.650 | 18.950 | 0.475 | 0.179 | 24.150 | 0.805 |
| P-value | 0.518 | <0.001 | 0.636 | 0.858 | <0.001 | 0.424 |
Changes of plasma NO content in the
two groups during the perioperative period
The plasma NO content of patients in Group A at the
T1, T2, and T3 time-points were
38.13±3.05, 17.34±3.16 and 36.41±3.28 µmol/l, respectively, while
the plasma NO content of patients in Group B at the T1,
T2 and T3 time-points were 38.43±3.18,
26.59±1.86 and 36.49±2.96 µmol/l, respectively, showing that no
significant difference existed in the plasma NO content between
Groups A and B at the T1 and T3 time-points
(P>0.05); the plasma NO levels in both Groups A and B at the
T2 time-point were significantly lower than that at the
T1 and T3 time-points (P<0.05); and that
the plasma NO content in Group A was significantly lower than that
of Group B at the T2 time-point (P<0.05) (Fig. 1).
Changes of plasma ET-1 levels in the
two groups during the perioperative period
According to the plasma ET-1 levels in Group A at
the T1, T2 and T3 time-points
(92.57±10.52, 179.05±9.27 and 98.24±11.57 µmol/l), and the plasma
ET-1 levels in Group B at the T1, T2 and
T3 time-points (93.15±12.63, 136.59±8.09, and
97.45±10.28 µmol/l), no significant difference in the plasma ET-1
levels between Groups A and B was presented at the T1
and T3 time-points (P>0.05); the plasma ET-1 levels
at the T2 time-point were significantly higher than
those at the T1 and T3 time-points
(P<0.05), and the plasma ET-1 levels at the T2
time-point in Group A were significantly higher than those in Group
B (P<0.05) (Fig. 2).
Changes of plasma IL-6 levels in the
two groups during the perioperative period
The plasma IL-6 levels of patients in Group A at the
T1, T2 and T3 time-points were
21.53±5.23, 54.15±8.63 and 22.69±6.18 pg/ml, respectively, and the
plasma IL-6 levels of patients in Group B at the T1,
T2 and T3 time-points were 22.41±5.08,
39.57±7.51 and 23.47±6.15 pg/ml, respectively. No significant
difference was found in the plasma IL-6 levels between Groups A and
B at the T1 and T3 time-points (P>0.05).
The plasma IL-6 levels at the T2 time-point were
significantly higher than at the T1 and T3
time-points (P<0.05), and the plasma IL-6 levels at the
T2 time-point in Group A were significantly higher than
that in Group B (P<0.05) (Fig.
3).
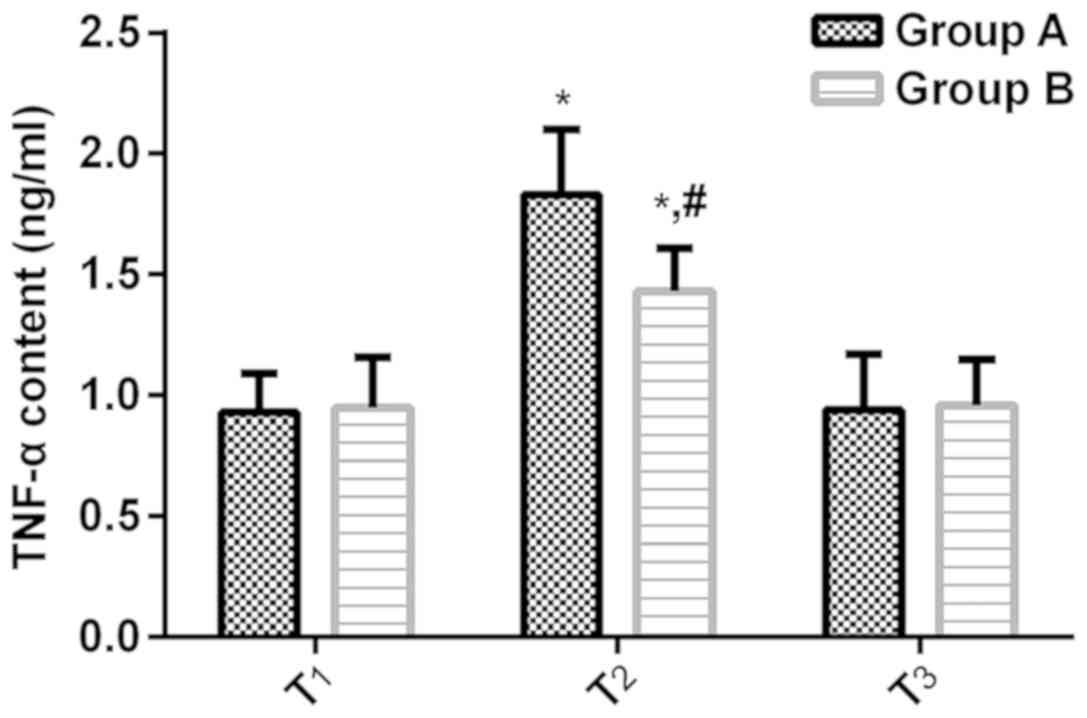
Changes of plasma TNF-α levels in the
two groups of patients during the perioperative period
According to the plasma TNF-α levels in Group A at
the T1, T2 and T3 time-points
(0.93±0.16, 1.83±0.27 and 0.94±0.23 ng/ml), and the plasma TNF-α
levels in Group B at the T1, T2 and
T3 time-points (0.95±0.21, 1.43±0.18 and 0.96±0.19
ng/ml), no significant difference in the plasma TNF-α levels
between Groups A and B surfaced at the T1 and
T3 time-points (P>0.05); the plasma TNF-α levels at
the T2 time-point were significantly higher than at the
T1 and T3 time-points (P<0.05), and the
plasma TNF-α levels at the T2 time-point in Group A were
significantly higher than that in Group B (P<0.05) (Fig. 4).
Discussion
A commonly chosen operation for patients with
cirrhosis in the clinic, hepatectomy requires to block the hepatic
vascular during the surgery with the consideration to reduce the
amount of bleeding, which brings problems since the re-opening of
hepatic vascular after the surgery may cause ischemia-reperfusion
injury of the liver, impaired vascular endothelial function and
liver function, affecting the liver's regeneration ability after
the surgery (17,18). Previous studies have shown that
NO/ET-1 ratio and inflammatory response are closely related to
hepatic ischemia-reperfusion injury, which can reflect the liver
function of the body (19,20). Therefore, to protect the vascular
endothelial function and relieve the inflammatory response without
interfering with the smooth operation is of great help to improve
the prognosis of patients with cirrhosis.
Characterized by quick effect, short duration, and
stable awaking status and less adverse reactions, the powerful and
effective anesthetic propofol, has been widely used in clinical
practice (21). Remifentanil, a
fentanyl opioid receptor agonist that can be rapidly absorbed by
intravenous administration, has a synergistic effect with inhaled
anesthetics, hypnotics, and benzodiazepines, and its dose is
closely related to analgesic effects (22,23). At
present, the mechanism of action of propofol and remifentanil in
liver surgery has not been elucidated. The study made by Hao et
al (24) showed that propofol
could prevent the apoptosis of liver cells and reduce
ischemia-reperfusion injury in rats. Xu et al (25) and Liu et al (26) considered that propofol and
remifentanil had protective effects on the hepatic
ischemia-reperfusion injury, which could improve the liver function
and liver microcirculation.
As active substances secreted by vascular
endothelial cells, both NO and ET-1 have a function of regulating
vasomotion. In the early stage of hepatic ischemia-reperfusion
injury, hepatocytes are in an ischemic and hypoxic environment,
where the intracellular calcium concentration gradually increases
to push the endothelial cells to secrete a large amount of ET-1
that can worsen vasoconstriction and cause secondary damage to the
liver cells as the liver microcirculation is disturbed (27,28). The
decrease of NO, reducing the function of ET-1 by promoting the
secretion and synthesis of ET-1 through feedback, can aggravate the
vasoconstriction, which further damages the liver cells and causes
liver ischemia-reperfusion injury (29). According to the results of this
study, the plasma NO levels in both Groups A and B at the
T2 time-point were significantly lower than that at the
T1 and T3 time-points, while the ET-1 level
was the opposite; at the T2 time-point, the plasma NO
content in Group A was significantly lower than that of Group B,
but the plasma ET-1 content in Group A was significantly higher
than that of Group B, indicating that the balance of the ratio of
NO/ET-1 was significantly broken during the hepatolobectomy
surgery, and the disorder of the ratio of NO/ET-1 was significantly
relieved after the anesthesia with propofol and remifentanil.
Hepatectomy can cause hepatic ischemia-reperfusion
injury, which manifests as the release of a large number of
inflammatory cytokines, the hepatocyte degeneration and necrosis,
and a large number of leukocyte infiltrations (30). IL-6 and TNF-α, the most active
pro-inflammatory cytokines currently studied, mainly released by
monocytes, can cause a strong inflammatory reaction in the body,
resulting in secondary damage to impaired organs (31,32). The
study by Taub et al (33)
showed that within a few minutes after the hepatectomy, IL-6 and
TNF-α could be released from the non-parenchymal liver, causing
liver inflammation. The results of this study showed that plasma
levels of IL-6 and TNF-α in both Groups A and B at the
T2 time-point were significantly higher than those at
the T1 and T3 time-points, and the levels of
plasma IL-6 and TNF-α at the T2 time in Group A were
significantly higher than those in Group B, suggesting that
propofol and remifentanil could inhibit the significant
inflammatory response occurred during the hepatectomy operation. As
shown in the this study, in both Groups A and B, the plasma levels
of AST and ALT at the T2 time-point were significantly
higher than those at the T1 and T3
time-points, and the plasma AST and ALT levels at the T2
time-point in Group A were significantly higher than that in Group
B, suggesting that propofol and remifentanil could prevent the
liver function from the influence of hepatectomy to a certain
degree, which was similar to the conclusion of the study by Poon
et al (34) that hepatectomy
might affect liver function in patients.
The research subjects of this study were selected in
strict accordance with the inclusion and exclusion criteria. The
general clinical baseline data of patients between the two groups
were not significantly different in such terms as sex, age, Glu,
body weight, smoking status, drinking status, Hb, RBC count, PLT
count, operation time, amount of bleeding during the operation, and
awake time to ensure the rigor and reliability of this study.
Limitations of this study were that no follow-up of the prognosis
of patients was performed and the specific regulatory mechanisms
for propofol and remifentanil were not elucidated. Thus, future
studies should extend the study time to clarify the specific
regulatory mechanisms for propofol and remifentanil.
In summary, the anesthesia of propofol combined with
remifentanil could contribute to the balance of NO/ET-1 and the
inhibition of inflammatory factors during the hepatectomy operation
in patients with liver cirrhosis, and help to protect the liver
function of patients, and reduce the incidence of liver
ischemia-reperfusion injury in patients.
Acknowledgements
Not applicable.
Funding
This study was supported by Research Foundation of
Health Commission of Hunan Province (grant no. B2019065).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW was involved in the writing of the manuscript. YW
and RQ performed ELISA. GK collected the general data of the
patients. YW and JL analyzed and interpreted the patient data
regarding the NO, endothelin and inflammatory cytokines. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hunan Provincial People's Hospital (Changsha, China). Patients who
participated in this study, had complete clinical data. Signed
informed consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morales BP, Planas R, Bartoli R, Morillas
RM, Sala M, Cabré E, Casas I and Masnou H: Early hospital
readmission in decompensated cirrhosis: Incidence, impact on
mortality, and predictive factors. Dig Liver Dis. 49:903–909. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Durand F, Buyse S, Francoz C, Laouénan C,
Bruno O, Belghiti J, Moreau R, Vilgrain V and Valla D: Prognostic
value of muscle atrophy in cirrhosis using psoas muscle thickness
on computed tomography. J Hepatol. 60:1151–1157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
González-González JA, García-Compean D,
Vázquez-Elizondo G, Garza-Galindo A, Jáquez-Quintana JO and
Maldonado-Garza H: Nonvariceal upper gastrointestinal bleeding in
patients with liver cirrhosis. Clinical features, outcomes and
predictors of in-hospital mortality. A prospective study. Ann
Hepatol. 10:287–295. 2011.PubMed/NCBI
|
|
5
|
Cheung TT, Dai WC, Tsang SH, Chan AC, Chok
KS, Chan SC and Lo CM: Pure laparoscopic hepatectomy versus open
hepatectomy for hepatocellular carcinoma in 110 patients with liver
cirrhosis: A propensity analysis at a single center. Ann Surg.
264:612–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Girdler NM, Rynn D, Lyne JP and Wilson KE:
A prospective randomised controlled study of patient-controlled
propofol sedation in phobic dental patients. Anaesthesia.
55:327–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Comelon M, Raeder J, Stubhaug A, Nielsen
CS, Draegni T and Lenz H: Gradual withdrawal of remifentanil
infusion may prevent opioid-induced hyperalgesia. Br J Anaesth.
116:524–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hannivoort LN, Vereecke HE, Proost JH,
Heyse BE, Eleveld DJ, Bouillon TW, Struys MM and Luginbühl M:
Probability to tolerate laryngoscopy and noxious stimulation
response index as general indicators of the anaesthetic potency of
sevoflurane, propofol, and remifentanil. Br J Anaesth. 116:624–631.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olthof PB, van Golen RF, Meijer B, van
Beek AA, Bennink RJ, Verheij J, van Gulik TM and Heger M: Warm
ischemia time-dependent variation in liver damage, inflammation,
and function in hepatic ischemia/reperfusion injury. Biochim
Biophys Acta Mol Basis Dis. 1863:375–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vasiljevic B, Maglajlic-Djukic S, Gojnic
M, Stankovic S, Ignjatovic S and Lutovac D: New insights into the
pathogenesis of perinatal hypoxic-ischemic brain injury. Pediatr
Int. 53:454–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Chen Z, Feng N, Tang J, Zhao X,
Liu C, Xu H and Zhang M: Protective effect of propofol
preconditioning on ischemia-reperfusion injury in human hepatocyte.
J Thorac Dis. 9:702–710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Losada DM, Souza ME, Jordani MC, Picinato
MA, Fina CF, Feres O, Michelone PR and Silva OC: Hyperbaric oxygen
therapy and ischemia and reperfusion: A valuable association to
attenuate ischemic lesion and hepatic reperfusion. Acta Cir Bras.
28:126–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pikwer A, Castegren M, Namdar S, Blennow
K, Zetterberg H and Mattsson N: Effects of surgery and
propofol-remifentanil total intravenous anesthesia on cerebrospinal
fluid biomarkers of inflammation, Alzheimer's disease, and neuronal
injury in humans: A cohort study. J Neuroinflammation. 14:1932017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan AS, Williams G, Woolsey C, Liu J,
Fields RC, Doyle MMB, Hawkins WG and Strasberg SM: Flange
gastroenterostomy results in reduction in delayed gastric emptying
after standard pancreaticoduodenectomy: A prospective cohort study.
J Am Coll Surg. 225:498–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Au KP, Chan SC, Chok KS, Chan AC, Cheung
TT, Ng KK and Lo CM: Child-Pugh parameters and platelet count as an
alternative to ICG test for assessing liver function for major
hepatectomy. HPB Surg. 2017:29480302017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ndongo-Thiam N, Clement A, Pin JJ,
Razanajaona-Doll D and Miossec P: Negative association between
autoantibodies against IL-17, IL-17/anti-IL-17 antibody immune
complexes and destruction in rheumatoid arthritis. Ann Rheum Dis.
75:1420–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simillis C, Robertson FP, Afxentiou T,
Davidson BR and Gurusamy KS: A network meta-analysis comparing
perioperative outcomes of interventions aiming to decrease ischemia
reperfusion injury during elective liver resection. Surgery.
159:1157–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohana G, Cohen S, Rath-Wolfson L and
Fishman P: A3 adenosine receptor agonist, CF102, protects against
hepatic ischemia/reperfusion injury following partial hepatectomy.
Mol Med Rep. 14:4335–4341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ratti F, Pulitanò C, Catena M, Paganelli M
and Aldrighetti L: Serum levels of endothelin-1 after liver
resection as an early predictor of postoperative liver failure. A
prospective study. Hepatol Res. 46:529–540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wiggers JK, van Golen RF, Verheij J,
Dekker AM, van Gulik TM and Heger M: Atorvastatin does not protect
against ischemia-reperfusion damage in cholestatic rat livers. BMC
Surg. 17:352017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnston DF, Stafford M, McKinney M,
Deyermond R and Dane K: Peripheral nerve blocks with sedation using
propofol and alfentanil target-controlled infusion for hip fracture
surgery: A review of 6 years in use. J Clin Anesth. 29:33–39. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freeman LM, Bloemenkamp KW, Franssen MT,
Papatsonis DN, Hajenius PJ, Hollmann MW, Woiski MD, Porath M, van
den Berg HJ, van Beek E, et al: Patient controlled analgesia with
remifentanil versus epidural analgesia in labour: Randomised
multicentre equivalence trial. BMJ. 350:h8462015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eleveld DJ, Proost JH, Vereecke H, Absalom
AR, Olofsen E, Vuyk J and Struys MMRF: An allometric model of
remifentanil pharmacokinetics and pharmacodynamics. Anesthesiology.
126:1005–1018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao W, Zhao ZH, Meng QT, Tie ME, Lei SQ
and Xia ZY: Propofol protects against hepatic ischemia/reperfusion
injury via miR-133a-5p regulating the expression of MAPK6. Cell
Biol Int. 41:495–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Z, Yu J, Wu J, Qi F, Wang H and Wang Z
and Wang Z: The effects of two anesthetics, propofol and
sevoflurane, on liver ischemia/reperfusion injury. Cell Physiol
Biochem. 38:1631–1642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Pan Z, Su D, Yang Z, Zheng B, Wang
X and Tian J: Remifentanil ameliorates liver ischemia-reperfusion
injury through inhibition of interleukin-18 signaling.
Transplantation. 99:2109–2117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gogus N, Akan B, Bayrakci S, Girgin G and
Baydar M: The effects of a small-dose ketamine-propofol combination
on tourniquet-induced ischemia-reperfusion injury during
arthroscopic knee surgery. J Clin Anesth. 26:46–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar A, Aggarwal R, Naik SR, Saraswat V,
Ghoshal UC and Naik S: Hepatitis E virus is responsible for
decompensation of chronic liver disease in an endemic region.
Indian J Gastroenterol. 23:59–62. 2004.PubMed/NCBI
|
|
29
|
Marasciulo FL, Montagnani M and Potenza
MA: Endothelin-1: The yin and yang on vascular function. Curr Med
Chem. 13:1655–1665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lentsch AB, Kato A, Yoshidome H, McMasters
KM and Edwards MJ: Inflammatory mechanisms and therapeutic
strategies for warm hepatic ischemia/reperfusion injury.
Hepatology. 32:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Charrad R, Berraïes A, Hamdi B, Ammar J,
Hamzaoui K and Hamzaoui A: Anti-inflammatory activity of IL-37 in
asthmatic children: Correlation with inflammatory cytokines TNF-α,
IL-β, IL-6 and IL-17A. Immunobiology. 221:182–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thimmulappa RK, Lee H, Rangasamy T, Reddy
SP, Yamamoto M, Kensler TW and Biswal S: Nrf2 is a critical
regulator of the innate immune response and survival during
experimental sepsis. J Clin Invest. 116:984–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taub R, Greenbaum LE and Peng Y:
Transcriptional regulatory signals define cytokine-dependent and
-independent pathways in liver regeneration. Semin Liver Dis.
19:117–127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poon RT, Fan ST, Lo CM, Liu CL and Wong J:
Long-term survival and pattern of recurrence after resection of
small hepatocellular carcinoma in patients with preserved liver
function: Implications for a strategy of salvage transplantation.
Ann Surg. 235:373–382. 2002. View Article : Google Scholar : PubMed/NCBI
|