Introduction
Spontaneous isolated superior mesenteric artery
dissection (SISMAD) refers to a type of dissecting lesion involving
the SMA and its branches. The SISMAD classification proposed by Yun
et al (1) in 2009 is as
follows: Type I, true and false lumen revealing entry and re-entry
sites; Type II, true lumen but no re-entry flow from the false
lumen; Type IIa, visible false lumen but no visible re-entry site;
Type IIb, no visible false luminal flow, usually accompanied with
true lumen narrowing; Type III, SMA dissection with occlusion of
SMA. Misdiagnosis of SISMAD is common in clinical practice, which
may lead to severe intestinal ischemic necrosis or even death
(2,3). With the development of medical imaging,
increasing cases of SISMAD are confirmed in patients with acute
abdominal pain. At present, the diagnosis of SISMAD is mainly based
on computed tomography angiography (CTA) or digital subtraction
angiography (DSA) (4,5). Although CTA has served as an effective
diagnostic tool for SISMAD, its application in clinical practice is
limited by contrast agent allergy, overdose of radiation and high
cost (6). DSA has been considered as
a ‘gold standard’ tool for SISMAD and allows for administration of
treatments whilst diagnostic evaluation is ongoing (7). However, the application of DSA in
emergency settings is limited, as it is invasive, requires a
complex preparation process and is time-consuming (8). Therefore, a diagnostic tool that is
non-invasive, efficient and cost-effective, and has high
sensitivity and specificity is required. Color Doppler sonography
(CDS) has been used for peripheral vascular examination (9). However, the application of CDS in the
assessment of SISMAD has remained to be fully evaluated (10). The current study did not compare
ultrasound with DSA but with CTA in order to identify a more
convenient method to diagnose SISMAD. Of note, CTA is more commonly
used in the diagnosis of SISMAD and DSA is more valuable as an
interventional treatment of SISMAD. According to various studies,
CTA is now almost equal to DSA in its diagnostic ability (11) and in fact, may be a faster and more
noninvasive method. Furthermore, DSA cannot be effectively used in
cases of thrombosis in the false lumen (12). Hence, the current study aimed to
assess the value of CDS in the diagnosis of SISMAD compared with
CTA.
Materials and methods
Patients
A total of 19 SISMAD patients admitted to the
Shandong Medical Imaging Research Institute that were confirmed by
CTA between May 2014 and July 2017, were enrolled in the present
study. All patients first underwent CDS and then CTA. Demographic
and clinical data of all patients were collected, including age,
sex, SISMAD classification, inner diameter (ID), diameter stenosis,
area and area stenosis.
CDS examinations
A GE Vivid 7-dimension Color Doppler ultrasonic
imaging instrument (GE Healthcare, Little Chalfont, UK) with an
adult cardiac probe was used, with the settings of coronary
examination and a tissue harmonic image of 2.0–3.4 MHz. The patient
was placed in a supine position with bent knees and the sampling
cursor was placed on the abdomen. The patient was scanned from the
upper to lower abdomen to assess the aorta, celiac trunk and SMA.
The SMA with branches and aorta was studied in its long axis in the
sagittal plane to evaluate the ID, echo and blood flow signal. The
characteristics and extent of thrombotic false lumen and narrowed
true lumen were evaluated when there was hyperecho segmentation.
The inlet and outlet of true lumen were identified on
cross-sectional scanning. The minimal ID and cross-sectional area
(CSA), diameter stenosis and area stenosis rate and flow rate of
true lumen were assessed and recorded. The insonation angle was
<60°. All sonographers who performed the examinations for the
present study had >10 years of experience in vascular
ultrasonography.
CTA
All SISMAD patients underwent CTA. An iodine
contrast agent allergy test was performed prior to CTA. The iodine
contrast solution was administered by bolus injection via the
antecubital vein. The CTA examination was performed from the aorta
to the bilateral femoral artery. The minimal ID and CSA, diameter
stenosis and area stenosis rate, and flow rate of true lumen were
evaluated though multiplane reorganization, maximum density
projection and volume rendering of captured images.
Statistical analysis
Statistical analysis of the data was performed with
SPSS 20.0 (IBM Corp., Armonk, NY, USA). P<0.05 (two-sided) was
considered to indicate statistical significance. Continuous
variables are expressed as the mean ± standard deviation and
differences between the two imaging modalities were analyzed using
a paired Student's t-test.
Results
Patient data
In the present cohort of 19 patients, the mean age
was 60.4 years (range, 39–87 years) and 16 patients were male. All
patients were admitted for acute abdominal pain (lasting for 3–4
h), and 7 patients were complicated with ileus. Of the 19 patients,
18 were diagnosed as SISMAD with correct classification by CDS,
except one obese patient [body mass index (BMI), 42.5
kg/m2] complicated with severe ileus due to intestinal
gas and incompliance (as the patient did not tolerate the pressure
exerted on the abdomen by the probe). The success rate of the
examination was 94.7%.
Imaging Features
According to the SISMAD classification mentioned
above, the cohort comprised 5 patients with a type I lesion
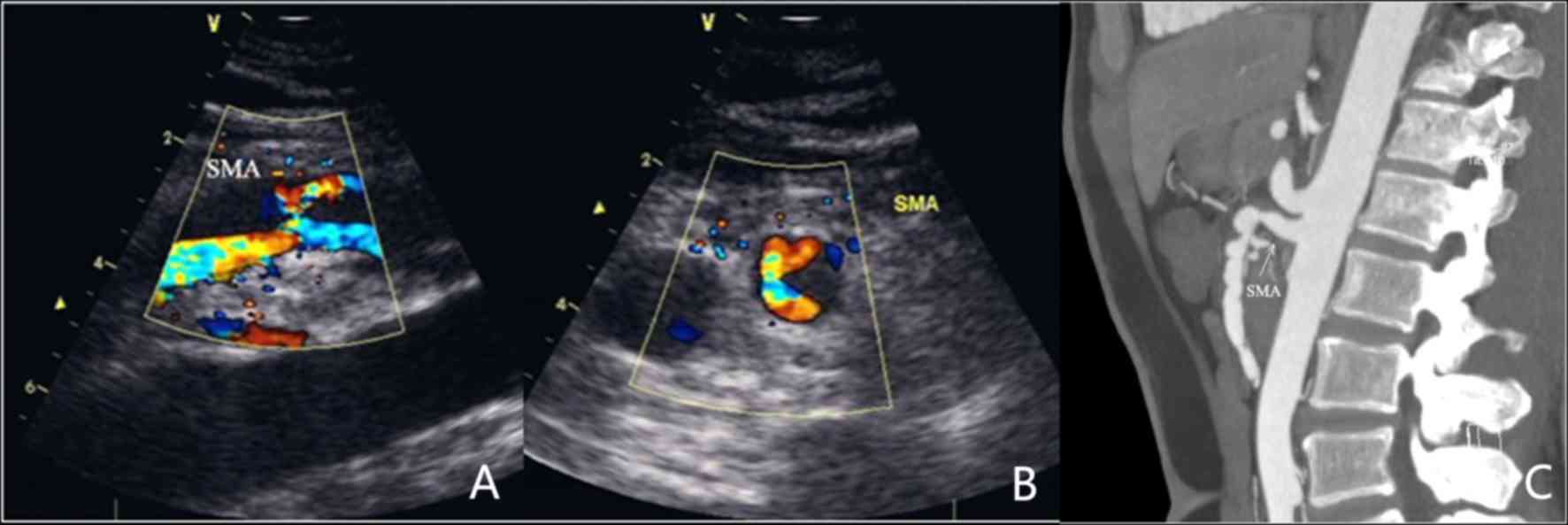
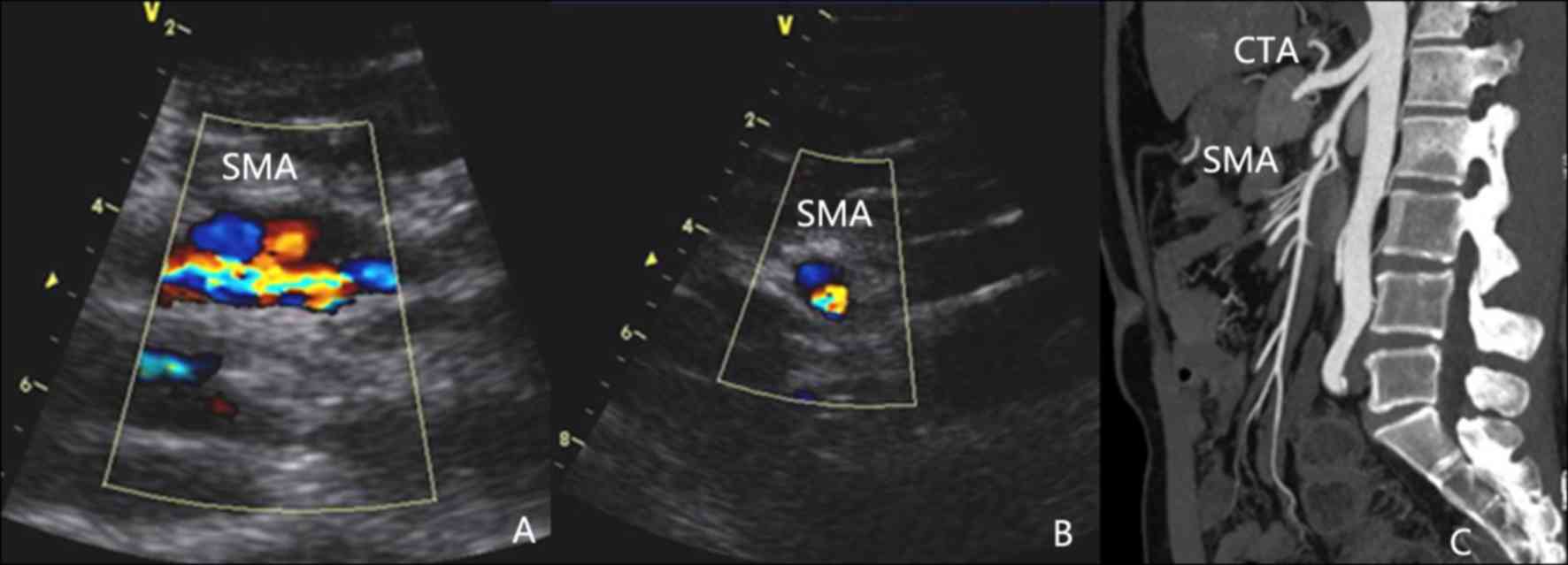
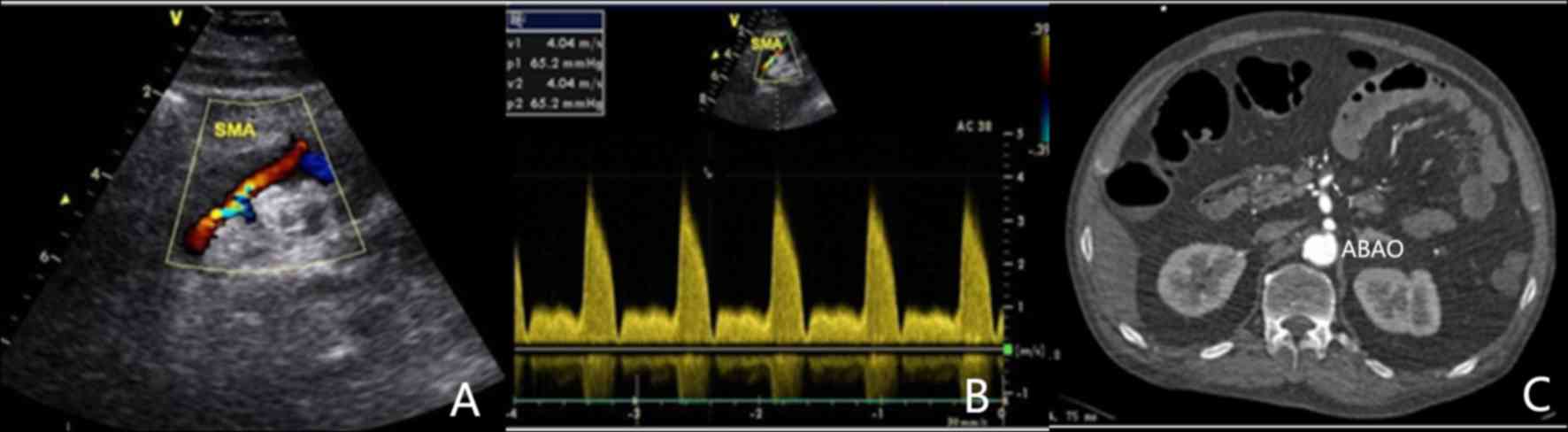
(Fig. 1), 4 with IIa (Fig. 2), 9 with IIb (Fig. 3) and one with a type III lesion
(Fig. 4). The demographic data, as
well as CDS and CTA evaluation indicators, including minimal ID and
CSA, diameter stenosis and area stenosis rate, and flow rate of
true lumen of the 18 SISMAD patients are summarized in Table I.
 | Table I.Characteristics of 18 patients with
SISMAD. |
Table I.
Characteristics of 18 patients with
SISMAD.
|
|
|
| CTA | CDS |
|---|
|
|
|
|
|
|
|---|
| Case no. | Sex | Age (years) | ID (mm) | Area
(mm2) | Diameter stenosis
(%) | Area stenosis
(%) | ID (mm) | Area
(mm2) | Diameter stenosis
(%) | Area stenosis
(%) | V (cm/sec) |
|---|
| 1 | M | 63 | 2.01 | 3.16 | 74.00 | 94.00 | 2.01 | 3.12 | 75.00 | 93.80 | 293 |
| 2 | M | 60 | 2.11 | 3.29 | 73.65 | 93.20 | 2.10 | 3.25 | 73.75 | 93.50 | 286 |
| 3 | F | 58 | 3.00 | 7.50 | 61.70 | 83.00 | 3.11 | 7.54 | 61.73 | 85.00 | 260 |
| 4 | M | 73 | 1.79 | 2.49 | 77.82 | 92.10 | 1.80 | 2.54 | 77.78 | 94.90 | 321 |
| 5 | M | 72 | 1.98 | 3.10 | 75.10 | 92.90 | 2.01 | 3.13 | 74.69 | 93.80 | 310 |
| 6 | M | 48 | 1.18 | 1.15 | 84.90 | 96.40 | 1.20 | 1.13 | 84.62 | 97.70 | 404 |
| 7 | M | 52 | 2.32 | 4.16 | 70.00 | 92.10 | 2.30 | 4.15 | 69.74 | 91.70 | 286 |
| 8 | F | 57 | 3.02 | 7.06 | 62.60 | 86.00 | 3.02 | 7.06 | 62.50 | 85.90 | 240 |
| 9 | M | 69 | 2.41 | 4.55 | 69.10 | 90.00 | 2.42 | 4.52 | 70.00 | 91.00 | 275 |
| 10 | M | 55 | 3.48 | 9.63 | 55.00 | 80.20 | 3.47 | 9.61 | 54.95 | 81.90 | 200 |
| 11 | M | 63 | 2.58 | 5.29 | 67.35 | 88.30 | 2.60 | 5.30 | 67.50 | 89.40 | 240 |
| 12 | M | 54 | 3.62 | 10.14 | 55.00 | 80.00 | 3.61 | 10.17 | 57.32 | 60.92 | 210 |
| 13 | M | 49 | 4.00 | 12.44 | 52.00 | 80.00 | 4.00 | 12.56 | 50.00 | 75.00 | 190 |
| 14 | M | 57 | 2.80 | 6.19 | 65.66 | 89.00 | 2.81 | 6.15 | 65.86 | 87.80 | 220 |
| 15 | M | 39 | 2.75 | 5.73 | 66.00 | 86.00 | 2.72 | 5.72 | 66.25 | 88.60 | 226 |
| 16 | M | 76 | 2.45 | 4.57 | 70.00 | 93.00 | 2.41 | 4.52 | 70.00 | 91.00 | 280 |
| 17 | M | 87 | 2.20 | 3.91 | 74.00 | 90.00 | 2.23 | 3.80 | 72.83 | 92.40 | 324 |
| 18 | F | 66 | 6.00 | 29.00 | 23.50 | 41.62 | 6.10 | 29.20 | 23.75 | 41.88 | 160 |
On CDS, the 18 SISMAD patients were characterized by
enlarged lumen from the origin of the SMA, dorsal true lumen and
ventral false lumen divided by hyperecho segmentation. In addition,
it was possible to distinguish the velocity of blood flow in the
true lumen and false lumen, with the faster one being that in the
true lumen. All dissecting lesions had a large false lumen, parts
of which were present as solid echo filling and absence of blood
flow signal. The true lumen was subjected to different degrees of
compression. A total of 8 lesions had a severely narrowed true
lumen, accounting for 10–30% of the entire lumen and with a luminal
blood flow velocity of 275–404 cm/sec.
All SISMAD patients were diagnosed by CTA, which
revealed similar characteristics to those identified on CDS,
including enlarged lumen from the origin of the SMA, true lumen and
false lumen distinguished by hyperecho segmentation and thrombotic
false lumen. In certain patients, a clear perforation of the
diaphragm was visible on ultrasonic imaging.
Statistical data
Within the cohort, there was no significant
difference between CDS and CTA in terms of the mean minimal ID
(2.77±1.08 vs. 2.76±1.06), CSA (6.86±6.30 vs. 6.85±6.25), diameter
stenosis (64.52±14.33 vs. 67.33±9.15) and area stenosis
(85.34±13.82 vs. 85.99±12.19; all P>0.05; Table II).
 | Table II.Comparison of parameters measured by
CDS and CTA for patients with spontaneous isolated superior
mesenteric artery dissection. |
Table II.
Comparison of parameters measured by
CDS and CTA for patients with spontaneous isolated superior
mesenteric artery dissection.
| Parameter | CDS | CTA | t | P-value |
|---|
| ID (mm) | 2.77±1.08 | 2.76±1.06 | 1.339 | 0.198 |
| Diameter stenosis
(%) | 64.52±14.33 | 67.33±9.15 | −0.680 | 0.507 |
| Area
(mm2) | 6.86±6.30 | 6.85±6.25 | 0.376 | 0.712 |
| Area stenosis
(%) | 85.34±13.82 | 85.99±12.19 | −0.551 | 0.588 |
Discussion
Patients with SISMAD always present with atypical
clinical symptoms, most of which are sudden abdominal pain
(13–16). It is a relatively rare underlying
cause of acute abdominal pain. While the pathogenesis of SISMAD has
not been fully elucidated, it may be caused by hypertension,
vasculitis, arterial mid-lamellar cystic necrosis, fibromuscular
dysplasia and atherosclerosis (2,17–19).
According to certain scholars, the anatomical features of the SMA
arch may induce focused shear forces, which may ultimately produce
a dissecting lesion that is similar to the DeBakey type III aortic
dissection (20).
At present, CTA is considered a preferred method for
diagnosing SISMAD, which is able to clearly display the
characteristics of the SMAD and distal small branch vessels, and
facilitate classification (21).
However, contrast agent allergy, overdose of radiation and
expensive cost hamper the wide application of CTA in SISMAD
(22). CDS is a type of
non-invasive, efficient and cost-effective diagnostic tool for
SISMAD, while having a comparatively a higher sensitivity and
specificity (23). In the present
study, two-dimensional images of the trunk and branches of the SMA
were more clearly displayed by using a cardiac probe and the
coronary harmonic imaging setting. As the cardiac probe is
relatively small, it may effectively squeeze away intestinal gas
and is also more sensitive to blood flow than the convex array
probe (24). Furthermore, in the
present study, that there was no significant difference between
mean minimal ID, CSA, diameter stenosis and area stenosis rate
determined by CTA and CDS. Therefore, the use of CDS, which is more
convenient and inexpensive compared with CTA, is feasible for the
diagnosis of SISMAD.
SISMAD should be distinguished from atherosclerosis,
embolism and abdominal aortic dissection involving the SMA.
Atherosclerotic stenosis usually develops at the origin part of the
SMA, and is more common in the elderly (25). The ultrasonic characteristics include
a convex plaque located at the origin part of the SMA, narrowing of
the lumen and increased blood flow velocity (26). The SMA embolism mostly results from
cardiac causes (27). For instance,
in patients with atrial fibrillation or myocardial infarction, the
left atrial or ventricular thrombus may fall off into the SMA,
which may appear on ultrasound as an intraluminal hypoechoic solid
tissue filling without blood flow (28). The detached thrombus usually remains
at the bifurcation (29). Patients
with abdominal aortic dissection involving the SMA feature
segmentation in the abdominal aorta extending into the SMA
(30). However, in patients with
SISMAD, the location of the dissecting lesion is only between the
beginning and the distal end of the SMA, with a normal abdominal
aorta (31).
In the present study, one obese patient (BMI, 42.5
kg/m2) was not able to successfully undergo CDS even
after fasting and gastrointestinal decompression due to long
disease duration (32), intestinal
gas and intolerance to pressure on the abdomen exerted by the
probe. In this patient, it was not possible to clearly display the
SMA. For such cases, CTA or DSA should be performed if mesenteric
arterial dissection is suspected to avoid delay of diagnosis.
Of note, the present study had certain limitations.
First, the sample size was relatively small. Furthermore,
ultrasound examination was greatly affected by intestinal gas, and
it has certain limitations for patients with obvious
flatulence.
For the clinical treatment of SISMAD, conservative
treatment or endovascular repair has been commonly used. In the
present study, a total of 9 patients underwent luminal stenting, 9
patients received conservative treatment and 1 patient died due to
prolonged bowel ischemia. In the latter case, a marked delay
between disease onset and presentation of the patient at the clinic
was present, and at the time-point of diagnosis, the patient had
developed widespread bowel ischemic necrosis and infection. In the
setting of CTA examination, the degree of ischemia may only be
quantitatively evaluated according to the proportion of true and
false lumens (33). CDS is not only
able to evaluate the proportion of true and false lumens, but to
also accurately measure the blood flow velocity in the true lumen
(34). In particular, the
significantly decreased ratio of the CSA of true and false lumen
and the blood flow velocity of >275 cm/sec in the fasting state
indicates that the true lumen is severely compressed. A higher
blood flow velocity is associated with a more severe compression of
the true lumen (35), and a greater
extend of bowel ischemia. For patients with ultrasonic features of
solid echo filling without flow in the true and false lumen, open
surgery or endovascular repair is suggested due to severe SMA
ischemia. Otherwise, conservative treatment may be performed first
and the blood supply of the SMA should be carefully observed
(36,37).
In conclusion, CDS is able to not only diagnose
SISMAD, but also to measure minimal ID and CSA, diameter stenosis
and area stenosis rate, and flow rate of the true lumen. CDS may
serve as a useful tool for diagnosis of SISMAD.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shandong Provincial
Medical Science and Technology Development Program (grant no.
2015WS0185).
Availability of data and materials
The data used and analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
HQ designed the present study, acquired, analyzed
and interpreted the data, and approved the final version of the
manuscript. CL acquired the data and performed image analysis. SB
performed image examinations. DD analyzed the data. XM interpreted
the data. TW searched the literature. XJ, SZ and SB prepared the
manuscript. XZ performed statistical analysis. All authors have
read and approved the final manuscript.
Ethics approval and informed consent
Informed consent was obtained from each patient and
the study was approved by the ethics review board of the Provincial
Hospital Affiliated to Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CDS
|
color Doppler sonography
|
|
SISMAD
|
spontaneous isolated superior
mesenteric artery dissection
|
|
CSA
|
cross-sectional area
|
|
SMA
|
superior mesenteric artery
|
|
CTA
|
computed tomography angiography
|
|
DSA
|
digital subtraction angiography
|
|
ID
|
inner diameter
|
References
|
1
|
Yun WS, Kim YW, Park KB, Cho SK, Do YS,
Lee KB, Kim DI and Kim DK: Clinical and angiographic follow-up of
spontaneous isolated superior mesenteric artery dissection. Eur J
Vasc Endovasc Surg. 37:572–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HK, Jung HK, Cho J, Lee JM and Huh S:
Clinical and radiologic course of symptomatic spontaneous isolated
dissection of the superior mesenteric artery treated with
conservative management. J Vasc Surg. 59:465–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park UJ, Kim HT, Cho WH, Kim YH and Miyata
T: Clinical course and angiographic changes of spontaneous isolated
superior mesenteric artery dissection after conservative treatment.
Surg Today. 44:2092–2097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li S, Gu X, Jiang G and Tian F: Comment on
‘The value of a new image classification system for planning
treatment and prognosis of spontaneous isolated superior mesenteric
artery dissection’. Vascular. 23:5582015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Zhao S, Li J, Huang Z, Luo C and
Yang L: Value of Multi-detector CT in detection of isolated
spontaneous superior mesenteric artery dissection. Chin Med Sci J.
32:28–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoo J, Lee JB, Park HJ, Lee ES, Park SB,
Kim YS and Choi BI: Classification of spontaneous isolated superior
mesenteric artery dissection: Correlation with multi-detector CT
features and clinical presentation. Abdom Radiol (NY).
43:3157–3165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichiba T, Hara M, Yunoki K, Urashima M and
Naitou H: Serial follow-up evaluation with computed tomography
after conservative medical treatment in patients with symptomatic
spontaneous isolated superior mesenteric artery dissection. Vasc
Endovascular Surg. 51:538–544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng K, Gao Y, Chu E, Shen B, Gao D and
Luo J: CT angiography features of the involved arterial branches of
the spontaneous isolated superior mesenteric artery dissection.
Zhonghua Wei Chang Wai Ke Za Zhi. 17:264–267. 2014.(In Chinese).
PubMed/NCBI
|
|
9
|
Funahashi H, Shinagawa N, Saitoh T, Takeda
Y and Iwai A: Conservative treatment for isolated dissection of the
superior mesenteric artery: Report of two cases. Int J Surg Case
Rep. 26:17–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko SH, Hye R and Frankel DA: Management of
spontaneous isolated visceral artery dissection. Ann Vasc Surg.
29:470–474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia Z, Huang Y, Shi H, Tang L, Shi H, Qian
L and Jiang G: Comparison of CTA and DSA in the diagnosis of
superior mesenteric artery dissecting aneurysm. Vascular.
26:346–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zerbib P, Perot C, Lambert M, Seblini M,
Pruvot FR and Chambon JP: Management of isolated spontaneous
dissection of superior mesenteric artery. Langenbecks Arch Surg.
395:437–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong Z, Fu W, Chen B, Guo D, Xu X and Wang
Y: Treatment of symptomatic isolated dissection of superior
mesenteric artery. J Vasc Surg. 57 (2 Suppl):69S–76S. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jain A, Tracci MC, Coleman DM, Cherry KJ
and Upchurch GR Jr: Renal malperfusion: Spontaneous renal artery
dissection and with aortic dissection. Semin Vasc Surg. 26:178–188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noh M, Kwon H, Jung CH, Kwon SU, Kim MS,
Lee WJ, Park JY, Han Y, Kim H, Kwon TW and Cho YP: Impact of
diabetes duration and degree of carotid artery stenosis on major
adverse cardiovascular events: A single-center, retrospective,
observational cohort study. Cardiovasc Diabetol. 16:742017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rong JJ, Qian AM, Sang HF, Meng QY, Zhao
TJ and Li XQ: Immediate and middle term outcome of symptomatic
spontaneous isolated dissection of the superior mesenteric artery.
Abdom Imaging. 40:151–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afshinnia F, Sundaram B, Rao P, Stanley J
and Bitzer M: Evaluation of characteristics, associations and
clinical course of isolated spontaneous renal artery dissection.
Nephrol Dial Transplant. 28:2089–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Min SI, Yoon KC, Min SK, Ahn SH, Jae HJ,
Chung JW, Ha J and Kim SJ: Current strategy for the treatment of
symptomatic spontaneous isolated dissection of superior mesenteric
artery. J Vasc Surg. 54:461–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satokawa H, Takase S, Seto Y, Yokoyama H,
Gotoh M, Kogure M, Midorikawa H, Saito T and Maehara K: Management
strategy of isolated spontaneous dissection of the superior
mesenteric artery. Ann Vasc Dis. 7:232–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu XM, Wang TD and Chen MF: Percutaneous
endovascular treatment for isolated spontaneous superior mesenteric
artery dissection: Report of two cases and literature review.
Catheter Cardiovasc Interv. 73:145–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katsura M, Mototake H, Takara H and
Matsushima K: Management of spontaneous isolated dissection of the
superior mesenteric artery: Case report and literature review.
World J Emerg Surg. 6:162011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia ZZ, Zhao JW, Tian F, Li SQ, Wang K,
Wang Y, Jiang LQ and Jiang GM: Initial and middle-term results of
treatment for symptomatic spontaneous isolated dissection of
superior mesenteric artery. Eur J Vasc Endovasc Surg. 45:502–508.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo BR, Han HY, Cho YK and Park SJ:
Spontaneous rupture of a middle colic artery aneurysm arising from
superior mesenteric artery dissection: Diagnosis by color Doppler
ultrasonography and CT angiography. J Clin Ultrasound. 40:255–259.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim H, Park H, Park SJ, Park BW, Hwang JC,
Seo YW and Cho HR: Outcomes of spontaneous isolated superior
mesenteric artery dissection without antithrombotic use. Eur J Vasc
Endovasc Surg. 55:132–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka Y, Yoshimuta T, Kimura K, Iino K,
Tamura Y, Sakata K, Hayashi K, Takemura H, Yamagishi M and
Kawashiri MA: Clinical characteristics of spontaneous isolated
visceral artery dissection. J Vasc Surg. 67:1127–1133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimura Y, Kato T and Inoko M: Outcomes of
treatment strategies for isolated spontaneous dissection of the
superior mesenteric artery: A systematic review. Ann Vasc Surg.
47:284–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tomita K, Obara H, Sekimoto Y, Matsubara
K, Watada S, Fujimura N, Shibutani S, Nagasaki K, Hayashi S, Harada
H, et al: Evolution of computed tomographic characteristics of
spontaneous isolated superior mesenteric artery dissection during
conservative management. Circ J. 80:1452–1459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu SY, Hsu MY, Chen CM, Yeow KM, Hung CF,
Su IH, Shie RF and Pan KT: Endovascular repair of spontaneous
isolated dissection of the superior mesenteric artery. Clin Radiol.
67:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CF, Lai HC, Yao HY, Cheng YT, Lee
WL, Wang KY and Liu TJ: True lumen stenting for a spontaneously
dissected superior mesenteric artery may compromise major
intestinal branches and aggravate bowel ischemia. Vasc Endovascular
Surg. 48:83–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garrett HE Jr: Options for treatment of
spontaneous mesenteric artery dissection. J Vasc Surg.
59:1433–1439.e1-e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kimura Y, Kato T, Nagao K, Izumi T, Haruna
T, Ueyama K, Inada T and Inoko M: Outcomes and radiographic
findings of isolated spontaneous superior mesenteric artery
dissection. Eur J Vasc Endovasc Surg. 53:276–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dua A, Desai SS, Nodel A and Heller JA:
The impact of body mass index on lower extremity duplex
ultrasonography for deep vein thrombosis diagnosis. Ann Vasc Surg.
29:1136–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitsuoka H, Nakai M, Terai Y, Gotou S,
Miyano Y, Tsuchiya K and Yamazaki F: Retrograde stent placement for
symptomatic spontaneous isolated dissection of the superior
mesenteric artery. Ann Vasc Surg. 35:203.e17–e21. 2016. View Article : Google Scholar
|
|
34
|
Ranschaert E, Verhille R, Marchal G,
Rigauts H and Ponette E: Sonographic diagnosis of ischemic colitis.
J Belge Radiol. 8:166–168. 1994.
|
|
35
|
Nagai T, Torishima R, Uchida A, Nakashima
H, Takahashi K, Okawara H, Oga M, Suzuki K, Miyamoto S, Sato R, et
al: Spontaneous dissection of the superior mesenteric artery in
four cases treated with anticoagulation therapy. Intern Med.
43:473–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heo SH, Kim YW, Woo SY, Park YJ, Park KB
and Kim DK: Treatment strategy based on the natural course for
patients with spontaneous isolated superior mesenteric artery
dissection. J Vasc Surg. 65:1142–1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mitchell EL and Moneta GL: Mesenteric
duplex scanning. Perspect Vasc Surg Endovasc Ther. 18:175–183.
2006. View Article : Google Scholar : PubMed/NCBI
|