Introduction
Fibroblast growth factor-2 (FGF-2) is reported to
have various functions and serve numerous regulatory functions in
complex organisms (1). FGF-2 is
reported to protect cells from various forms of death, such as
apoptosis or necrosis through inhibition of autophagy (2) and implicated in satellite cell
self-renewal and differentiation (3). FGF-2 is a key human mesenchymal stem
cell mitogen, often supplemented to increase human mesenchymal stem
cell growth rates (4). The
pro-survival effect of FGF-2 was seen from apoptotic cell death
(5). Suppression of FGF-2 expression
inhibited neural stem cell proliferation (6), and preconditioning with FGF-2
significantly increased production of the periodontal stem cells'
angiogenic secretome, especially vascular endothelial growth factor
and placental growth factor secretion (7).
Moreover, FGF-2 has been involved in the
differentiation of tested cells. FGF-2 was associated with
accelerated dedifferentiation during expansion culture and superior
re-differentiation upon induction (8). Fully dedifferentiated chondrocytes
expanded in a specific mesenchymal stem cell growth medium with
FGF-2 obtained the mesenchymal stem cell phenotype in an in
vitro environment (8). However,
in an in vivo environment, fully dedifferentiated
chondrocytes retained the chondrocyte phenotype. FGF-2 is an
important neurotrophic factor that can stimulate neurogenesis and
angiogenesis, and has been shown to have neuroprotective effects
after brain injuries (2).
In recent years, a cell spheroid culture system
using stem cells has been used for cell therapy (9). These stem-cell spheroids were shown to
maintain stem cell characteristics and differentiation ability
(10). This study was performed to
evaluate the effects of FGF-2 on cellular viability and osteogenic
differentiation using three-dimensional cell spheroids of stem
cells.
Materials and methods
Three-dimensional cultures of human
bone marrow-derived stem cells
The Institutional Review Board (Seoul St Mary's
Hospital, College of Medicine, The Catholic University of Korea)
reviewed and approved the present work (KC18SESI0083), and all of
the experimental schemes used were performed according to relevant
guidelines. Human mesenchymal stem cells derived from bone marrow
(BMSCs, Catholic MASTER Cells) were procured from the Catholic
Institute of Cell Therapy (CIC). The Catholic MASTER Cells supplied
by Catholic Institute of Cell Therapy were derived from human bone
marrow donated by healthy donors after informed consent was
obtained. Isolation and propagation of the BMSCs were performed
following a previously reported method (11). The Catholic Institute of Cell Therapy
has verified that all the samples showed CD73 and CD90 expression
was >90% positive. The cells were plated on the culture dish,
and cells that were detached from the dish were eliminated. The
culture medium was refreshed every 2 or 3 days, and the BMSCs were
nurtured with 95% O2 and 5% CO2 at 37°C in
the incubator.
Fig. 1 shows the
overview of the study design. Six mouth rinses were applied for
this study. We used silicon elastomer-based concave microwells
(H389600, StemFIT 3D; MicroFIT) with 600 µm diameters to make stem
cell spheroids. A total of 1×106 cells were loaded into
each well and cultured to evaluate the cell response. Cell
spheroids made of bone marrow-derived stem cells were treated with
FGF-2 at 0, 30, 60 and 90 ng/ml concentration. The spheroids'
changes in morphology were evaluated using an inverted microscope
(Leica DMIRM; Leica Microsystems). The changes in the spheroids'
diameter were evaluated on Days 1, 3, 5, and 7.
Determination of cellular
viability
Stem cell spheroids were cultured in growth media
composed of an alpha-minimal essential medium (α-MEM; Gibco; Thermo
Fisher Scientific, Inc.) comprising 200 mM of L-Glutamine, 10 mM of
ascorbic acid 2-phosphate, 100 µg/ml of streptomycin (all from
Sigma-Aldrich; Merck KGaA), 15% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.; 100 U/ml of penicillin, 2 mg/ml of
glycerophosphate disodium salt hydrate, and 38 µg/ml of
dexamethasone.
Qualitative analysis for cellular viability was done
using the commercially available Live/Dead Kit assay (Molecular
Probes) on Day 7. In short, these spheroids were washed twice with
the growth media, followed by suspension in 1 ml of α-MEM
containing 2 µl of 50 mM calcein acetoxymethyl ester working
solution and 4 µl of 2 mM ethidium homodimer-1 for 30 min at room
temperature. The intracellular esterase makes non-fluorescent,
cell-permeant calcein acetoxymethyl ester in intact cells to
produce green fluorescence. The ethidium homodimer enters the
damaged cells and produces a red fluorescence. We observed the
spheroids stained with calcein acetoxymethyl ester and ethidium
homodimer-1 using a fluorescence microscope (Axiovert 200;
Zeiss).
The commercially available cell counting kit-8
(CCK-8; Dojindo) was used for the quantitative analysis of cell
viability on Days 1, 3, 5, and 7. WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium, monosodium salt] was added and the stem cell spheroids
were cultured for 45 min at 37°C. The absorbance of the samples was
spectrophotometrically measured at 450 nm using a microplate reader
(BioTek).
Alkaline phosphatase activity assays
and Alizarin Red S staining
Cell spheroids grown on culture plates with
osteogenic media composed of α-MEM (Gibco; Thermo Fisher
Scientific, Inc.), containing 200 mM of L-Glutamine, 10 mM of
ascorbic acid 2-phosphate, 100 µg/ml of streptomycin (all from
Sigma-Aldrich; Merck KGaA), 15% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml of penicillin, 2 mg/ml of
glycerophosphate disodium salt hydrate, and 38 µg/ml of
dexamethasone, were obtained on Days 1, 3, and 7. We used a
commercially available kit (K412-500; BioVision, Inc.) for the
evaluation of alkaline phosphatase activity. The cells were
suspended again with an assay buffer, sonicated, and then
centrifuged to remove insoluble material. The mixture of
supernatant and a p-nitrophenylphosphate substrate was incubated at
25°C for 40 min. The resultant p-nitrophenol was
spectrophotometrically measured at 405 nm.
On Days 13, the stem cell spheroids were washed
twice with phosphate-buffered saline (Welgene, Gyeongsan-si,
Gyeongsangbuk-do, Republic of Korea), fixed with 4%
paraformaldehyde, and washed with deionized water. The cultures
were then stained with Alizarin Red S for 30 min at room
temperature. Evaluation of the morphology was then performed using
an inverted microscope (Leica DM IRM). Before removing
nonspecifically-bound stains, the cultures were washed three times
with deionized water. Quantification was done by solubilizing the
bound dye using 10 mM sodium phosphate containing 10%
cetylpyridinium chloride and evaluated spectrophotometrically at
560 nm.
Statistical analysis
The data were shown as means ± standard deviations
of the experiments. A test of normality and the equal of variances
in the samples were conducted. Two-way analysis of variance test
with Tukey's post hoc test was used to assess statistical
differences between the test groups and the control groups with
different time-points using the statistical program SPSS 12 for
Windows (SPSS Inc.). Statistical significance was set at
P<0.05.
Results
Formation of cell spheroids with human
gingiva-derived stem cells
Spheroids were well formed in silicon
elastomer-based concave microwells on Day 1 (Fig. 2). No significant morphological change
of the cell spheroids cultured in growth media was observed after
the addition of FGF-2 at 10 and 100 ng/ml concentration. The
morphology results of Days 3, 5, and 7 are shown in Fig. 1, and no noticeable changes were noted
with longer incubation time.
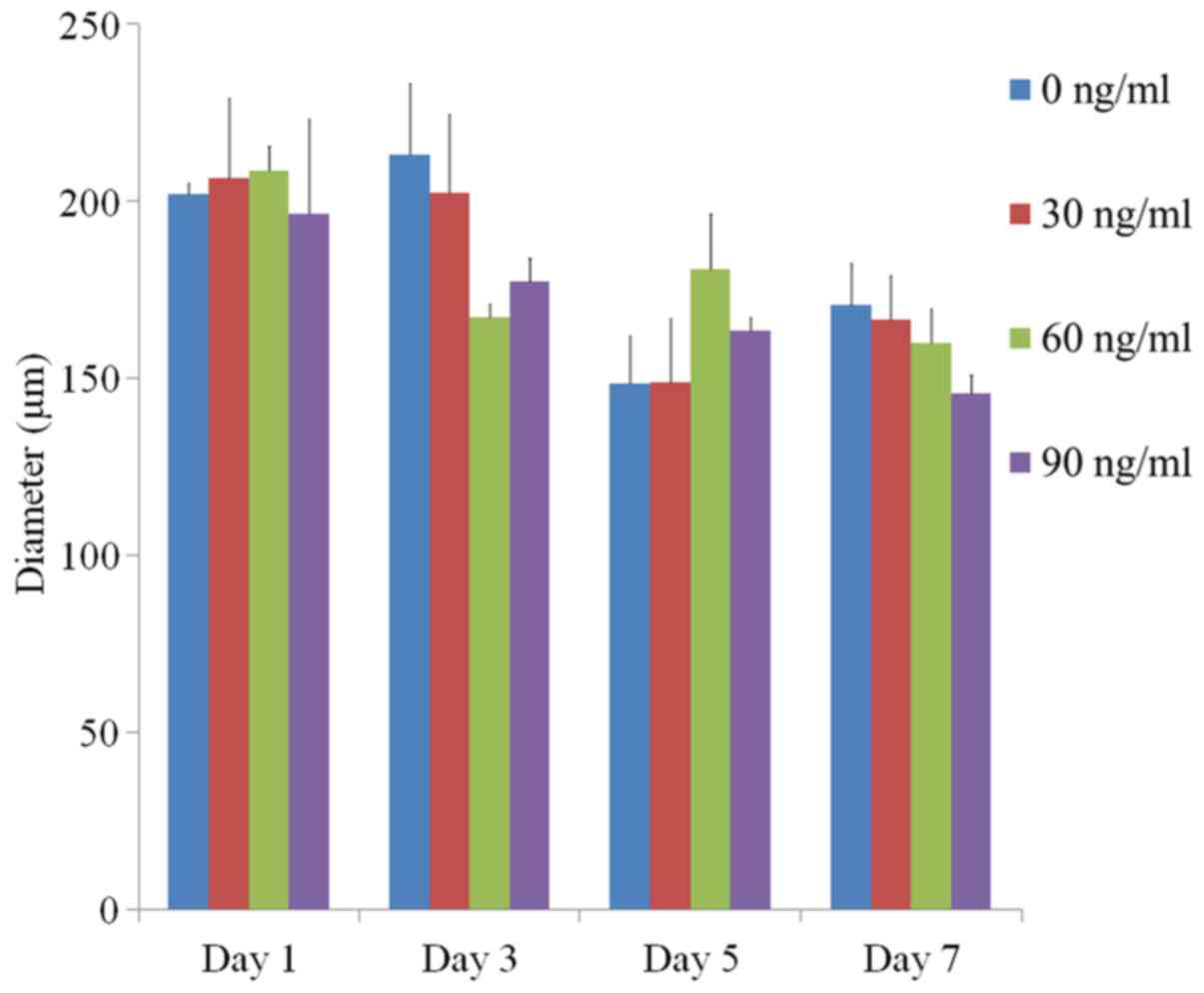
The average spheroid diameters at Day 1 for FGF-2 at
0, 10 and 100 ng/ml were 202.2±3.0, 206.6±22.6, 208.8±6.8 and
196.6±26.7 µm, respectively (P>0.05) (Fig. 3). The average spheroid diameters at
Day 3 were 213.1±20.3, 202.5±22.3, 167.2±3.9 and 177.6±6.3 µm,
respectively (P>0.05). The values for Day 5 were 148.7±13.5,
148.8±18.1, 181.0±15.5 and 163.7±3.7 µm, respectively (P>0.05).
The average diameters at Day 7 were 170.8±12.0, 166.6±12.7,
160.0±9.8 and 145.9±5.2 µm, respectively (P>0.05).
Determination of cellular
viability
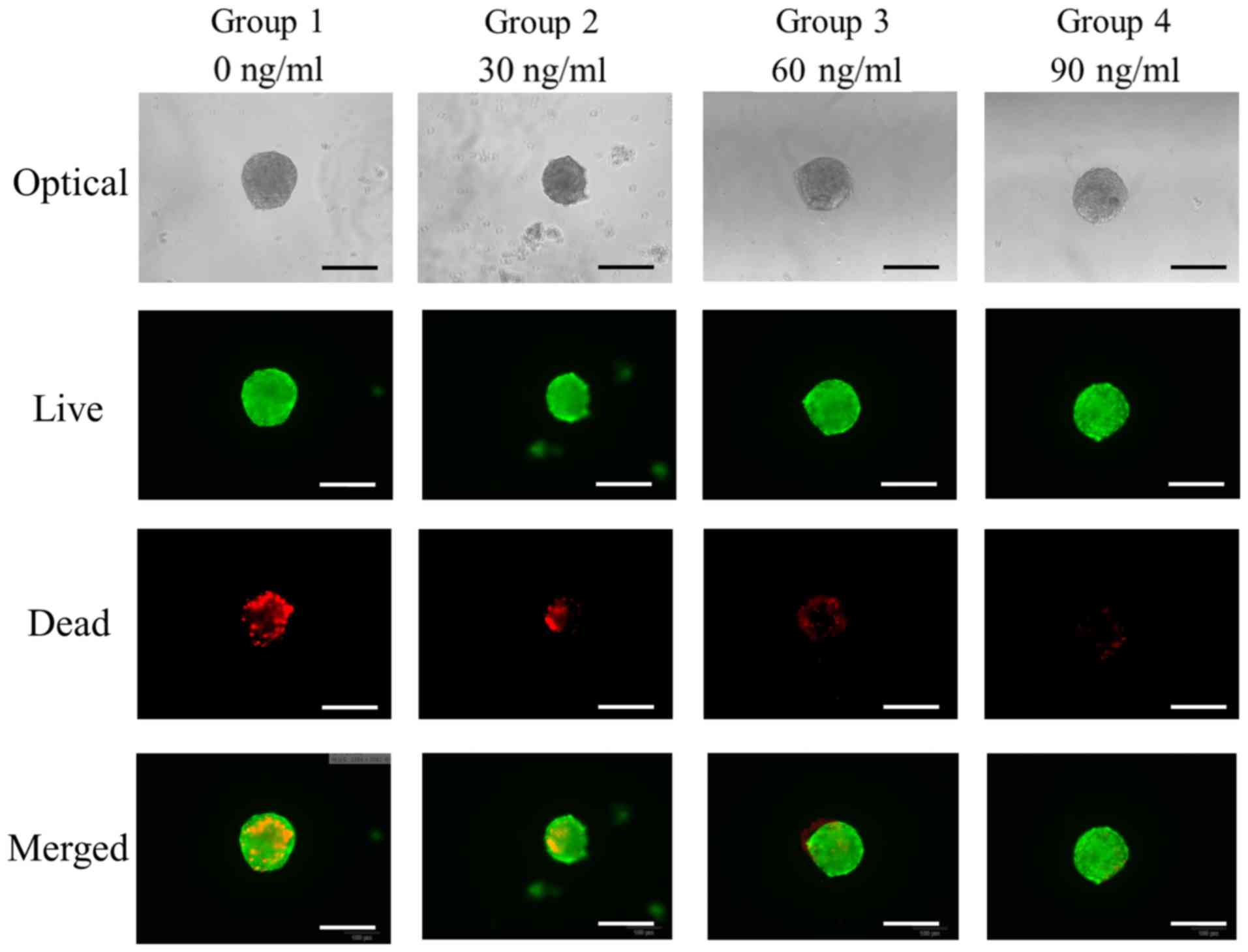
The qualitative results of viability of cell
spheroids were analyzed using a Live/Dead Kit assay at Day 7
(Fig. 4). In all cases, most of the
cells in the cell spheroids emitted green fluorescence.
The quantitative value for cellular viability on
Days 1, 3, 5, and 7 is shown in Fig.
5. The relative Cell Counting Kit-8 assay values for FGF-2 at
0, 30, 60, and 90 ng/ml at Day 1 were 100.0±5.5, 101.8±8.8,
99.2±4.8 and 103.4±9.6%, respectively. There were no statistically
significant differences between the groups on Day 1 (P>0.05).
Significant differences were noted between the groups with longer
incubation time in each group (P<0.05).
Alkaline phosphatase activity assays
and Alizarin Red S staining
The results of the alkaline phosphatase activity
assays at Days 1, 3, 5, and 7 are shown in Fig. 6. The absorbance values at 405 nm at
Day 7 for groups 1, 2, 3, and 4 were 0.422±0.005, 0.420±0.008,
0.492±0.005 and 0.397±0.028, respectively. The highest value was
noted for FGF-2 groups at 60 ng/ml concentration, but this did not
reach the statistical significance (P>0.05).
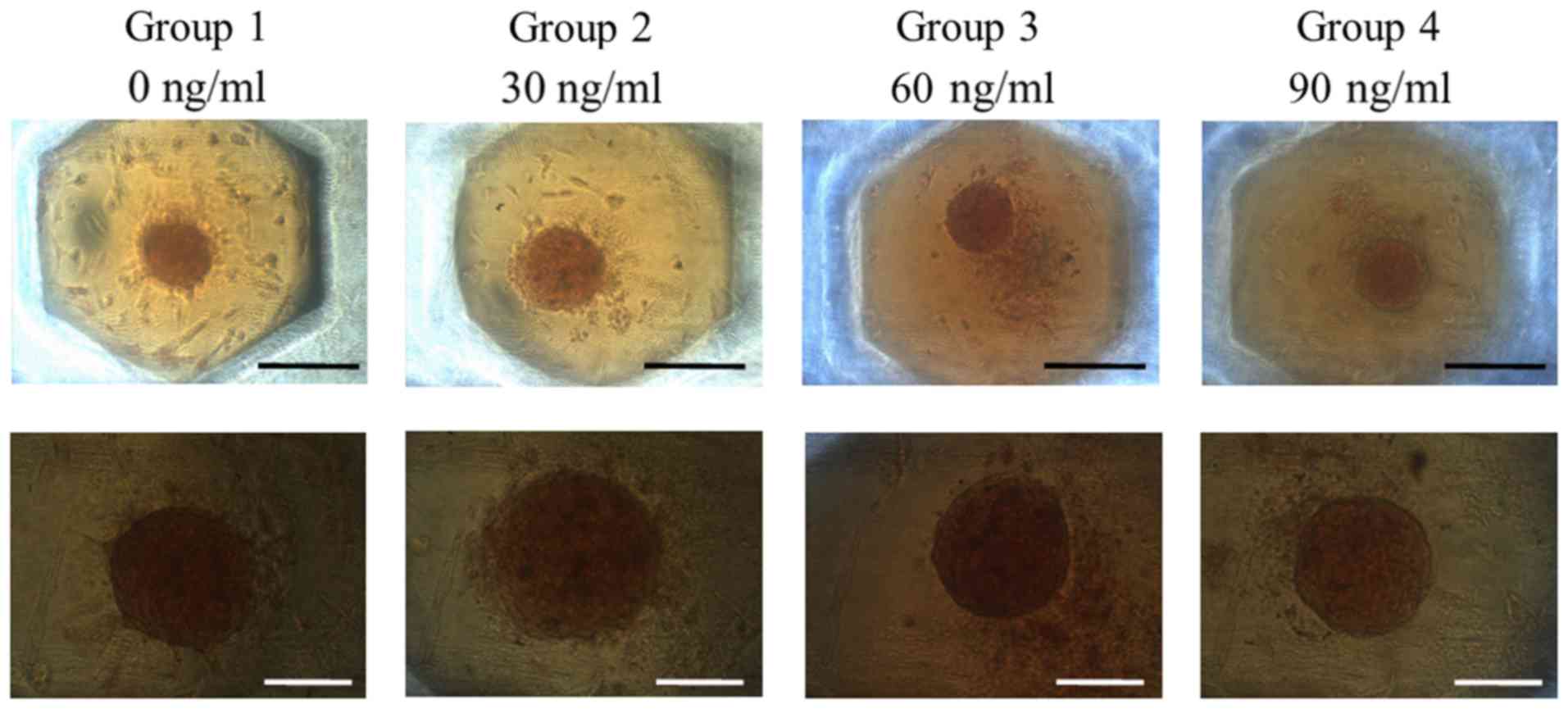
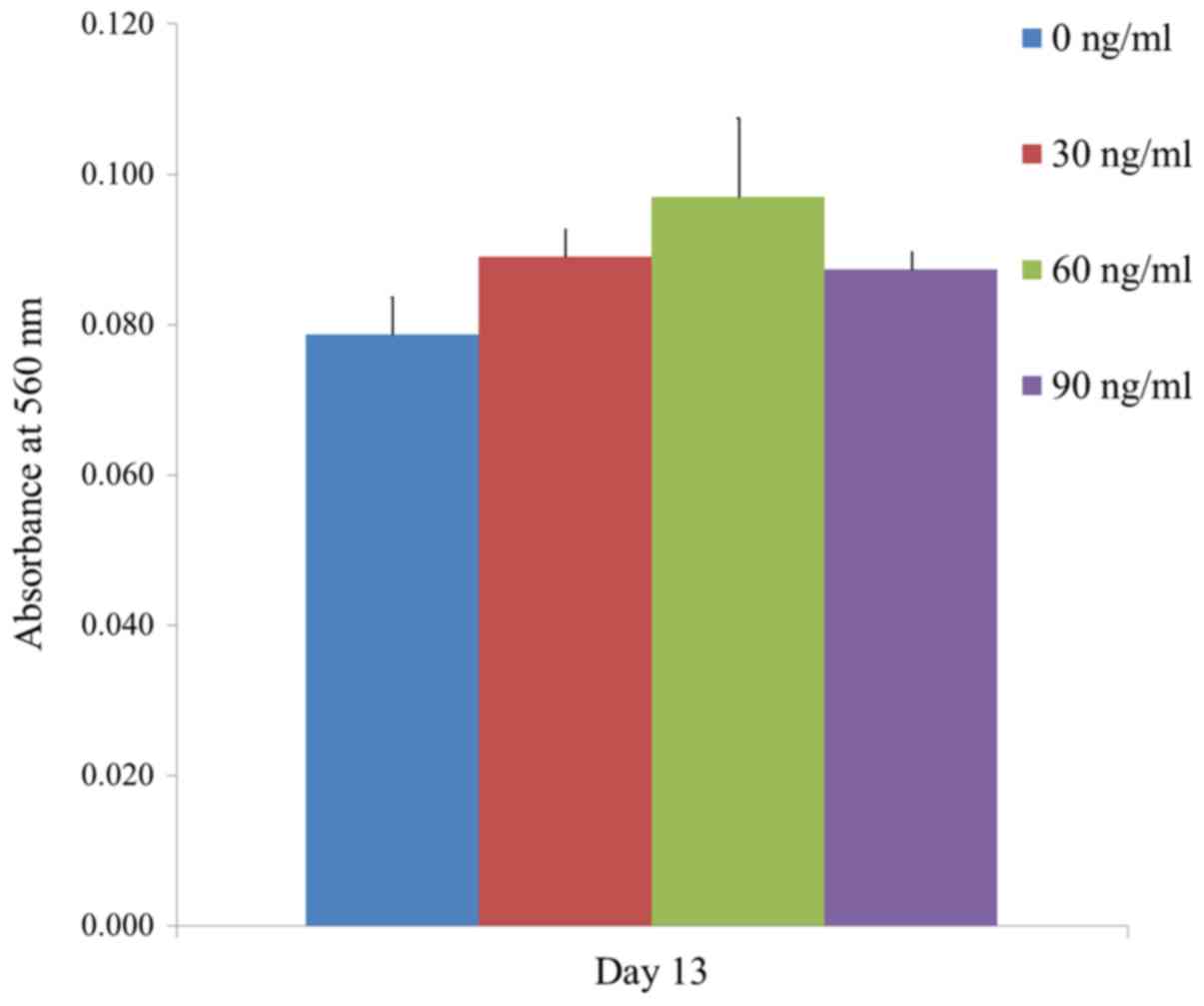
The results of the mineralization assay at Day 13
are shown in Fig. 7. Mineralized
extracellular deposits were evenly noted in each group. The
absorbance values at 405 nm at Day 13 for groups 1, 2, 3, and 4
were 0.079±0.005, 0.089±0.004, 0.097±0.010 and 0.087±0.002,
respectively. The quantification results showed that the highest
value was noted for FGF-2 groups at 60 ng/ml concentration when
compared with the value of the control, but this did not reach the
statistical significance (Fig. 8)
(P>0.05).
Discussion
This report discusses the effects of FGF-2 on
cellular viability and osteogenic differentiation using cell
spheroids of stem cells. The application of FGF-2 increased
alkaline phosphatase activity or Alizarin Red S staining at 60
ng/ml concentration.
Previous reports showed that FGF-2 induced cell
proliferation, survival, migration, and differentiation in various
cell types and tissues, and FGF-2 has been applied for clinical use
in the regeneration of damaged tissues (12). FGF-2 played an important role in
promoting wound healing and reducing scar tissue (13), and was suggested to be a potential
therapeutic agent for improving stem cell-based approaches for the
treatment of diabetes mellitus and its complications (14). Synergistic actions of FGF-2 and bone
marrow transplantation mitigate radiation-induced intestinal injury
(15).
Controversial results have been reported on cell
differentiation. Recombinant human FGF-2 inhibited osteogenic
induction and mineralization in human periodontal ligament-derived
cells (12). FGF-2 is reported to
show an antagonistic effect on the hard tissue differentiation
induced by bone morphogenetic protein-2 and bone morphogenetic
protein-4 (16). Suppression of
FGF-2 expression inhibited neural stem cell differentiation
(6). In another report, FGF-2
regulated and supported osteoblastic niche cells during
hematopoietic homeostasis recovery after bone marrow suppression
(17). The stimulatory effects of
FGF-2 signaling were seen on odontoblast differentiation from early
progenitors in dental pulp (18).
There has been efficient generation of neurons from human bone
marrow derived mesenchymal stem cells using FGF-2 alone (19).
Previous reports have attempted to reveal the
mechanisms of FGF-2. FGF-2 induced proliferative action, dependent
on SK1 and SK2, as well as xphingosine 1-phosphate and sphingosine
kinase (5). FGF-2 reduced scar
tissue by inhibiting the differentiation of epidermal stem cells to
myofibroblasts via the Notch1/Jagged1 pathway (13). FGF-2 shows the effects by repressing
MyoD, and Linc-RAM is required for FGF-2 function in regulating
myogenic cell differentiation (3).
FGF-2 acts due to suppression of endocytoplasmic reticulum stress
(20).
Combination therapy is used for the application of
FGF-2. Dual delivery of FGF-2 and nerve growth factor coacervater
ameliorates diabetic peripheral neuropathy via inhibiting Schwann
cells apoptosis (20). When
accompanied by connective tissue growth factor, FGF-2 has a
slightly additive effect on fibrogenic differentiation of
mesenchymal stem cells (21).
Adenovirus-mediated expression of FGF-2 and bone morphogenetic
protein-2 in bone marrow-derived mesenchymal stem cells, combined
with a demineralized bone matrix, was applied for repair of femoral
head osteonecrosis by promoting bone formation and angiogenesis
(22). Combined delivery of FGF-2,
transforming growth factor-β1, and adipose-derived stem cells from
an engineered periosteum to a critical-sized mouse femur defect
yielded improved bone allograft healing (23). The concurrent application of hypoxia
and FGF-2 could provide a favorable condition for culturing human
bone marrow-derived stem cells to be used in clinical applications
associated with bone tissue engineering, due to the enhancement of
cellular proliferation and regenerative potential (24). The effects of temporal manipulation
were achieved by applying FGF-2 and transforming growth factor-β3
on the derivation of proliferative chondrocytes from mesenchymal
stem cells (25).
Based on these findings, it was concluded that FGF-2
could produce increased alkaline phosphatase activity or Alizarin
Red S staining and further studies are needed to elucidate the
mechanisms of FGF-2.
Acknowledgements
Not applicable.
Funding
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Science, Information and Communication
Technology and Future Planning (grant no.
NRF-2017R1A1A1A05001307).
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
JYT, YK and JBP designed the study, were responsible
for data access and analysis, performed the experiments and wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at Seoul St Mary's Hospital, College of Medicine, and
The Catholic University of Korea (approval no. KC18SESI0083).
Informed consent was obtained from the participants as specified in
the Declaration of Helsinki and all of the experimental schemes
were performed according to the relevant guidelines as specified in
the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dvorak P, Bednar D, Vanacek P, Balek L,
Eiselleova L, Stepankova V, Sebestova E, Kunova Bosakova M, Konecna
Z, Mazurenko S, et al: Computer-assisted engineering of hyperstable
fibroblast growth factor 2. Biotechnol Bioeng. 115:850–862. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang C, Shan Y, Hu Y, Fang Z, Tong Y, Chen
M, Wei X, Fu X and Xu X: FGF2 attenuates neural cell death via
suppressing autophagy after rat mild traumatic brain injury. Stem
Cells Int. 2017:29231822017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Y, Cao F, Yu X, Chen C, Meng J, Zhong
R, Zhang Y and Zhu D: Linc-RAM is required for FGF2 function in
regulating myogenic cell differentiation. RNA Biol. 15:404–412.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Titmarsh DM, Tan CL, Glass NR, Nurcombe V,
Cooper-White JJ and Cool SM: Microfluidic screening reveals heparan
sulfate enhances human mesenchymal stem cell growth by modulating
fibroblast growth factor-2 transport. Stem Cells Transl Med.
6:1178–1190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruno M, Rizzo IM, Romero-Guevara R,
Bernacchioni C, Cencetti F, Donati C and Bruni P: Sphingosine
1-phosphate signaling axis mediates fibroblast growth factor
2-induced proliferation and survival of murine auditory
neuroblasts. Biochim Biophys Acta. 1864:814–824. 2017. View Article : Google Scholar
|
|
6
|
Li C, Che LH, Shi L and Yu JL: Suppression
of basic fibroblast growth factor expression by antisense
oligonucleotides inhibits neural stem cell proliferation and
differentiation in rat models with focal cerebral infarction. J
Cell Biochem. 118:3875–3882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ratajczak J, Hilkens P, Gervois P, Wolfs
E, Jacobs R, Lambrichts I and Bronckaers A: Angiogenic capacity of
periodontal ligament stem cells pretreated with deferoxamine and/or
fibroblast growth factor-2. PLoS One. 11:e01678072016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee J, Lee JY, Chae BC, Jang J, Lee E and
Son Y: Fully dedifferentiated chondrocytes expanded in specific
mesenchymal stem cell growth medium with FGF2 obtains mesenchymal
stem cell phenotype in vitro but retains chondrocyte
phenotype in vivo. Cell Transplant. 26:1673–1687. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SI, Ko Y and Park JB: Evaluation of
the maintenance of stemness, viability, and differentiation
potential of gingiva-derived stem-cell spheroids. Exp Ther Med.
13:1757–1764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA
and Jeun SS: Mesenchymal stem cells expressing brain-derived
neurotrophic factor enhance endogenous neurogenesis in an ischemic
stroke model. Biomed Res Int. 2014:1291452014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JH, Lee JE, Kang KJ and Jang YJ:
Functional efficacy of human recombinant FGF-2s tagged with
(His)6 and (His-Asn)6 at the N- and C-termini
in human gingival fibroblast and periodontal ligament-derived
cells. Protein Expr Purif. 135:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P, Shu B, Xu Y, Zhu J, Liu J, Zhou Z,
Chen L, Zhao J, Liu X, Qi S, et al: Basic fibroblast growth factor
reduces scar by inhibiting the differentiation of epidermal stem
cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell
Res Ther. 8:1142017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nawrocka D, Kornicka K, Szydlarska J and
Marycz K: Basic fibroblast growth factor inhibits apoptosis and
promotes proliferation of adipose-derived mesenchymal stromal cells
isolated from patients with type 2 diabetes by reducing cellular
oxidative stress. Oxid Med Cell Longev. 2017:30271092017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim BH, Jung HW, Seo SH, Shin H, Kwon J
and Suh JM: Synergistic actions of FGF2 and bone marrow
transplantation mitigate radiation-induced intestinal injury. Cell
Death Dis. 9:3832018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hyun SY, Lee JH, Kang KJ and Jang YJ:
Effect of FGF-2, TGF-β-1, and BMPs on teno/ligamentogenesis and
osteo/cementogenesis of human periodontal ligament stem cells. Mol
Cells. 40:550–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon KA, Son Y, Choi YJ, Kim JH and Cho
JY: Fibroblast growth factor 2 supports osteoblastic niche cells
during hematopoietic homeostasis recovery after bone marrow
suppression. Cell Commun Signal. 15:252017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vidovic-Zdrilic I, Vining KH, Vijaykumar
A, Kalajzic I, Mooney DJ and Mina M: FGF2 enhances odontoblast
differentiation by αSMA(+) progenitors in vivo. J Dent Res.
97:1170–1177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh M, Kakkar A, Sharma R, Kharbanda OP,
Monga N, Kumar M, Chowdhary S, Airan B and Mohanty S: Synergistic
effect of BDNF and FGF2 in efficient generation of functional
dopaminergic neurons from human mesenchymal stem cells. Sci Rep.
7:103782017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li R, Ma J, Wu Y, Nangle M, Zou S, Li Y,
Yin J, Zhao Y, Xu H, Zhang H, et al: Dual delivery of NGF and bFGF
coacervater ameliorates diabetic peripheral neuropathy via
inhibiting schwann cells apoptosis. Int J Biol Sci. 13:640–651.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu R, Zhao H, Muhammad H, Dong M,
Besenbacher F and Chen M: Dual-delivery of FGF-2/CTGF from silk
fibroin/PLCL-PEO coaxial fibers enhances MSC proliferation and
fibrogenesis. Sci Rep. 7:85092017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng WX and Wang L: Adenovirus-mediated
expression of BMP-2 and BFGF in bone marrow mesenchymal stem cells
combined with demineralized bone matrix for repair of femoral head
osteonecrosis in beagle dogs. Cell Physiol Biochem. 43:1648–1662.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Romero R, Travers JK, Asbury E, Pennybaker
A, Chubb L, Rose R, Ehrhart NP and Kipper MJ: Combined delivery of
FGF-2, TGF-β1, and adipose-derived stem cells from an engineered
periosteum to a critical-sized mouse femur defect. J Biomed Mater
Res A. 105:900–911. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JS, Kim SK, Jung BJ, Choi SB, Choi EY
and Kim CS: Enhancing proliferation and optimizing the culture
condition for human bone marrow stromal cells using hypoxia and
fibroblast growth factor-2. Stem Cell Res. 28:87–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tay LM, Wiraja C, Wu Y, Yang Z, Lee EH and
Xu C: The effect of temporal manipulation of transforming growth
factor beta 3 and fibroblast growth factor 2 on the derivation of
proliferative chondrocytes from mensenchymal stem cells-A study
monitored by quantitative reverse transcription polymerase chain
reaction and molecular beacon based nanosensors. J Biomed Mater Res
A. 106:895–904. 2018. View Article : Google Scholar : PubMed/NCBI
|