Introduction
Corneal ulcer (CU) is a common ophthalmological
disease and is one of the prime causes of monocular blindness
worldwide, particularly in developing countries (1). CU, including infectious and
non-infectious CU, is the prime cause of corneal opacity and the
fourth largest cause of blindness in the world (2). The clinical manifestations include
blurred vision, pain, photophobia and lacrimation, as well as
obvious visual impairment (3,4).
Ophthalmic examination may indicate disappearance of
the corneal luster, a decrease in transparency or ulcer formation
(5). Severe cases may cause corneal
perforation, intraocular infection or even blindness (6).
Functional magnetic resonance imaging (fMRI) is a
method for detecting brain changes (7,8), and may
be utilized to enhance the current knowledge on ophthalmological
diseases. fMRI is a neuroimaging research technique for exploring
the brain and central nervous system; it may be used for monitoring
the spontaneous neuronal activity of the human brain and provide
novel explanations for the pathophysiology of certain conditions
(9). It is suitable for the study of
central mechanisms, since it does not require any radioactive
tracers, and it may detect and accurately locate spontaneous
neuronal activity in the human brain by combining functional with
structural imaging (10). Amplitude
of low-frequency fluctuation (ALFF) is one of the useful
resting-state fMRI analytic methods for assessing the activity of
brain regions during rest (11).
Studies have demonstrated that the ALFF performs well based on its
test-retest reliability (9,12). The ordinary calculation and reliable
feature of the ALFF measurement make it an appropriate and helpful
tool for analyzing resting-state fMRI data of disease
characteristics. In previous studies by our group, the ALFF method
has been successfully used to assess the neurological status in
certain eye diseases, including optic neuritis (13), glaucoma (14) and comitant strabismus (15). ALFF is considered a reliable and
sensitive measurement that may be applied to accurately assess
spontaneous neural activity.
The aim of the present study was to investigate the
altered spontaneous brain activity and its association with the
behavioral performance in CU patients by using ALFF technology and
provide a potential neural mechanism underlying the pathogenesis of
CU.
Patients and methods
Subjects
A total of 40 CU patients examined at the First
Affiliated Hospital of Nanchang University (Nanchang, China)
between April 2016 and June 2017 were enrolled in the present
study.
The diagnostic criteria for CU were as follows: i) A
history of corneal trauma; ii) corneal infiltration, turbidity,
local tarnish, tissue defects and mixed congestion; iii) symptoms
of irritation, including increased sensitivity to light,
lacrimation decreased vision and eye secretions, and iv) anterior
chamber empyema in the patients with severe disease.
The inclusion criteria for CU patients was as
follows: i) A duration of CU of at least 14 days, ii) no
abnormality of brain parenchyma on craniocerebral MRI, iii)
right-handedness, iv) no foreign implant in the body and v) fMRI
was performed prior to CU surgery. The exclusion criteria were as
follows: i) Long-term medical treatment for blindness, ii) a
history of surgery in bilateral eyes, iii) bilateral late
blindness, iv) bilateral congenital blindness and v) psychiatric
disorders (including bipolar disorder, depression and sleep
disorder) and vi) cerebral infarction-associated diseases (e.g.,
cerebral infarction, cerebral hemorrhage or cerebral vascular
malformations). A total of 40 healthy control subjects (HCs) that
were matched with the corresponding CU group in terms of age, sex
and education status were also recruited. All of the participants
fulfilled the following criteria: i) MRI findings without apparent
deformities in the brain parenchyma; ii) no psychiatric disease,
cerebral infarction or cardiovascular disease; iii) no drugs or
alcohol addiction; iv) capable of undergoing MRI examination.
The present study was in accordance with the
Declaration of Helsinki and was formally approved by the Medical
Ethics Committee of the First Affiliated Hospital of Nanchang
University. All volunteers provided written informed consent.
MRI parameters
MRI was performed using a Trio 3-Tesla MR scanner
(Siemens AG, Munich, Germany). All of the subjects were instructed
to keep their eyes closed while awake and maintain natural
breathing during the scan (14). A
3-dimensional spoiled gradient recalled-echo pulse sequence was
applied to acquire the functional data with the parameters set as
follows: Acquisition matrix, 256×256; field of view, 250×250 mm;
echo time, 2.26 msex; repetition time, 1,900 msec; thickness, 1.0
mm; gap, 0.5 mm; flip angle, 9°). A total of 240 functional images
(acquisition matrix, 64×64; field of view, 220×220 mm; thickness,
4.0 mm; gap, 1.2 mm; repetition time, 2,000 msec; echo time, 30
msec; flip angle, 90°; axial views, 29. A total of 176 structural
images were thereby obtained and the examination lasted for 15
min.
fMRI data analysis
Functional data analysis was performed using a
method described in a previous study by our group (15). First, MRIcro software (Nottingham
University) was applied to remove any incomplete data. During
magnetization equilibration, the first 15 time-points were
discarded. Data Processing Assistant for advanced edition of
resting-state (rs)-fMRI software (DPARSFA 4.0; http://rfmri.org/DPARSF) was used for head motion
correction, spatial normalization, slice timing, data conversion
into the digital imaging communications in medicine format, and
full-width smoothing with a Gaussian kernel of 6×6×6 mm3
at half-maximum, based on the rs-fMRI data analysis toolkit (REST;
http://www.restfmri.net) and Statistical
Parametric Mapping software (SPM; http://www.fil.ion.ucl.ac.uk/spm). Subjects were
excluded if they had 1.5 angular motion or the maximum offset of x,
y or z direction exceeded 1.5 mm during the fMRI examination. The
number of patients in the CU group and the HC group was originally
46. After removal of certain patients due to head motion and the
consequent removal of respective control individuals to maintain
equal group sizes, 40 patients remained in each group. Although it
is not a one-to-one correspondence, it has consulted a statistical
expert to indicate that it has no effect on the study. The Friston
six head-motion parameters were used to regress out head-motion
effects based on previous literature that revealed that
higher-order models were more effective (16,17).
Furthermore, linear regression was applied to remove other various
sources of spurious covariates and their temporal derivatives,
including the signal from a ventricular region of interest and a
region centered in the white matter (18). It is worth noting that in the data of
the present study, the global signal did not shrink as was the case
in previous studies by our group (9,19,20),
which may be due to the debated elimination of global signals
during the resting-state data pre-processing (21,22).
After the head motion correction, the fMRI images were normalized
to the space standard of the Montreal Institute of Neurological
Institute (23) using a standard
echo plane imaging template, and at the same time, the images were
resampled with a resolution of 3×3×3 mm. After pre-treatment, the
time series of each voxel was linearly decreased in order to reduce
the low frequency drift, heart noise and physiological
high-frequency respiration, along with linear detrending of the
time series. In order to reduce the global impact of variability
across the participants, the ALFF of each voxel was divided by the
global mean ALFF value for each participant.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Variables of the demographic and clinical features of
the CU and HC groups were compared using SPSS software version 20.0
(IBM Corp., Armonk, NY, USA) via an independent-samples t-test. The
statistical threshold of the voxel level for multiple comparisons
according to the Gaussian random field (GRF) theory was set at a
level of P<0.05. Furthermore, AlphaSim was corrected at a
cluster size of >40 voxels and a significance level of
P<0.01. All ALFF maps were z-transformed with Fisher's r-to-z
transformation to reduce the influence of individual variation for
group statistical comparisons and the independent t-test was used
to compare group differences in the zALFF maps using the GRF method
to correct for multiple comparisons and regressed covariates of age
and sex with Statistical Parametric Mapping 12 software (The
MathWorks, Inc.; two-tailed, voxel-level P<0.01, GRF correction,
cluster-level P<0.05). The regions of the cerebrum with a
distinctly different mean ALFF value between the CU subjects and
HCs were analyzed using receiver operating characteristic (ROC)
curves.
Results
Demographics and disease
characteristics
No statistically significant differences were
present between the HC and CU groups in body weight (P=0.892) or
age (P=0.824), as indicated in Table
I. The mean duration of CU was 25.75±5.65 days (Table I).
 | Table I.Characteristics of participants
included in the study. |
Table I.
Characteristics of participants
included in the study.
| Parameter | CU group
(n=40) | HCs (n=40) | t | P-value |
|---|
| Males/females | 26/14 | 26/14 | N/A | >0.99 |
| Age (years) | 51.25±5.46 | 51.98±5.18 | 0.251 | 0.824 |
| Body weight
(kg) | 63.12±7.35 | 63.89±6.73 | 0.181 | 0.892 |
| Right-handedness
(n) | 40 | 40 | N/A | >0.99 |
| Duration of CU
(days) | 25.75±5.65 | N/A | N/A | N/A |
Differences in ALFF
In contrast to those of HCs, ALFF values of the CU
patients were significantly lower in the left cerebellar anterior
lobe, right middle frontal gyrus and left middle frontal gyrus, but
higher in the right cerebellar inferior lobe, left cerebellar
inferior lobe, left inferior temporal gyrus, right fusiform gyrus,
left superior frontal gyrus, right angular gyrus and bilateral
superior frontal gyrus (Figs. 1 and
2; Table
II).
 | Figure 1.Significant differences in
spontaneous brain activity between the CU group and HCs.
Differences were observed in the left cerebellum anterior lobe,
right middle frontal gyrus, left middle frontal gyrus, right
cerebellar inferior lobe, left cerebellar inferior lobe, left
inferior temporal gyrus, right fusiform gyrus, left superior
frontal gyrus, right angular gyrus and bilateral superior frontal
gyrus. The red areas denote brain regions with higher ALFF and the
blue areas denote brain regions with lower ALFF [P<0.001 for
multiple comparisons using Gaussian random field theory (z>2.3;
P<0.001; cluster, >13 voxels; Alphasim corrected)]. ALFF,
amplitude of low-frequency fluctuation; HCs, healthy controls; L,
left; R, right; CU, corneal ulcer. |
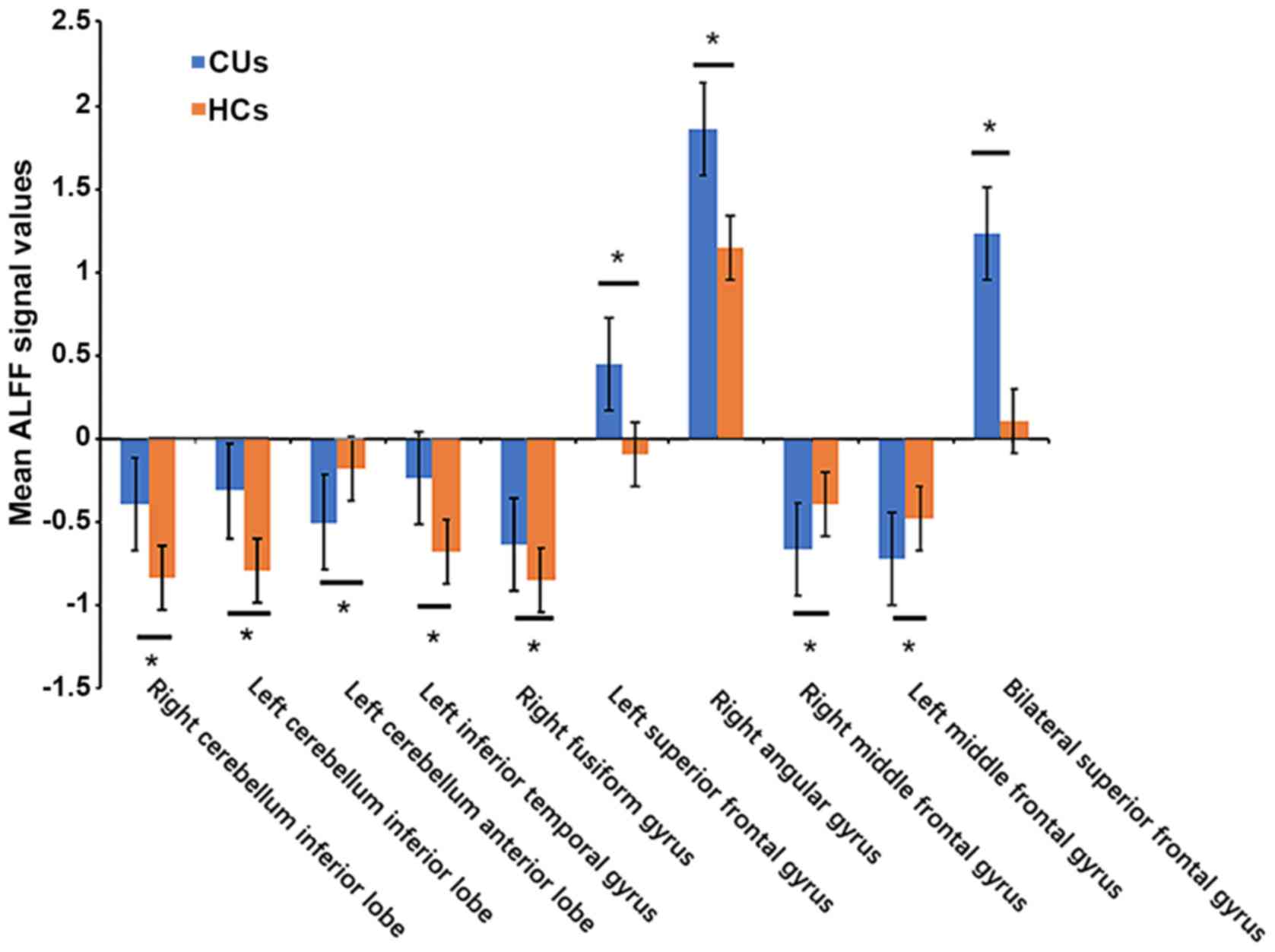 | Figure 2.Means of altered spontaneous brain
activity compared between the CU group and HCs. The statistical
threshold was set at voxel with P<0.01 for multiple comparisons
using family-wise error correction (z>2.3; P<0.01; cluster,
>40 voxels). *P<0.05. HCs, healthy controls; CU, corneal
ulcer; ALFF, amplitude of low-frequency fluctuation; RCIL, right
cerebellum inferior lobe; LCIL, left cerebellum inferior lobe;
LITG, left inferior temporal gyrus; RFG, right fusiform gyrus;
LSFG, left superior frontal gyrus; RAG, right angular gyrus; BSFG,
bilateral superior frontal gyrus; LCAL, left cerebellum anterior
lobe; RMFG, right middle frontal gyrus; LMFG, left middle frontal
gyrus. |
 | Table II.Brain areas with significantly
different ALFF values between groups. |
Table II.
Brain areas with significantly
different ALFF values between groups.
|
|
|
| MNI
coordinates |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Comparison of
ALFF | L/R | Brain region | X | Y | Z | BA | Peak voxels | T-value | P-values |
|---|
| UDs<HCs |
|
|
|
|
|
|
|
|
|
| 1 | L | Cerebellar anterior
lobe | −36 | −51 | −27 | / | 61 | −5.35 | P<0.001 |
| 2 | R | Middle frontal
gyrus | 30 | −6 | 54 | 6 | 19 | −4.5773 | P<0.001 |
| 3 | L | Middle frontal
gyrus | −24 | −9 | 51 | 6 | 18 | −4.1103 | P<0.001 |
| UDs>HCs |
|
|
|
|
|
|
|
|
|
| 1 | R | Cerebellar inferior
lobe | 21 | −69 | −60 | / | 94 | 5.557 | P<0.001 |
| 2 | L | Cerebellar inferior
lobe | −36 | −72 | −57 | / | 218 | 5.786 | P<0.001 |
| 3 | L | Inferior temporal
gyrus | −57 | −48 | −27 | 20 | 24 | 4.4602 | P<0.001 |
| 4 | R | Fusiform gyrus | 51 | −36 | −27 | 18 | 16 | 4.4716 | P<0.001 |
| 5 | L | Superior frontal
gyrus | −18 | 57 | 3 | 9 | 25 | 4.8905 | P<0.001 |
| 6 | R | Angular gyrus | 48 | −63 | 39 | 39 | 16 | 5.2397 | P<0.001 |
| 7 | B | Superior frontal
gyrus | 0 | −21 | 78 | 6 | 132 | 4.8908 | P<0.001 |
ROC curve analysis
The mean ALFF values of different brain regions were
analyzed using the ROC curve method. The area under the ROC curve
(AUC) represented the diagnosis rate. The AUC of ALFF values in
different brain regions was as follows: i) CUs>HCs (Fig. 3A): right cerebellar inferior lobe,
0.760 (P<0.001); left cerebellar inferior lobe, 0.828
(P<0.001); left inferior temporal gyrus, 0.754 (P<0.001);
right fusiform gyrus, 0.771 (P<0.001); left superior frontal
gyrus, 0.812 (P<0.001); right angular gyrus, 0.807 (P<0.001);
bilateral superior frontal gyrus, 0.785 (P<0.001); ii)
CUs<HCs (Fig. 3B): Left
cerebellar anterior lobe, 0.848 (P<0.001); right middle frontal
gyrus, 0.778 (P<0.001); and left middle frontal gyrus, 0.773
(P<0.001).
 | Figure 3.ROC curve analysis of the mean ALFF
values for altered brain regions. (A) The area under the ROC curve
for the RCIL was 0.760 (P<0.001; 95% CI, 0.650–0.862), that for
the LCIL was 0.828 (P<0.001; 95% C,: 0.741–0.915), that for the
LITG was 0.754 (P<0.001; 95% CI, 0.651–0.857), that for the RFG
was 0.771 (P<0.001; 95% CI, 0.670–0.872), that for the LSFG was
0.812 (P<0.001; 95% CI, 0.721–0.904), that for the RAG was 0.807
(P<0.001; 95% CI, 0.708–0.906) and that for the BSFG was 0.785
(P<0.001; 95% CI, 0.684–0.885) [CUs>HCs]. (B) The area under
the ROC curve was 0.848 (P<0.001; 95% CI, 0.763–0.933) for the
LCAL, while that for the RMFG was 0.778 (P<0.001; 95% CI,
0.679–0.877) and that for the LMFG 0.773 (P<0.001; 95% CI,
0.674–0.873) [CUs<HCs]. ALFF, amplitude of low-frequency
fluctuation; HCs, healthy controls; CU, corneal ulcer; ROC,
receiver operating characteristic; RCIL, right cerebellar inferior
lobe; LCIL, left cerebellar inferior lobe; LITG, left inferior
temporal gyrus; RFG, right fusiform gyrus; LSFG, left superior
frontal gyrus; RAG, right angular gyrus; BSFG, bilateral superior
frontal gyrus; LCAL, left cerebellar anterior lobe; RMFG, right
middle frontal gyrus; LMFG, left middle frontal gyrus. |
Discussion
The middle frontal gyrus (Fig. 4), which is involved in attention
control (24) and working memory
(25), comprises one-third of the
frontal lobe. Chronic CU patients, appear to have difficulty
avoiding inattention due to the impaired vision. Certain previous
studies have indicated that depressed individuals had dysfunction
in the middle frontal gyrus (26,27).
Visual impairment may directly affect physical function and
performance through decreased ability to see where to walk and
visualize the location of objects in space (28). Low vision has been associated with
increased disability. Bruce and Hoff (29) indicated that disability may lead to
depression. In support of these results, the present study
indicated a decrease of the ALFF value in the left/right middle
frontal gyrus of CU patients, which may also be associated with
depression. The depression caused by CU might result in middle
frontal gyrus dysfunction.
 | Figure 4.ALFF results of brain activity in the
CU group. Compared with the HCs, the ALFF of the following regions
was decreased to various extents: 1) Left cerebellar anterior lobe
(t=−5.35), 2) right middle frontal gyrus (t=−4.5573), 3) left
middle frontal gyrus (t=−4.1103), 4) right cerebellar inferior lobe
(t=5.557), 5) left cerebellar inferior lobe (t=5.786), 6) left
inferior temporal gyrus (t=4.4602), 7) right fusiform gyrus
(t=4.4716), 8) left superior frontal gyrus (t=4.8905), 9) right
angular gyrus (t=5.2397) and 10) bilateral superior frontal gyrus
(t=4.8908). The sizes of the spots denote the degree of
quantitative changes. HCs, healthy controls; ALFF, amplitude of
low-frequency fluctuation; CU, corneal ulcer. |
The anterior lobe is a part of the cerebellum that
mediates unconscious proprioception. The present study demonstrated
that the ALFF value in the left anterior cerebellar lobe in the CU
group was significantly reduced.
Traditionally, the function of the cerebellum is
considered to be movement coordination (30). However, with the development and
application of neuroimaging technology in recent years, the
understanding on the role of cerebellum, particularly the
cerebellar inferior lobe, has expanded to include emotional
processing (31). Previous studies
have indicated that patients with social anxiety examined by
positron emission tomography had abnormal signals in the
cerebellum, which was characterized by increased cerebral blood
flow and led to the conclusion that the cerebellum is associated
with anxiety (32). This is in line
with the present results. Therefore, the high ALFF value of the
cerebellar inferior lobe may be the result of anxiety in
patients.
The inferior temporal gyrus (ITG), located on the
lateral and inferior surface of the temporal neocortex, may be
regarded as the language formulation central area and tertiary
visual association cortex. Its functions include visual perception,
cognitive language and memory (33).
ITG is an important area of association that promotes cognitive
processing and regulation of emotions. In addition, compared with
HCs, patients with somatoform pain disorder exhibit increased
activation of the temporal region, suggesting that the temporal
region activity may be associated with physical pain (34).
The fusiform gyrus is located at the midsole of the
visual cortex (35), fusiform face
area. Previous studies have indicated that the fusiform gyrus is
involved in the visual cognitive function of facial recognition
(36). Face recognition areas have
separate brain processing areas, and individuals with agnosia are
likely to retain the ability to recognize faces at the same time.
The possible explanation may be that their normal object
recognition neural pathways is impaired, but the fusiform gyrus
face area is not (37). Jiang et
al (38) indicated that face
classification in visual scenes may begin with the high-order
region of the right fusiform gyrus, which is consistent with the
observation that the ALFF value is increased in the fusiform gyrus.
It may be assumed that blurred vision and ulceration caused by CUs
affect facial recognition of patients.
The angular gyrus (ANG) is an important associative
region in the back of the brain above the Wernicke region and at
the apex occipital lobe (39). In
terms of response and perception, attention is sensitive to trial
history. Although the basis of the sensory motor interactions
remains to be elucidated, from a cognitive and neurologic
perspective, converging solid evidence from various methods
suggested that the right ANG may be of great importance (40,41).
The superior frontal gyrus (SFG), accounts for one
third of the frontal lobe of the human brain, is bounded laterally
by the superior frontal sulcus. Studies including fMRI experiments
have indicated that the superior frontal gyrus is engaged in
self-awareness (42) and laughter
(43). In the present study, ALFF
values in CU patients were markedly higher in bilateral SFG, which
suggested intrinsic brain activation in this area. The ALFF method
has been successfully applied in ophthalmological diseases, as
outlined in an overview of previous studies provided in Table III, and has a huge development
prospect.
 | Table III.Summary of previous studies on the
application of the amplitude of low-frequency fluctuation method in
ophthalmological diseases. |
Table III.
Summary of previous studies on the
application of the amplitude of low-frequency fluctuation method in
ophthalmological diseases.
| First author
(year) | Disease | (Refs.) |
|---|
| Guo (2010) | High myopia | (44) |
| Huang (2015) | Glaucoma | (14) |
| Huang (2015) | Optic neuritis | (13) |
| Tan (2016) | Strabismus | (15) |
| Li (2016) | Monocular
blindness | (45) |
| Tan (2016) | Open-globe
injury | (46) |
| Wang (2017) | Diabetic
retinopathy | (47) |
| Liang (2016) | Amblyopia | (48) |
| Pan (2018) | Acute eye pain | (49) |
ROC curve analysis provides a statistical method to
distinguish diseased from healthy subjects. The accuracy is
considered perfect for AUC values of 0.7–0.9, while values between
0.5 and 0.7 indicate moderate predictability and those <0.5
indicate that the discrimination ability is low. The ROC curve
analysis of the present study indicated that the AUCs of each brain
region were >0.7, which suggested that these specific ALFF
differences have a proper diagnostic accuracy in characterizing CU.
In a word, the present results illustrated that the ALFF method may
be a sufficient biomarker of fMRI for the basic research and study
of eye diseases. In conclusion, the present study indicates the
presence of brain activity disorders in CU patients. These novel
results may offer significant information to explain the potential
neural effects of CU.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors made substantial contributions to this
research. WQS, WW and LY performed the experiments and collected
the data. WFL, YQS, TS and QL designed the current study. YLM and
BL contributed to designing the study and were involved in data
interpretation and writing the discussion. PWZ, NJ and YS performed
the MRI scanning. WQS wrote the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the First Affiliated Hospital of Nanchang
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu J, Zhang WS, Zhao J and Zhou HY: Review
of clinical and basic approaches of fungal keratitis. Int J
Ophthalmol. 9:1676–1683. 2016.PubMed/NCBI
|
|
2
|
World Health Organization, . Causes of
blindness and visual impairment. 2016.
|
|
3
|
Ngoie Maloba V, Ngayuna Nkiene J, Tunku
Kabamba G and Chenge Borasisi G: Frequency of corneal ulcer:
Retrospective study of 380 cases carried out in two centers in the
DR Congo. J Fr Ophtalmol. 41:57–51. 2018.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El Sheha H: Self-retained amniotic
membrane for dendritic keratitis. Ascrs. 2015.
|
|
5
|
Mascarenhas J, Lalitha P, Prajna NV,
Srinivasan M, Das M, D'Silva SS, Oldenburg CE, Borkar DS, Esterberg
EJ, Lietman TM and Keenan JD: Acanthamoeba, fungal, and bacterial
keratitis: A comparison of risk factors and clinical features. Am J
Ophthalmol. 157:56–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas PA and Kaliamurthy J: Mycotic
keratitis: Epidemiology, diagnosis and management. Clin Microbiol
Infect. 19:210–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bekiesińskafigatowska M, Helwich E,
Rutkowska M, Stankiewicz J and Terczyńska I: Magnetic resonance
imaging of neonates in the magnetic resonance compatible incubator.
Arch Med Sci. 12:1064–1070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H and Wang X: Correlation of iron
deposition and change of gliocyte metabolism in the basal ganglia
region evaluated using magnetic resonance imaging techniques: An in
vivo study. Arch Med Sci. 12:163–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B,
Gao L and Wang YX: Long-term sleep deprivation decreases the
default spontaneous activity and connectivity pattern in healthy
male subjects: A resting-state fMRI study. Neuropsychiatr Dis
Treat. 11:761–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biswal BB: Resting state fMRI: A personal
history. Neuroimage. 62:938–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Zhu C, Chen H, Duan X, Lu F, Li
M, Liu F, Ma X, Wang Y, Zeng L, et al: Frequency-dependent
alterations in the amplitude of low-frequency fluctuations in
social anxiety disorder. J Affect Disord. 174:329–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zuo XN, Di Martino A, Kelly C, Shehzad ZE,
Gee DG, Klein DF, Castellanos FX, Biswal BB and Milham MP: The
oscillating brain: Complex and reliable. Neuroimage. 49:1432–1445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Cai FQ, Hu PH, Zhong YL, Zhang Y,
Wei R, Pei CG, Zhou FQ and Shao Y: Disturbed spontaneous
brain-activity pattern in patients with optic neuritis using
amplitude of low-frequency fluctuation: A functional magnetic
resonance imaging study. Neuropsychiatr Dis Treat. 11:3075–3083.
2015.PubMed/NCBI
|
|
14
|
Huang X, Zhong YL, Zeng XJ, Zhou F, Liu
XH, Hu PH, Pei CG, Shao Y and Dai XJ: Disturbed spontaneous brain
activity pattern in patients with primary angle-closure glaucoma
using amplitude of low-frequency fluctuation: A fMRI study.
Neuropsychiatr Dis Treat. 11:1877–1883. 2015.PubMed/NCBI
|
|
15
|
Tan G, Huang X, Zhang Y, Wu AH, Zhong YL,
Wu K, Zhou FQ and Shao Y: A functional MRI study of altered
spontaneous brain activity pattern in patients with congenital
comitant strabismus using amplitude of low-frequency fluctuation.
Neuropsychiatr Dis Treat. 12:1243–1250. 2016.PubMed/NCBI
|
|
16
|
Satterthwaite TD, Elliott MA, Gerraty RT,
Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB,
Hakonarson H, Gur RC, Gur RE and Wolf DH: An improved framework for
confound regression and filtering for control of motion artifact in
the preprocessing of resting-state functional connectivity data.
Neuroimage. 64:240–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan CG, Cheung B, Kelly C, Colcombe S,
Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX and Milham
MP: A comprehensive assessment of regional variation in the impact
of head micromovements on functional connectomics. Neuroimage.
76:183–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fox MD, Snyder AZ, Vincent JL, Corbetta M,
Van Essen DC and Raichle ME: The human brain is intrinsically
organized into dynamic, anticorrelated functional networks. Proc
Natl Acad Sci USA. 102:9673–9678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li HJ, Dai XJ, Gong HH, Nie X, Zhang W and
Peng DC: Aberrant spontaneous low-frequency brain activity in male
patients with severe obstructive sleep apnea revealed by
resting-state functional MRI. Neuropsychiatr Dis Treat. 11:207–214.
2015.PubMed/NCBI
|
|
20
|
Dai XJ, Peng DC, Gong HH, Wan AL, Nie X,
Li HJ and Wang YX: Altered intrinsic regional brain spontaneous
activity and subjective sleep quality in patients with chronic
primary insomnia: A resting--state fMRI study. Neuropsychiatr Dis
Treat. 10:2163–2175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo
HJ, Martin A and Cox RW: Trouble at rest: How correlation patterns
and group differences become distorted after global signal
regression. Brain Connect. 2:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ,
Liang M, Tian LX, Jiang TZ and Wang YF: Altered baseline brain
activity in children with ADHD revealed by resting-state functional
MRI. Brain Dev. 29:83–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siddharth K, Michael AM, Cahill ND, Kiehl
KA, Pearlson G, Baum SA and Calhoun VD: ICA-fNORM: Spatial
normalization of fMRI data using intrinsic group-ICA networks.
Front Syst Neurosci. 5:932011.PubMed/NCBI
|
|
24
|
Japee S, Holiday K, Satyshur MD, Mukai I
and Ungerleider LG: A role of right middle frontal gyrus in
reorienting of attention: A case study. Front Syst Neurosci.
9:232015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan HM, Jackson MC, van Koningsbruggen
MG, Shapiro KL and Linden DE: Frontal and parietal theta burst TMS
impairs working memory for visual-spatial conjunctions. Brain
Stimul. 6:122–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang CC, Yu SC, Mcquoid DR, Messer DF,
Taylor WD, Singh K, Boyd BD, Krishnan KR, MacFall JR, Steffens DC
and Payne ME: Reduction of dorsolateral prefrontal cortex gray
matter in late-life depression. Psychiatry Res. 193:1–6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nelson JD, Craig JP, Akpek EK, Azar DT,
Belmonte C, Bron AJ, Clayton JA, Dogru M, Dua HS, Foulks GN, et al:
TFOS DEWS II introduction. Ocul Surf. 15:269–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leat SJ: A proposed model for integrated
low-vision rehabilitation services in Canada. Optom Vis Sci.
93:77–84. 2016.PubMed/NCBI
|
|
29
|
Bruce ML and Hoff RA: Social and physical
health risk factors for first-onset major depressive disorder in a
community sample. Soc Psychiatry Psychiatr Epidemiol. 29:165–171.
1994.PubMed/NCBI
|
|
30
|
Ferrari C, Oldrati V, Gallucci M, Vecchi T
and Cattaneo Z: The role of the cerebellum in explicit and
incidental processing of facial emotional expressions: A study with
transcranial magnetic stimulation. Neuroimage. 169:256–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adamaszek M, D'Agata F, Ferrucci R, Habas
C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E,
et al: Consensus paper: Cerebellum and emotion. Cerebellum.
16:552–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kilts CD, Kelsey JE, Knight B, Ely TD,
Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ and Nemeroff
CB: The neural correlates of social anxiety disorder and response
to pharmacotherapy. Neuropsychopharmacology. 31:2243–2253. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dien J, Brian ES, Molfese DL and Gold BT:
Combined ERP/fMRI evidence for early word recognition effects in
the posterior inferior temporal gyrus. Cortex. 49:2307–2321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stoeter P, Bauermann T, Nickel R, Corluka
L, Gawehn J, Vucurevic G, Vossel G and Egle UT: Cerebral activation
in patients with somatoform pain disorder exposed to pain and
stress: An fMRI study. Neuroimage. 36:418–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
James CE, Oechslin MS, Van De Ville D,
Hauert CA, Descloux C and Lazeyras F: Musical training yields
opposite effects on grey matter density in cognitive versus
sensorimotor networks. Brain Struct Funct. 219:353–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weiner KS: On (ab)normality: Einstein's
fusiform gyrus. Brain Cogn. 94:1–3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Caspers J, Palomero-Gallagher N, Caspers
S, Schleicher A, Amunts K and Zilles K: Receptor architecture of
visual areas in the face and word-form recognition region of the
posterior fusiform gyrus. Brain Struct Funct. 220:205–219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang F, Dricot L, Weber J, Righi G, Tarr
MJ, Goebel R and Rossion B: Face categorization in visual scenes
may start in a higher order area of the right fusiform gyrus:
Evidence from dynamic visual stimulation in neuroimaging. J
Neurophysiol. 106:2720–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yazar Y, Bergström ZM and Simons JS:
Continuous theta burst stimulation of angular gyrus reduces
subjective recollection. PLoS One. 9:e1104142014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Makris N, Preti MG, Wassermann D, Rathi Y,
Papadimitriou GM, Yergatian C, Dickerson BC, Shenton ME and Kubicki
M: Human middle longitudinal fascicle: Segregation and
behavioral-clinical implications of two distinct fiber connections
linking temporal pole and superior temporal gyrus with the angular
gyrus or superior parietal lobule using multi-tensor tractography.
Brain Imaging Behav. 7:335–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bocca F, Töllner T, Müller HJ and Taylor
PC: The right angular gyrus combines perceptual and
response-related expectancies in visual search: TMS-EEG evidence.
Brain Stimul. 8:816–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goldberg I, Harel M and Malach R: When the
brain loses its self: Prefrontal inactivation during sensorimotor
processing. Neuron. 50:329–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fried I, Wilson C, MacDonald K and Behnke
EJ: Electric current stimulates laughter. Nature. 391:6501998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guo MX, Dong HH, Zhang YT, Zhang Q and Yin
XH: ALFF changes in brain areas of human with high myopia revealed
by resting-state functional MRI. International Conference on
Biomedical Engineering and Informatics. IEEE. 91–94. 2010.
|
|
45
|
Li Q, Xin H, Lei Y, Wei R, Zhang Y, Zhong
YL, Jiang N and Shao Y: Altered spontaneous brain activity pattern
in patients with late monocular blindness in middle-age using
amplitude of low-frequency fluctuation: A resting-state functional
MRI study. Clin Interv Aging. 11:1773–1780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tan G, Huang X, Ye L, Wu AH, He LX, Zhong
YL, Jiang N, Zhou FQ and Shao Y: Altered spontaneous brain activity
patterns in patients with unilateral acute open globe injury using
amplitude of low-frequency fluctuation: A functional magnetic
resonance imaging study. Neuropsychiatr Dis Treat. 12:2015–2020.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang ZL, Zou L, Lu ZW, Xie XQ, Jia ZZ, Pan
CJ, Zhang GX and Ge XM: Abnormal spontaneous brain activity in type
2 diabetic retinopathy revealed by amplitude of low-frequency
fluctuations: A resting-state fMRI study. Clin Radiol.
72:340.e1–340.e7. 2017. View Article : Google Scholar
|
|
48
|
Liang M, Xie B, Yang H, Yu L, Yin X, Wei L
and Wang J: Distinct patterns of spontaneous brain activity between
children and adults with anisometropic amblyopia: A resting-state
fMRI study. Graefes Arch Clin Exp Ophthalmol. 254:569–576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q,
Freeberg S, Zhu PW, Ye L, Ma MY, Huang X and Shao Y: Altered
intrinsic brain activities in patients with acute eye pain using
amplitude of low-frequency fluctuation: A resting-state fMRI study.
Neuropsychiatr Dis Treat. 14:251–257. 2018. View Article : Google Scholar : PubMed/NCBI
|


















