Introduction
Diabetes mellitus (DM) has reached endemic levels,
with its worldwide prevalence estimated to increase to 552 million
by 2030 (1). Among the major burdens
of diabetes patients are microvascular and macrovascular
complications, particularly diabetic nephropathy (DN). DN is
functionally characterized by initial glomerular hyperfiltration
and persistent albuminuria, followed by a progressive decline in
the glomerular filtration rate (GFR), leading to the development of
end-stage renal disease (ESRD).
The major treatment for DN is tight control of blood
glucose and blood pressure, but these methods do not slow down the
progression of DN to ESRD. Renal replacement therapy is inevitable
once DN has progressed to ESRD, but the medical cost of renal
replacement is high; thus, treatment should be provided during the
early stages of DN. The urinary albumin-to-creatinine ratio (UACR)
and estimated (e)GFR are used for screening for incipient DN.
Furthermore, five distinct stages of chronic kidney disease have
been defined based on the progression of renal impairment (2). In general, stages I–III are classified
as early-stage DN, stage IV as intermediate-stage (moderate DN) and
stage V as late-stage/severe DN, with an eGFR of ≤30 ml/min/1.73
m2 and persistent macroalbuminuria (UACR ≥300 mg/g).
Traditional glucose-lowering treatments include
insulin, metformin, sulfonylureas, meglitinides and
thiazolidinediones. Although these drugs are effective in reducing
the risk of diabetic complications, they are associated with
significant side effects, including hypoglycemia and weight gain.
Over the last decade, several novel glucose-lowering drugs have
been introduced and have been increasingly used as treatments for
diabetes and its complications. Glucagon-like peptide-1 (GLP-1)
receptor agonists are among these more recent drug classes.
GLP-1 receptor agonists are a class of
anti-hyperglycemic drugs for type 2 diabetes. They include
exenatide, lixisenatide, liraglutide, dulaglutide and albiglutide.
Liraglutide was the second GLP-1 receptor agonist to receive
regulatory approval by the Food and Drug Administration for type 2
diabetes in January 2010 (3).
However, whether liraglutide offers therapeutic advantages compared
with other drugs for DN has remained to be confirmed. Recently,
Mann et al (4) performed a
randomized controlled trial to evaluate the change of renal
outcomes of treatment with liraglutide. 9,340 DN patients were
assigned to receive liraglutide or placebo, which results suggested
that the liraglutide group had fewer patients who exhibited
persistent macroalbuminuria when compared with the placebo group.
That is to say, liraglutide may decrease persistent
macroalbuminuria and improve renal outcomes. By contrast, the study
by Davies et al (5) was
conducted to establish the efficacy and safety of liraglutide. A
total of 279 patients with moderate DN were divided into
liraglutide and placebo groups, and the results demonstrated that
no changes in renal function were observed in the liraglutide and
placebo groups. The aforementioned two studies had certain
limitations: First, although the study of Mann et al
(4) covered early-stage, moderate
and late-stage DN patients, it did not particularly proceed
subgroup analyses. In other words, the result showed that
liraglutide lowered the level of proteinuria and improved renal
function, but it did not clarify which stage of DN was affected by
liraglutide. Second, the study by Davies et al (5) only showed that liraglutide had no
effect on moderate DN, but it did not clarify whether liraglutide
had therapeutic effects on DN in other stages, such as the early
stage. Therefore, the effects of liraglutide against incipient DN
were not determined in these studies. The present study was the
first analysis investigating the effect of liraglutide in patients
with type 2 diabetes who also had incipient DN. Of note, it was
indicated that liraglutide has renoprotective effects in patients
with early-stage DN.
Materials and methods
Search strategy
The PubMed, OVID, Cochrane Library, Chinese National
Knowledge Infrastructure (CNKI) and WanFang databases were searched
by two investigators independently. These databases were
extensively searched and articles from the time the databases were
established until October 2018 were examined. The following search
terms were used: (‘liraglutide’) and (‘diabetic nephropathies’ or
‘diabetic nephropathy’ or ‘diabetic kidney diseases’ or ‘diabetic
complications’). The publications were first filtered based on
title, abstract and key words, and the full-text versions were then
assessed while applying the inclusion and exclusion criteria
(described below). The publication language was restricted to
English and Chinese.
Selection criteria
All relevant articles focusing on the association
between liraglutide and renal function index were collected. The
following studies were included: a) Studies performed as randomized
controlled trials; b) studies that involved type 2 diabetes
patients with stage I–III nephropathy: Stages I and II were defined
by an eGFR of ≥60 ml/min/1.73 m2 with normal and mildly
increased albuminuria (UACR<30 mg/g), respectively, while stage
III was defined by an eGFR of 30–60 ml/min/1.73 m2 and
an increase in UACR from 30 to 300 mg/g in two morning spot urine
collections sustained over 12 weeks (2); c) studies that included patients who
were under a controlled diet and exercise therapy, treatment with
anti-hypertensive drugs or other anti-hyperglycemic treatments
(control group), and those treated with liraglutide (experimental
group); d) studies that reported on renal function outcomes,
including UACR, urinary albumin excretion rate (UAER), serum
creatinine (Scr); and e) studies with a duration of >8 weeks.
The following studies were excluded: a) Those that included no
information on renal function or the biochemical index of type 2
DN; b) those with duplicate clinical data published by the same
authors but in different periodicals; c) those with unclear or
inappropriate diagnostic criteria, intervention measures or outcome
indicators; d) case reports, letters, reviews, expert opinion,
conference abstracts, editorials, and studies published in a
language other than English or Chinese; and e) articles using cell
lines and/or in vitro/ex vivo studies.
Data extraction
Datum included in the present study were extracted
independently by authors JY and JM. If disagreement was
encountered, the third author (TT) was consulted for consensus. The
general information, including the name of the first author, the
year of publication, type of trial, number of patients, the
treatment methods, course of treatment and outcome data were
extracted from each of the included papers. Study characteristics
and clinical examination data were generalized and are described in
table format.
Statistical analysis
RevMan 5.3 software was downloaded from the Cochrane
Collaboration website and used for meta-analysis. Clinical
heterogeneity and methodological heterogeneity of the included
studies were analyzed using the χ2 and I2
tests, respectively. If acceptable statistical heterogeneity
existed among the studies (P>0.1 and I2<50%), the
fixed-effect method was used to pool the data (6). Otherwise, the random-effects model was
used (6,7). The mean difference (MD) and 95%
confidence intervals (CIs) were used to compare continuous
variables, while risk ratios and 95% CIs were used to compare
dichotomous variables (7). Whenever
heterogeneity was significant, it was attempted to determine its
source using the study-by-study exclusion method. P<0.05 was
considered to indicate statistical significance. Egger's test
performed using Stata 12.0 software (Stata Corp) and funnel plots
drawn with RevMan 5.3 software were used to detect publication
bias.
Assessment of quality of evidence
The risk of bias was assessed by two investigators
independently, as recommended by the Cochrane Handbook for
Systematic Reviews of Interventions (7). Disagreements were resolved by a third
reviewer. The quality appraisal of the literature included random
sequence generation, allocation concealment, blinding of
participants and personnel, blinding of outcome assessment,
incomplete outcome data and selective reporting. Articles that had
clearly described details and met or surpassed the quality criteria
were defined as low-risk; otherwise, they were deemed high-risk.
Equivocal articles in terms of quality criteria were deemed to be
of unclear risk.
Results
Literature search and selection
Initially, 261 relevant records were retrieved from
the PubMed, Ovid, Cochrane Library, CNKI and WanFang databases. A
total of 31 full-text articles were then extracted for detailed
assessment and were filtered via their titles and abstracts for
eligibility assessment for final inclusion. Following exclusion of
9 crossover trials without control groups, as well as 8 trials that
lacked renal function marker analysis, one study of which had a
control group of patients with DM rather than DN, 13 publications
that satisfied the inclusion criteria were finally selected for
inclusion in the meta-analysis (8–20). The
article search and study selection process are displayed in
Fig. 1.
Study characteristics
Of the total 1,187 patients included, 590 belonged
to the treatment group, while 597 belonged to the control group and
received routine treatment, including a controlled diet and
exercise therapy, anti-hypertensive drugs or other
anti-hyperglycemic treatments. The treatment group received
liraglutide combined with routine treatment, anti-hypertensive
drugs or other anti-hyperglycemic treatment. The doses of
liraglutide used in the trials included were largely consistent.
Subcutaneous injection of liraglutide was administered prior to
bedtime on a daily basis. The initial dose in the first week was
0.6 mg/day and was increased from 1 to 1.2 mg/day in the second
week. Among the 13 trials, 12 adopted a two-armed parallel group
design, while one [Zhang et al (8)] adopted a three-armed group design. The
durations of interventions varied among the diabetes trials,
ranging from 8 to 24 weeks. A total of 7 studies lasted for 24
weeks, 3 lasted for 8 weeks, 1 lasted for 10 weeks and the
remaining ones lasted for 12 weeks. Basic information about the
studies included are presented in Table
I.
 | Table I.Characteristics of the studies
included. |
Table I.
Characteristics of the studies
included.
| First author
(year) | Sample cases | Testing scheme | Test group | Control group | Duration
(weeks) | Outcomes | (Refs.) |
|---|
| Zhang (2017) | 22/20 | RCT | LIR (0.6 to 1.2 mg
qd) plus RT | RT | 10 | ACIKLM | (8) |
| Zha (2018) | 30/30 | RCT | LIR (0.6 to 1.2 mg
qd) plus Huang kui capsule | Huang kui capsule
(2.5 g tid) | 24 | ADGH | (9) |
| Dong (2018) | 43/43 | RCT | LIR (0.6 to 1.2 mg
qd) plus INS | INS | 24 | BDGHI | (10) |
| Li (2017) | 21/22 | RCT | LIR (0.6 to 1.2 mg
qd) plus RT | RT | 12 | ABCDEFI | (11) |
| Zheng (2015) | 110/110 | RCT | LIR (0.6 to 1.2 mg
qd) plus INS | INS | 8 | ADFH | (12) |
| Yan (2016) | 100/100 | UNK | LIR (0.6 to 1.2 mg
qd) plus TEL | TEL | 10 | CIKLM | (13) |
| Shen (2017) | 30/30 | RCT | LIR (0.6 to 1.2 mg
qd) plus OLM and INS | OLM (20 mg qd) plus
INS | 24 | ACEFG | (14) |
| Chen (2016) | 30/31 | RCT | LIR (0.6 to 1.2 mg
qd) plus RT | RT | 24 | ACDEFI | (15) |
| Ren (2015) | 24/24 | RCT | LIR (0.6 to 1.2 mg
qd) plus INS | INS | 24 | ACDGI | (16) |
| Zhao (2014) | 19/26 | UNK | LIR (0.6 to 1.2 mg
qd) plus VAL | VAL | 24 | CDJ | (17) |
| Aiyitan (2017) | 89/73 | UNK | LIR (0.6 to 1.2 mg
qd) plus INS | INS | 8 | BGH | (18) |
| Liu (2016) | 59/75 | RCT | LIR (0.6 to 1.2 mg
qd) plus INS | INS | 8 | BGH | (19) |
| Liu (2015) | 13/13 | RCT | LIR (0.6 to 1.2 mg
qd) plus OLM and INS | OLM (20 mg qd) plus
INS | 24 | DGJ | (20) |
Risk of bias
The risk of bias assessments is presented in
Fig. 2. All trials were randomly
designed, but four (9,13,18,19) were
judged to have unclear risk of bias owing to allocation
concealment.
Blinding of participants and personnel was not
performed in one study (17);
furthermore, another study did not perform blinding of outcome
assessors (18). In addition, four
studies did not specify whether the participants and personnel were
blinded (14,16,18,19), and
four studies did not specify whether blinding of the outcome
assessors was performed (12–14,17).
Attrition bias was ambiguous in seven of the trials (9,12–14,17–19);
however, most trials had a low risk of reporting bias or other
biases, and only one had an unclear risk of reporting bias
(8).
Effect of interventions
Effect on proteinuria and renal
function
According to the stage of DN of the subjects, the 13
trials (8–20) were divided into two subgroups: Four
trials (11,13,18,19)
belonged to stages I and II, and the remaining ones belonged to
stage III. In addition, UACR, UAER and Scr were evaluated to
determine the effect of liraglutide on proteinuria and renal
function.
A total of 2 trials (18,19)
investigated the effect of liraglutide during DN stages I and II on
the UACR and UAER. The treatment and control groups comprised 148
patients each. No significant heterogeneity was identified among
the studies (UACR: χ2=0.01; I2=0%, P=0.93;
UAER: χ2=0.00; I2=0%, P=0.96); hence, a
fixed-effects model was used for the meta-analyses. The UACR and
UAER were lower in the treatment group than those in the control
group (UACR: MD=−90.96, 95% CI=−94.12 to −87.80, P<0.00001;
UAER: MD=−64.86, 95% CI=−66.63 to −63.08, P<0.00001; Fig. 3A and B). A total of 2 trials
(11,13) were used to compare Scr levels between
the two groups. The treatment group included 121 patients and the
control group included 122 patients. No significant heterogeneity
was identified between the two trials (χ2=0.14;
I2=0%, P=0.71). Patients receiving liraglutide had
better Scr levels than the subjects in the control group
(MD=−13.67, 95% CI=−17.88 to −9.46, P<0.00001; Fig. 3C).
As for stage-III DN, 5 trials (9,10,14,16,20)
reported on the UACR with 140 patients each in the treatment and
control group. Without statistical heterogeneity among the studies
(χ2=7.08; I2=43%, P=0.13), a fixed-effects
model was selected for the pooled analysis, which revealed that the
treatment group was better than the control group in terms of UACR
(MD=−11.23, 95% CI=−13.14 to −9.32, P<0.00001; Fig. 4A). A total of 3 trials (9,10,12)
compared the UAER between the treatment group and the control group
(183 patients per group). No significant heterogeneity was observed
(χ2=3.28; I2=39%, P=0.19) and a fixed-effects
model was used for the pooled analysis. The treatment group had a
lower UAER than the control group (MD=−14.06; 95% CI=−16.93 to
−11.18; P<0.00001; Fig. 4B).
Furthermore, analysis of 4 trials reporting on Scr levels (8,10,15,16)
indicated that there was a significant difference in Scr levels
between the treatment group (n=119) and control group (n=118).
Heterogeneity testing again showed no statistically significant
difference between these studies (χ2=5.32;
I2=44%, P=0.15), and thus, the fixed-effects model was
selected for pooled analysis, which revealed that the treatment
group exhibited better Scr levels than the control group (MD=−9.17,
95% CI=−14.61 to −3.72, P=0.001; Fig.
4C). Overall, the results suggested that liraglutide
ameliorates renal function.
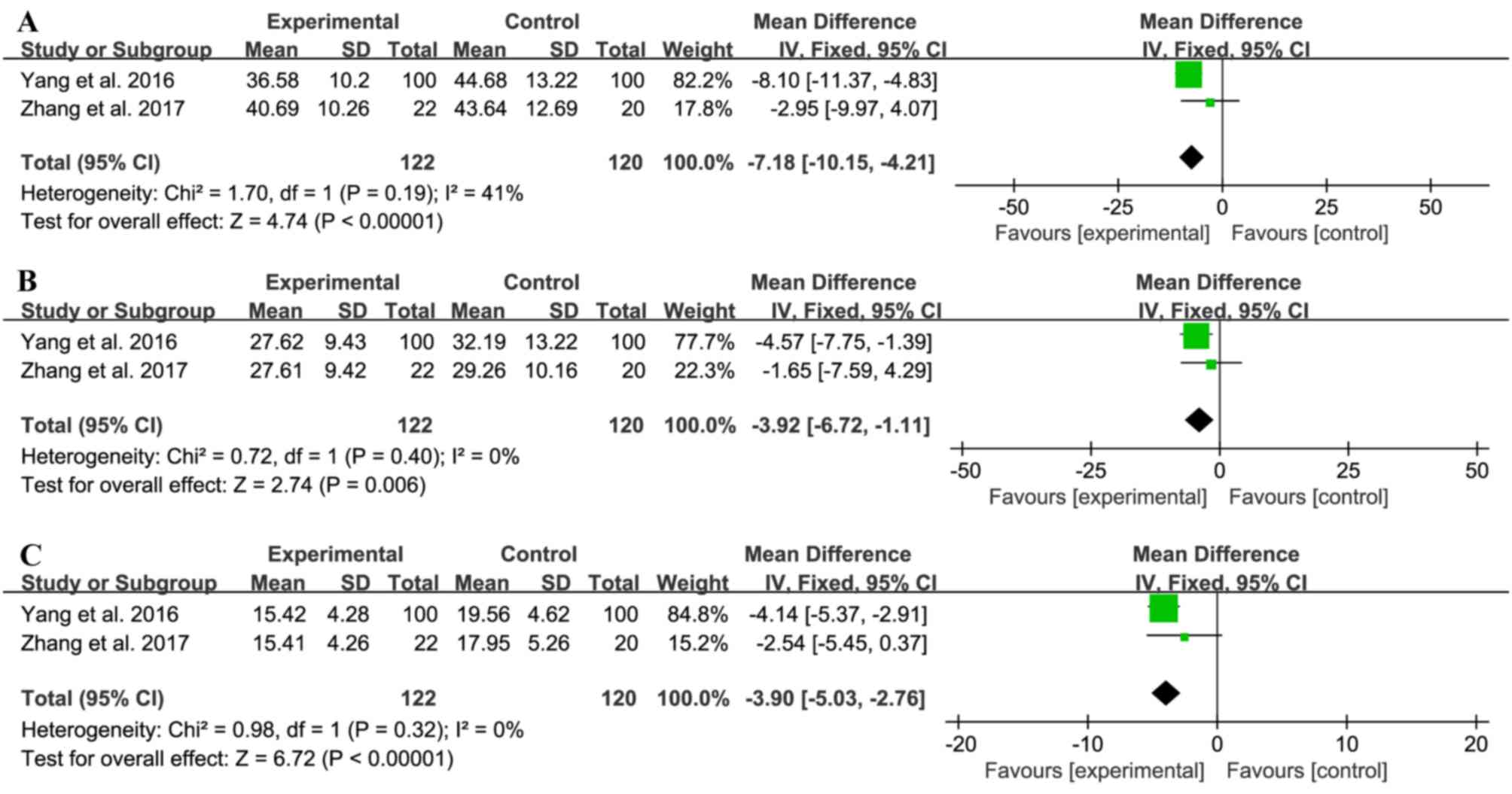
Effect on inflammation
A total of 2 trials (8,13)
compared transforming growth factor-β1
(TGF-β1), tumor necrosis factor-α (TNF-α) and
interleukin-6 (IL-6) levels between the treatment group (n=122) and
control group (n=120). No significant heterogeneity was observed
(TGF-β1: χ2=1.70; I2=41%, P=0.19;
TNF: χ2=0.72; I2=0%, P=0.40; IL-6:
χ2=0.98; I2=0%, P=0.32), and a fixed-effects
model was used for the pooled analyses. The treatment group had
lower levels of the inflammatory factors compared with those in the
control group (TGF-β1: MD=−7.18; 95% CI=−10.15 to −4.21;
P<0.00001; TNF: MD=−3.92; 95% CI=−6.72 to −1.11; P=0.006; IL-6:
MD=−3.90; 95% CI=−5.03 to −2.76; P<0.00001), indicating that
liraglutide exhibited anti-inflammatory effects in patients with
early-stage of DN (Fig. 5).
Effect on body mass index (BMI) and
blood lipids
A total of 8 trials (9–12,15–17,20)
reported on the BMI in the treatment group (n=290) and control
group (n=299), and no significant heterogeneity was determined
(χ2=8.96, I2=22%, P=0.26). The fixed-effects
model was used for the pooled analysis, which indicated that
liraglutide was associated with a reduced BMI (MD=−2.09, 95%
CI=−2.29 to −1.88, P<0.00001; Fig.
6A). A total of 3 trials (11,14,15)
compared the total cholesterol (TC) levels between the treatment
group (n=83) and control group (n=84), and no significant
heterogeneity was observed (χ2=0.72, I2=0%,
P=0.70). A fixed-effects model was used for the meta-analysis,
indicating that liraglutide treatment reduced the level of TC
(MD=−0.49, 95% CI=−0.78 to −0.21, P=0.0006; Fig. 6B). Furthermore, 4 trials (11,12,14,15)
compared the triglyceride (TG) levels between the treatment group
(n=193) and control group (n=194), and no significant heterogeneity
was determined (χ2=4.21, I2=29%, P=0.24).
Therefore, the fixed-effects model was used for the meta-analysis,
revealing that liraglutide also decreased the level of TG
(MD=−0.30, 95% CI=−0.32 to −0.28, P<0.00001; Fig. 6C). These results suggested that,
compared with the control treatment, liraglutide lowers the blood
lipid levels and the BMI.
Effect on blood glucose and
glycosylated hemoglobin (HbA1c)
A total of 7 trials (8,9,11,12,14–16)
evaluated the effect of liraglutide on fasting blood glucose (FBG)
levels in the treatment group (n=267) compared with control
subjects (n=267). The statistical heterogeneity of the FBG data was
acceptable (χ2=4.72; I2=0%, P=0.58);
therefore, the fixed-effects model was used for meta-analysis. The
pooled analysis indicated no difference between the treatment group
and control group (MD=0.01; 95% CI=−0.15 to 0.16; P=0.91; Fig. 7A).
A total of 4 trials (10,11,18,19)
investigated the effect of liraglutide on post-prandial blood
glucose (PBG) levels. The liraglutide group comprised 212 patients,
while the control group comprised 213 patients; moderate
heterogeneity was observed (χ2=6.06; I2=50%,
P=0.11) and a random-effects model was used for analysis. The
results suggested that the treatment group had better PBG levels
than the control group (MD=−1.51; 95% CI=−1.81 to −1.22;
P<0.00001; Fig. 7B).
As presented in Fig.
7C, 7 trials (8,11,13–17)
reported on the effect of liraglutide on HbA1c, and all studies had
acceptable heterogeneity (χ2=9.14; I2=34%,
P=0.17); therefore, the fixed-effects model was used. The
meta-analysis indicated no significant difference between the
treatment group and control group in terms of HbA1c levels
(MD=−0.07; 95% CI=−0.15 to 0.01; P=0.10).
Adverse events
Only 5 trials (8,10,15–17)
reported on adverse events, 3 of which (8,15,17)
reported that no adverse events occurred during the treatment
period. Of the remaining 2 trials, 1 (10) indicated that patients in the
treatment group presented with hypoglycemia (n=1), nausea (n=5) and
diarrhea (n=7); however, the same adverse events were experienced
in patients in the control groups (n=7, n=7 and n=8, respectively).
The other trial (16) reported that
12.5% patients in the treatment group had exhibited
gastrointestinal-tract reactions (n=3). However, there were no
serious adverse events reported in any of these trials. The most
common adverse events were gastrointestinal tract reactions and
hypoglycemia, but they resolved quickly.
Evaluation of publication bias
In the present study, funnel plots and Egger's test
were used to identify publication bias (Fig. 8A, BMI; Fig. 8B, FBG; Fig. 8C, HbA1c). Symmetry was observed in
Fig. 8, and the values reported in
one study were beyond the 95% CI range (Fig. 8B). However, those of the other trials
were within the 95% CI range (Fig. 8A
and C). At the same time, Egger's test for BMI (P=0.085), FBG
(P=0.448) and HbA1c (P=0.709) also suggested that there was no
publication bias in the studies included.
Discussion
DN is one of the complications of DM. The
pathogenesis of DN is linked to various factors, including
metabolic and hemodynamic abnormalities (21). The primary treatment for DN is tight
control of blood glucose and blood pressure, but these methods do
not slow down the progression of DN. Over the last decade,
liraglutide has been introduced and has been increasingly used for
the treatment of DM and its complications.
Liraglutide is one of the representative GLP-1
receptor agonist drugs. Physiologically, GLP-1 exerts its actions
through the GLP-1 receptor in pancreatic β-cells, resulting in
glucose-dependent insulin secretion and thus reduction in blood
glucose levels (22–24). However, only 10–15% of endogenously
released GLP-1 reaches the systemic circulation; most GLP-1 is
degraded by the enzyme dipeptidyl peptidase-4 (25). Therefore, GLP-1 receptor agonists
were developed and introduced in the clinic for improving the
levels of internal GLP-1, increase insulin secretion and reduce
blood glucose levels. Furthermore, previous studies have indicated
that the GLP-1 receptor is produced not only in the pancreas, but
also in the kidneys (25,26). The aim of the present meta-analysis
was to determine whether liraglutide exhibits a renoprotective
effect.
The meta-analysis included 1,187 patients; most of
the trials included had a randomized double-blinded parallel
control design. UACR, UAER and Scr were selected as ideal markers
for assessment of kidney function, as they are relatively inert by
age, sex, BMI and inflammatory conditions (27,28). In
addition, according to the 5 distinct stages of chronic kidney
diseases, the 13 studies on early DN included were divided into
stages I–II and stage III. Regardless of the DN stage, the present
results suggested that liraglutide may have positive effects on
lowering UACR, UAER and Scr. It also offered the benefit of
producing anti-inflammatory responses in patients with stage-III
DN.
Importantly, the NF-κB signaling pathway is the
major pathway that regulates the effect of inflammatory cytokines,
including TNF-α. A previous study suggested that liraglutide
downregulated the levels of NF-κB by binding to GLP-1 receptor to
inhibit the levels of TNF-α, IL-6 and monocyte chemoattractant
protein-1 in the kidneys of a patient with diabetes (29). The results of a previous study
suggested that liraglutide had anti-fibrotic and anti-inflammatory
effects in the kidney (29).
With regard to blood glucose and lipid levels, the
present meta-analysis indicated that liraglutide reduced the BMI,
blood lipids and PBG levels. However, liraglutide only appeared to
have an effect on PBG but provided no apparent benefit on FBG and
HbA1c, probably due to most control groups receiving insulin.
Compared with insulin, liraglutide had little effect on FBG and
HbA1c, which is in agreement with a previous clinical study
(30). Furthermore, although the
Egger's test and funnel plots indicated no publication bias in BMI,
FBG and HbA1c, it is likely that other biases existed in the
studies examined. For example, allocation concealment, blinding of
participants and personnel and outcome assessment, and incomplete
outcome data were not clear in these included trials, which may be
the main reasons of potential biases. Regarding adverse events,
only 5 studies in all included trials reported adverse events. Up
until now, the number of trials referring on the adverse events is
too limited to make any conclusion for the safety of liraglutide.
Hence, future clinical trials should perform rigorous
investigations regarding the safety of liraglutide.
In conclusion, the present meta-analysis indicated
that compared with the control treatment, liraglutide reduced
proteinuria, improved renal function and produced an
anti-inflammatory effect in patients with incipient DN. These
results may serve as a basis to guide the clinical application of
liraglutide.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Jiangsu
Provincial Hospital of Traditional Chinese Medicine (grant no.
y2018rc02 to JY).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WL and JY were involved in the conceptualization of
the study. TT and WS performed the electronic database searches. JY
and JM performed analysis of the data. JY and TT assessed the
quality of evidence and participated in outlining the inclusion and
exclusion criteria. WL and JM provided final approval of all
procedures. WL wrote and reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMI
|
body mass index
|
|
DM
|
diabetes mellitus
|
|
DN
|
diabetic nephropathy
|
|
ESRD
|
end-stage renal disease
|
|
FBG
|
fasting blood glucose
|
|
GLP-1
|
glucagon-like peptide-1
|
|
HbA1c
|
glycosylated hemoglobin
|
|
IL-6
|
interleukin-6
|
|
MD
|
mean difference
|
|
PBG
|
post-prandial blood glucose
|
|
Scr
|
serum creatinine
|
|
TC
|
total cholesterol
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TGF-β1
|
transforming growth factor-β1
|
|
UAER
|
urinary albumin excretion rate
|
|
UACR
|
urinary albumin-to-creatinine
ratio
|
References
|
1
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF Diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tong L and Adler S: Glycemic control of
type 2 diabetes mellitus across stages of renal impairment:
Information for primary care providers. Postgrad Med. 130:381–393.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
U.S. Food Drug Administration, .
Drugs@FDA: FDA Approved Drug Products. VICTOZA (Liraglutide).
http://www.accessdata.fda.gov/July 11–2016
|
|
4
|
Mann JFE, Ørsted DD, Brown-Frandsen K,
Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B and Buse JB;
LEADER Steering Committee and Investigators, : Liraglutide and
renal outcomes in type 2 diabetes. N Engl J Med. 377:839–848. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davies MJ, Bain SC, Atkin SL, Rossing P,
Scott D, Shamkhalova MS, Bosch-Traberg H, Syrén A and Umpierrez GE:
Efficacy and safety of liraglutide versus placebo as add-on to
glucose-lowering therapy in patients with type 2 diabetes and
moderate renal impairment (LIRA-RENAL): A randomized clinical
trial. Diabetes Care. 39:222–230. 2016.PubMed/NCBI
|
|
6
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions version 5.1.2. The cochrane
collaboration, 2011. http://handbook.cochrane.org/April 8–2018
|
|
8
|
Zhang JH, Liu XR, Sheng CX and Liu YE:
Analysis on difference in gastrointestinal hormone levels of
patients with the history of diabetes and concurrent nephropathy
and study on the role of liraglutide. Eur Rev Med Pharmacol Sci.
21:3523–3529. 2017.PubMed/NCBI
|
|
9
|
Zha M, Zhang S, Ruan Y, Shi M, Zhou L and
Huang LJ: Clinical effects of combination therapy of Huangkui
capsules and liraglutide on patients with early diabetic
nephropathy. Chin Trad Patent Med. 40:1493–1495. 2018.(In
Chinese).
|
|
10
|
Dong L and Zhao JH: Effects of liraglutide
on renal function in patients with microalbuminuria of diabetic
nephropathy. Lin Chang Hui Cui. 33:420–423. 2018.
|
|
11
|
Li Q, Cao WJ, Zhou GY and Yang LH: Effects
of liraglutide on early diabetic nephropathy and its relationship
with the changes of VEGF and VEGF-A. Xiangnan Xue Yuan Xue Bao (Yi
Xue Ban). 19:6–11. 2017.
|
|
12
|
Zheng Y and Yu SD: Clinical effects of
combination therapy of insulin glargine and liraglutide on type 2
diabetic patients with nephropathy. Xiandai Shi Yong Yi Xue.
27:251–253. 2015.
|
|
13
|
Yang R, Wang YF and Zhang W: Liraglutide
combined with low dosage telmisartan decreases the serum levels of
TNF-α, IL-6 and TGF-β1 in patients with early diabetic nephropathy.
Med J West China. 28:191–194. 2016.(In Chinese).
|
|
14
|
Shen YP, Qiao Q and Lu GY: Effect of
liraglutide on PI3K-Akt-mTOR pathway in patients with diabetic
nephropathy. Huazhong Ke Ji Da Xue Xue Bao (Yi Xue Ban).
46:466–470. 2017.
|
|
15
|
Chen ZP: Efficacy and safety analysis of
Liraglutide in treatment of patients with type 2 diabetes mellitus
and mild to moderate renal disease. Zhongguo Dang Dai Yi Yao.
23:131–133. 2016.
|
|
16
|
Ren W, Guo JJ, Zuo GX, Li YB, Gao LL, Li X
and Liu J: Effects of liraglutide on early diabetic nephropathy
with obese. Chin Remedies & Clinics. 15:1284–1286. 2015.(In
Chinese).
|
|
17
|
Zhao CY, Guo HT, Dai HS, Tian JR and Zhao
YQ: Clinical effects of liraglutide on early diabetic nephropathy.
Shandong Yi Yao. 54:47–49. 2014.(In Chinese).
|
|
18
|
Aiyitan A and Qing Q: Clinical effect of
liraglutide in treating early diabetic nephropathies and the
influence of renal function and adipocytokines. Chin J Clin
Rational Drug Use. 10:10–14. 2017.(In Chinese).
|
|
19
|
Liu R: Protective effect of hypoglycemic
therapy by liraglutide on renal function in early diabetic
nephropathy. J Hainan Med Univ. 22:43–46. 2016.
|
|
20
|
Liu CY: Liraglutide treatment efficacy of
early type 2 diabetic nephropathy and its mechanism analysis.
Dalian Yi Ke Da Xue. 9–11. 2015.
|
|
21
|
Fineberg D, Jandeleit-Dahm KA and Cooper
ME: Diabetic nephropathy: Diagnosis and treatment. Nat Rev
Endocrinol. 9:713–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jorsal T, Rhee NA, Pedersen J, Wahlgren
CD, Mortensen B, Jepsen SL, Jelsing J, Dalbøge LS, Vilmann P,
Hassan H, et al: Enteroendocrine K and L cells in healthy and type
2 diabetic individuals. Diabetologia. 61:284–294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sonne DP, Rehfeld JF, Holst JJ, Vilsbøll T
and Knop FK: Postprandial gallbladder emptying in patients with
type 2 diabetes: Potential implications for bile-induced secretion
of glucagon-like peptide 1. Eur J Endocrinol. 171:407–419. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen A, Lund A, Knop FK and Vilsbøll
T: Glucagon-like peptide 1 in health and disease. Nat Rev
Endocrinol. 14:390–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holst JJ: The physiology of glucagon-like
peptide 1. Physiol Rev. 87:1409–1439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kreymann B, Williams G, Ghatei MA and
Bloom SR: Glucagon-like peptide-1 7–36: A physiological incretin in
man. Lancet. 2:1300–1304. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopez-Giacoman S and Madero M: Biomarkers
in chronic kidney disease, from kidney function to kidney damage.
World J Nephrol. 4:57–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Javanmardi M, Azadi NA, Amini S and Abdi
M: Diagnostic value of cystatin C for diagnosis of early renal
damages in type 2 diabetic mellitus patients: The first experience
in Iran. J Res Med Sci. 20:571–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou SJ, Bai L, Lv L, Chen R, Li CJ, Liu
XY, Yu DM and Yu P: Liraglutide ameliorates renal injury in
streptozotocin-induced diabetic rats by activating endothelial
nitric oxide synthase activity via the downregulation of the
nuclear factor-κB pathway. Mol Med Rep. 10:2587–2594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Russell-Jones D, Vaag A, Schmitz O, Sethi
BK, Lalic N, Antic S, Zdravkovic M, Ravn GM and Simó R; Liraglutide
Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group, :
Liraglutide versus insulin glargine and placebo in combination with
metformin and sulfonylurea therapy in type 2 diabetes mellitus
(LEAD-5 met+SU): A randomised controlled trial. Diabetologia.
52:2046–2055. 2009. View Article : Google Scholar : PubMed/NCBI
|