Introduction
Idiopathic pulmonary fibrosis (IPF) is a specific
chronic, progressive and fibrosing interstitial lung disease
(1,2), where the normal lung parenchyma is
progressively replaced by an altered extracellular matrix with
destruction of the alveolar architecture. These changes lead to
decreased lung compliance, impaired gas exchange and, ultimately,
respiratory failure and death (3).
IPF occurs worldwide and its incidence has been increasing over
time, with ~2.8–9.3 cases per 100,000 people in Europe and North
America in 2012 (4). The
IPF-associated mortality rate is high and the median survival time
after diagnosis is 2–4 years (3,5).
However, the pathogenesis of IPF has remained to be fully
elucidated.
IPF is a consequence of multiple integrating genetic
and environmental factors, which cause a cascade of injury of the
alveolar epithelium (6,7). Most of these factors disrupt the
oxidant/anti-oxidant balance in the lung (8,9) and
evidence has indicated that patients with IPF exhibit higher levels
of oxidative biomarkers and lower levels of anti-oxidant biomarkers
(10). N-acetylcysteine
(NAC), an alternative anti-oxidant, has been demonstrated to
improve the glutathione homeostasis in the human body (11). Furthermore, it was reported to
decrease inflammation and collagen deposition in a mouse model of
bleomycin-induced lung fibrosis (12).
However, according to the clinical trials that have
emerged in previous years, the efficacy of NAC therapy for IPF
remains controversial. While certain studies have suggested that
NAC therapy provides a benefit for patients with IPF and slows the
deterioration of lung function, particularly the decline in forced
vital capacity (FVC) (13–17), others have indicated no benefit of
this treatment for IPF (18–20). In addition, the results of previous
meta-analyses on the efficacy of NAC therapy for IPF are
contradictory. Sun et al (21) determined that the only significant
effects of NAC therapy were to decrease the percentage of predicted
VC and improve the 6-min walking distance test (6MWT), whereas
Kandhare et al (22)
indicated that anti-oxidant therapy was only significantly
associated with the percentage of predicted VC and changes in the
percentage of predicted diffusing capacity for carbon monoxide
(DLCO) in IPF patients.
Considering this conflicting evidence, an updated
and comprehensive systematic review and meta-analysis of the
efficacy and safety of pharmaceutical treatments for IPF including
NAC were performed in order to provide information that may guide
further clinical decisions regarding this therapy.
Materials and methods
Data resources and search
strategies
A comprehensive search for relevant studies on NAC
therapy for IPF published until August 2018 was performed in
appropriate electronic databases and sources, including Pubmed,
EMbase, the Cochrane library, the Chinese National Knowledge
Infrastructure, the Wangfang Database, the VIP Database and the
Chinese Biology Medical Database, by two independent reviewers (FF
and JZ). The language was restricted to Chinese and English. The
following keywords or free terms were used: (‘acetylcysteine’ or
‘N-acetylcysteine’ or ‘NAC’) and (‘idiopathic pulmonary
fibrosis’ or ‘IPF’ or ‘usual interstitial pneumonia’). All clinical
studies except case reports were selected for analysis. The
bibliographies of primary studies, as well as the references listed
in the selected articles, were also searched for further relevant
publications.
Inclusion and exclusion criteria
The inclusion criteria for studies were as follows:
i) Controlled trials comparing a NAC-treated group with a control
group that received routine treatment or drugs other than
anti-oxidants, e.g., lecithinized superoxide dismutase, for the
treatment of IPF. ii) The diagnosis of IPF was in accordance with
an official statement of the American Thoracic Society/the European
Respiratory Society/the Japanese Respiratory Society/the Latin
American Thoracic Association Clinical Practice Guideline (2). iii) The following outcome measures were
reported in the trials: FVC and adverse effects were regarded as
primary outcomes, while secondary outcomes that were also retrieved
included DLCO, the percentage predicted DLCO (DLCO%), VC, partial
arterial oxygen pressure (PaO2), 6MWT and mortality.
Studies that did not meet the inclusion criteria
mentioned above were excluded. In addition, review articles, animal
experiments, duplicated publications, studies with inappropriate
interventions and studies with insufficient useful data were not
considered.
Assessment of methodological
quality
The methodological quality of all of the included
trials was independently assessed in duplicate by two reviewers (FF
and JZ). This quality assessment was according to a scoring system
from 0 to 14, which evaluated the following aspects: Randomization,
blinding, analysis, patient selection, comparability of groups at
baseline, extent of follow-up, treatment protocol, co-interventions
and outcomes (23). A consensus
between the two reviewers was reached for individual category
scores. This assessment strategy has been widely used in previous
meta-analysis publications (24,25).
Clinical outcomes
All data were extracted by two independent reviewers
(FF and ZW) and the discrepancies were resolved by discussion with
a third expert adjudicator (XZ). The primary outcomes were FVC and
adverse effects, while DLCO, DLCO%, VC, 6MWT, PaO2 and
mortality were the secondary outcomes.
Subgroup analysis
Pre-specified subgroup analyses were performed to
explore the potential causes of heterogeneity in the effects of NAC
therapy on the primary and secondary outcomes. It was hypothesized
that the treatment efficacy and safety of NAC is greater in trials
using i) NAC combined therapy vs. NAC monotherapy (due to the
potential pharmacokinetic synergy achieved with combined therapy);
ii) a higher NAC dose (1,800 mg per day) rather than below; iii)
NAC aerosol administration (due to the direct anti-oxidant effects
in alveoli); iv) those with a lower methodological quality (as
studies with a higher methodological quality tend to exhibit more
modest treatment effects).
Statistical analysis
All data were analyzed using RevMan 5.3 software
(The Cochrane Collaboration). Mean differences (MDs) or
standardized MDs (SMDs) with 95% confidence intervals (95% CIs)
were determined in the statistical analysis of continuous
variables, whereas pooled risk ratio (RR) with 95% CIs were used
for dichotomous variables. Clinical and methodological
heterogeneities among studies were analyzed using the χ2
and I2 tests. If the I2 value was >50%
and/or P<0.1 for pooled studies, a random-effects model was used
for the meta-analysis. Otherwise, a fixed-effects model was used.
Outcomes were calculated using P-values and P<0.05 was
considered to indicate statistical significance. As >10 studies
were included in the present meta-analysis, funnel plots were drawn
for the evaluation of potential publication bias with regard to
outcomes. While publication bias may result in asymmetric funnel
plots, clinical or methodological heterogeneity among studies
affects the shape of the funnel plots. Therefore, publication bias
was further evaluated by Egger's test using Stata 14.0 software
(StataCorp.).
Results
Data collection
Initially, 961 potentially relevant articles were
identified during the initial search of the seven databases. After
removal of duplicated articles, the titles and abstracts of the
remaining 734 articles were screened, following which 436 review
articles, 92 animal or in vitro studies, eight case reports,
three publications in German, 129 studies without relevant
outcomes, five studies without a control group and 26 studies with
inappropriate controls were excluded. Subsequently, the full-text
of 35 articles was screened; this led to the exclusion of 14
articles due to repeated data, inappropriate controls or lack of
assessable outcomes. Finally, a total of 21 eligible studies
published between 2005 and 2016 were included in the present
meta-analysis (13–20,26–38). A
flow chart depicting the selection process is presented in Fig. 1.
Characteristics of the studies
included
Among the 21 eligible studies, 13 were performed in
China and eight in the United States, Japan or various European
countries. Overall, 1,354 patients (including 695 patients who
received NAC therapy and 659 who received other therapies) were
identified. NAC was orally administered in all but five Japanese
studies, where NAC was administered via inhalation. The most
commonly used oral dose [76.19% (16/21)] was 600 mg three times a
day (1,800 mg per day). Combined therapy frequently included
corticosteroids (13 studies) and pirfenidone (two studies). The
duration of treatment ranged from 3 to 15 months. The demographic
and clinical baseline characteristics of the included studies,
comprising the first author, year of publication, type of study,
patient number and outcomes, are presented in Table I.
 | Table I.Characteristics of the studies
included. |
Table I.
Characteristics of the studies
included.
|
|
|
| Sample size |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Type of study | Population (n) | NAC | Control | Duration | Outcome | Quality score | NAC regimen | Complete
intervention | (Refs.) |
|---|
| Bando (2010) | Case-control | 25 | 14 | 11 | 12 months | ABDFH | Concealment: No;
ITT: Yes; Blinding: No (score, 6) | Daily dose: 704.8
mg Route: inhaled | Intervention: NAC
(352.4 mg, bid) Control: Without therapy | (18) |
| Behr (2016) | RCT | 123 | 61 | 62 | 24 weeks | ABDGH | Concealment: Yes;
ITT: Yes Blinding: Double (score, 12) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + pirfenidone (1,602–2,043 mg/d) Control: Pirfenidone
(1,602–2,043 mg/d) | (19) |
| Demedts (2005) | RCT | 155 | 80 | 75 | 12 weeks | BCDEH | Concealment: Yes;
ITT: Yes; Blinding: Double (score, 12) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) Control: Placebo | (13) |
| Fu (2015) | RCT | 30 | 15 | 15 | 6 months | E | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d
for 8 weeks; 0.125 mg/kg/d for maintenance) Control: Prednisone
(0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d for 8 weeks; 0.125 mg/kg/d
for maintenance) | (26) |
| Homma (2012) | RCT | 100 | 51 | 49 | 48 weeks | AB | Concealment: No;
ITT, Yes; Blinding: No (score, 10) | Daily dose: 704.8
mg Route: Inhaled | Intervention: NAC
(352.4 mg, bid) Control: Without therapy | (14) |
| Huang (2015) | RCT | 74 | 37 | 37 | 6 months | ABCF | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d
for 8 weeks; 0.125 mg/kg/d for maintenance) Control: Prednisone
(0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d for 8 weeks; 0.125 mg/kg/d
for maintenance) | (27) |
| Jiang (2009) | RCT | 26 | 13 | 13 | 12 weeks | ACF | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + methyprednisolone (0.4 mg/kg/d) Control:
Methyprednisolone (0.4 mg/kg/d) | (28) |
| Jiang (2008) | RCT | 20 | 12 | 8 | 12 weeks | DF | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d
for 4 weeks, 0.125 mg/kg/d for maintenance) Control: (0.5 mg/kg/d
for 4 weeks; 0.25 mg/kg/d for 4 weeks, 0.125 mg/kg/d for
maintenance) | (29) |
| Liu (2016) | RCT | 116 | 58 | 58 | 6 months | CEF | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 1 month; 0.25 mg/kg/d
for 2 months; 0.125 mg/kg/d for 3 months) Control: Prednisone (0.5
mg/kg/d for 1 month; 0.25 mg/kg/d for 2 months; 0.125 mg/kg/d for 3
months) + cyclophosphamide (25–150 mg/d) | (30) |
| Liu (2015) | RCT | 80 | 40 | 40 | 3 months | ABCF | Concealment: No;
ITT: No; Blinding: No (score, 8) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + budesonide (1 mg, tid for 4 weeks; 1 mg, bid for 8
weeks) Control: Budesonide (1 mg, tid for 4 weeks; 1 mg, bid for 8
weeks) | (31) |
| Long (2011) | RCT | 20 | 10 | 10 | 6 months | HF | Concealment: No;
ITT: Yes, Blinding: No (score, 8) | Daily dose: 600 mg
Route: Po | Intervention: NAC
600 mg, tiw + prednisone (0.5 mg/kg/d) + γ-IFN (200 U, subcutaneous
injection, tiw) Control: Prednisone (0.5 mg/kg/d) + γ-IFN (200 U,
subcutaneous injection, tiw) | (32) |
| Lu (2013) | RCT | 62 | 32 | 30 | 6 months | BDEF | Concealment: No;
ITT: No; Blinding: No (score, 8) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d
for 8 weeks; 0.125 mg/kg/d maintenance) Control: Prednisone (0.5
mg/kg/d for 4 weeks; 0.25 mg/kg/d for 8 weeks; 0.125 mg/kg/d
maintenance) | (33) |
| Luo (2006) | RCT | 40 | 20 | 20 | 12 months | CEH | Concealment: No;
ITT: Yes; Blinding: No (score, 8) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (30–45 mg/d) Control: Prednisone (30–45
mg/d) | (34) |
| Martinez
(2014) | RCT | 264 | 133 | 131 | 60 weeks | BGH | Concealment: Yes;
ITT: Yes; Blinding: Double (score, 11) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) Control: Placebo | (20) |
| Nan (2007) | RCT | 40 | 20 | 20 | 12 months | ACF | Concealment: No;
ITT: No; Blinding: No (score, 6) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 1 month; 0.4 mg/kg/d
for 1 month, 0.3 mg/kg/d for 1 month; 10 mg, qd for maintenance) +
azathioprine (2 mg/kg/d) Control: Prednisone (0.5 mg/kg/d for 1
month; 0.4 mg/kg/d for 1 month, 0.3 mg/kg/d for 1 month; 10 mg, qd
for maintenance) + azathioprine (2 mg/kg/d) | (35) |
| Sakamoto
(2013) | Retrospective
study | 18 | 11 | 7 | 6 months | AH | Concealment: No;
ITT: No; Blinding: No (score, 6) | Daily dose: 704.8
mg Route: Inhaled | Intervention: NAC
(352.4 mg, bid) + pirfenidone (1,200–1,800 mg/d) Control:
Pirfenidone (1,200–1,800 mg/d) | (15) |
| Sakamoto
(2015) | Case-control | 34 | 24 | 10 | 12 months | B | Concealment: No;
ITT: No; Blinding: No (score, 8) | Daily dose: 704.8
mg Route: Inhaled | Intervention: NAC
(352.4 mg, bid) + pirfenidone (1,200–1,800 mg/d) Control:
Pirfenidone (1,200–1,800 mg/d) | (16) |
| Tominoka
(2005) | RCT | 30 | 15 | 15 | 12 months | GH | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 352 mg
Route: Inhaled | Intervention: NAC
(352 mg/d) Control: Bromhexine hydrochloride (4 mg/d) | (17) |
| Wang (2015) | RCT | 42 | 21 | 21 | 6 months | BCEF | Concealment: No;
ITT: No; Blinding: No (score, 7) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d
for 4 weeks; 0.125 mg/kg/d for maintenance Control: Prednisone (0.5
mg/kg/d for 4 weeks; 0.25 mg/kg/d for 4 weeks; 0.125 mg/kg/d for
maintenance | (36) |
| Yang (2008) | RCT | 32 | 17 | 15 | 12 weeks | ABCF | Concealment: No;
ITT: No; Blinding: No (score, 6) | Daily dose: 1,800
mg Route: Po | Intervention: NAC
(600 mg, tid) Control: Prednisone (0.5 mg/kg/d) | (37) |
| Zhu (2009) | RCT | 23 | 11 | 12 | 6 months | BDFH | Concealment: No;
ITT: Yes; Blinding: No (score, 8) | Daily dose: 1,200
mg Route: Po | Intervention: NAC
(600 mg, tid) + prednisone (0.5 mg/kg/d for 4 weeks; 0.25 mg/kg/d
for 8 weeks; 0.125 mg/kg/d for maintenance Control: Prednisone (0.5
mg/kg/d for 4 weeks; 0.25 mg/kg/d for 8 weeks; 0.125 mg/kg/d for
maintenance | (38) |
Assessment of methodological
quality
Of the studies included, 18 were randomized
controlled trials, two were case-control studies and one was a
retrospective study. Only four of the 18 randomized controlled
trials provided details about the randomization method. These
studies were designed as randomized, double-blinded,
placebo-controlled trials that declared the concealment of
treatment allocation and blinding procedures. A total of 9 studies
included the drop-out data and provided the detailed information.
According to the quality scoring, the mean methodological quality
score of an individual trial was 7.9, while the median score was 7
(range, 6–12). The individual scores for each trial are provided in
Table I.
Meta-analysis of primary outcomes
Overall effect on FVC
The FVC was evaluated for a total of 470 patients in
nine studies (14,15,18,19,27,28,31,35,37). As
presented in Fig. 2A, these studies
had moderate heterogeneity (I2=55%, P=0.02); therefore,
a random-effects model was used to analyze the data. Meta-analysis
of the studies revealed that NAC treatment reduced the decline in
FVC (MD, 0.26; 95% CI, 0.10–0.41; P=0.001).
Overall adverse effects
A total of 12 studies (13,14,16,18–20,27,31,33,36–38),
including 1,003 patients, reported on the adverse effects of NAC
therapy. The heterogeneity among studies (I2=41%,
P=0.07) warranted the use of a random-effects model. The
meta-analysis did not reveal any significant difference (RR, 1.08;
95% CI, 0.84–1.38; P=0.57) in adverse effects between the NAC
therapy and control groups (Fig.
2B).
Meta-analysis of secondary outcomes
Overall effect on DLCO
A pooled analysis of DLCO data from seven studies
(13,27,28,31,34,36,37) was
performed. A fixed-effects model was used due to homogeneity among
the studies (I2=1%, P=0.42). The decline in DLCO was
significantly lesser in the NAC treatment group compared with that
in the control group (SMD, 0.41; 95% CI, 0.21–0.61; P<0.0001;
Fig. 3A).
 | Figure 3.Forest plots for the meta-analysis on
the efficacy and safety of NAC therapy for idiopathic pulmonary
fibrosis (IPF) with regard to the secondary outcomes. (A) DLCO; (B)
DLCO%; (C) VC; (D) PaO2; (E) 6MWT; (F) mortality. DLCO,
diffusing capacity for carbon monoxide; DLCO%, percentage predicted
DLCO; VC, vital capacity; PaO2, partial arterial oxygen
pressure; 6MWT, 6-minute walking distance test; IV, inverse
variance; Std., standard; M-H, Mantel-Haenszel; NAC,
N-acetylcysteine; df, degrees of freedom. |
Overall effect on DLCO%
Of the 21 studies, 6 studies (13,18,19,29,33,38)
evaluated the DLCO%. There was significant heterogeneity among the
studies (I2=54%, P=0.05), and thus, a random-effects
model was used. Pooled analysis revealed there was no significance
(SMD, 0.31; 95% CI, −0.07–0.68; P=0.11; Fig. 3B).
Overall effect on VC
VC was reported for 393 patients in six studies
(13,26,30,33,34,36). A
random-effects model was used (I2=69%, P=0.006) and the
meta-analysis identified no influence of NAC therapy on the change
in VC (MD, 0.06; 95% CI, −0.10 to 0.23; P=0.44; Fig. 3C).
Overall effect on PaO2
PaO2 was reported for 566 patients in 12
studies (18,27–33,35–38). A
random-effects model was used (I2=67%, P<0.001) and
the meta-analysis revealed that PaO2 was significantly
greater in the NAC therapy group than that in the control group
(SMD, 0.62; 95% CI, 0.30–0.94; P<0.001; Fig. 3D).
Overall effect on the change of
6MWT
Although the 6MWT was reported in four trials, only
three trials reported data as means and standard deviations, which
was required for statistical aggregation. A random-effects model
was used (I2=97%, P<0.001) and the meta-analysis
indicated that improvement in the 6MWT result was significantly
greater with NAC treatment than with control treatment (MD, 23.69;
95% CI, 7.92–39.47; P=0.003; Fig.
3E).
Overall effect on mortality
Mortality was also assessed for 697 patients in nine
studies (13,15,17–20,32,34,38).
These studies indicated homogeneity (Fig. 3F), and therefore, a fixed-effects
model was used (I2=31%, P=0.17). The results revealed
that NAC treatment had no influence on mortality (RR, 1.02; 95% CI,
0.66–1.59; P=0.92).
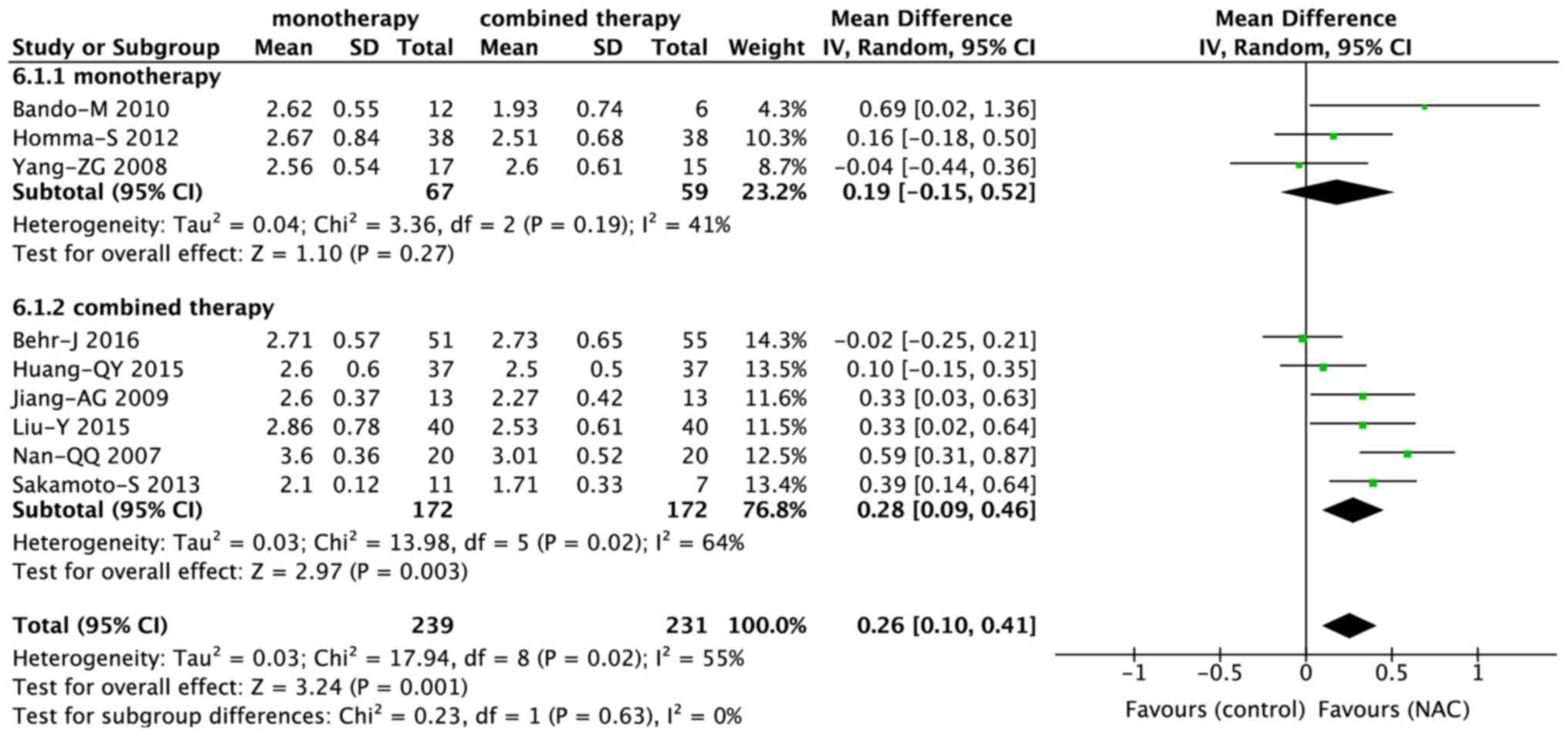
Subgroup analysis of FVC
Monotherapy vs. combined therapy
Among the nine studies that evaluated the FVC, six
trials (15,19,27,28,31,35) and
three trials (14,18,37)
involved combined therapy and monotherapy, respectively. While
combined therapy including NAC significantly reduced the decline in
FVC (MD, 0.28; 95% CI, 0.09–0.46; P=0.003), NAC monotherapy did not
achieve a significant difference (MD, 0.19; 95% CI, −0.15 to 0.52;
P=0.27; Fig. 4). However, there was
no significant difference between combined and monotherapy (P=0.63;
Fig. 5).
Low vs. high dose of NAC
A total of six (19,27,28,31,35,37) and
three (14,15,18)
studies used high (1,800 mg per day) and low (<1,800 mg per day)
doses of NAC, respectively. The NAC dose had no significant
influence on the change in FVC (Fig.
4).
Inhaled vs. oral administration
NAC was orally administered in six studies (19,27,28,31,35,37) and
administered via inhalation in three studies (14,15,18). The
route of administration did not influence the change in FVC
(Fig. 4).
Effect of the study quality on the
outcomes
The mean of the methodological score was 7.9 and
this was used as the cut-off score for dividing the studies into
high- (>7.9) and low- (<7.9) quality subgroups. Among the
nine studies that evaluated the FVC, two (14,19) were
included in the high-quality subgroup, while seven (15,18,27,28,31,35,37) were
included in the low-quality subgroup. The treatment effects of NAC
were significantly greater (P=0.03) in the low-quality subgroup
than in the high-quality subgroup (MD, 0.32; 95% CI, 0.16–0.48,
P<0.001 vs. MD, 0.04; 95% CI, −0.16 to 0.23, P=0.71; Figs. 4 and 6).
Subgroup analysis of adverse effects
Monotherapy vs. combined therapy
Among the 12 studies that evaluated the adverse
effects of NAC, seven (16,19,27,31,33,36,38)
involved combined therapy and five (13,14,18,20,37)
involved monotherapy. The use of NAC alone or in combination did
not influence the occurrence of adverse effects (Fig. 7).
Low vs. high dose of NAC
A total of eight (13,19,20,27,
31,33,36,37) and
four (14,16,18,38)
studies used high (1,800 mg per day) and low (<1,800 mg per day)
doses of NAC, respectively. The dose of NAC did not influence the
occurrence of side effects (Fig.
7).
Inhaled vs. oral administration
A total of nine (13,19,20,27,31,33,36–38) and
three (14,16,18)
studies involved oral and inhaled routes of NAC administration,
respectively. The incidence of adverse effects was significantly
higher (P=0.02) in the inhalation subgroup (RR, 7.66; 95% CI,
1.48–39.69; P=0.02) than that in the oral subgroup (RR, 1.00; 95%
CI, 0.91 to 1.10; P=0.98; Figs. 7
and 8).
Effect of the study quality on the
outcomes
The studies reporting on adverse effects were also
divided into a high-quality (13,14,16,19,20,31,33,38) and
a low-quality (18,27,36,37)
subgroup, and no significant differences were observed between the
two groups (Fig. 7).
Publication bias
Funnel plots and Egger's test were used to identify
potential publication bias in the included studies. The funnel
plots were asymmetrical for the studies reporting adverse effects
and PaO2 data (Fig. 9);
this indicated a potential risk of publication bias. However,
Egger's test revealed no publication bias for adverse effects
(P=0.224) or PaO2 data (P=0.61).
Discussion
IPF is a progressive and fatal disease, with
symptoms including cough, chronic dyspnea, fatigue and weight loss
(39). Several risk factors are
associated with IPF, including environmental factors, microbial
agents, gastroesophageal reflux and genetic factors (2). While affected patients may benefit from
certain non-pharmacological therapies, including long-term oxygen
administration or lung transplantation, there are concerns
regarding available pharmacological therapies. Pirfenidone
(40) and nintedanib (41), two newly approved drugs, have been
reported to lower the decline in FVC in patients with IPF; however,
they are associated with severe adverse effects and increased
mortality rates.
The current treatment options for IPF are limited.
NAC, an anti-oxidant, has been used for the treatment of IPF for
several years. Although the pathogenesis of IPF remains to be fully
elucidated, it has been indicated that oxidative agent-mediated
alveolar epithelial cell injury, along with an abnormal fibroblast
response, contributes to the development of pulmonary fibrosis
(42,43). NAC is a precursor of the anti-oxidant
glutathione; therefore, in patients with IPF, who exhibit oxidative
stress (10) and lower glutathione
levels, NAC therapy may prove effective by inhibiting oxidation and
restoring the redox balance. Furthermore, a study has indicated
that NAC is an efficacious drug for patients with IPF associated
with an rs3750920 single-nucleotide polymorphism in the gene
encoding Toll-interacting protein of the TT genotype (44).
In the present updated systematic review and
meta-analysis, 1,354 patients in 21 trials were evaluated regarding
the efficacy and safety of NAC therapy in IPF. Two previous
meta-analyses addressing the same topic have been published
(21,22). However, one of them only included
five studies (21), while the other
included 12 studies assessing anti-oxidant therapy for IPF
(22). For the present
meta-analysis, data for FVC, DLCO, DLCO%, VC and 6MWT were
extracted as important outcomes. Pooled analysis of the data
indicated that NAC therapy significantly reduced the decline in the
FVC, a result that is different from those of the previous studies
(21,22). Furthermore, NAC significantly reduced
the decline in DLCO, and improved the 6MWT results, which was in
accordance with the results of Sun et al (21). Most of the studies included in the
present meta-analysis also evaluated the PaO2, and the
pooled analysis revealed that NAC therapy stabilized this parameter
in patients with IPF. Although NAC is able to ameliorate hypoxia
and protect patients with IPF from further deterioration of lung
function, its adverse effects cannot be ignored. Commonly reported
adverse effects in the studies analyzed included cough, dyspnea,
bacterial pneumonia, diarrhea, headache, edema and abdominal pain.
The incidence of cough was high in two studies (14,18), in
which NAC was administered via inhalation. However, the present
meta-analysis identified no significant difference in the incidence
of adverse effects between NAC therapy and the control treatments.
Furthermore, the present analysis revealed that NAC therapy did not
increase the mortality of IPF patients. However, in the PANTHER
trial (45), combined therapy with
NAC, prednisone and azathioprine was discontinued due to an
increased incidence of mortality. When the trial was continued
using a two-arm design (NAC vs. placebo), NAC therapy was indicated
to have no influence on mortality (20). Of note, in most of the studies
included in the present meta-analysis, NAC was administered in
combination with prednisone, which may not increase mortality.
Furthermore, to address the possible heterogeneity
in the primary outcomes, a hypothesis-based subgroup analysis of
FVC was performed. The most important result was that NAC combined
therapy significantly reduced the decline in FVC (P=0.003), while
monotherapy did not result in a significant difference. This
indicates that combined therapy may be more effective than
monotherapy. However, in the PANORAMA study (19), a phase II, randomized,
double-blinded, placebo-controlled study, addition of NAC to
pirfenidone did not substantially alter the tolerability profile of
pirfenidone, and was unlikely to have benefits for IPF. This result
suggested that the combination of NAC and pirfenidone should be
used with caution. The present analysis also indicated that oral
administration of NAC at a high dose was beneficial in terms of
FVC. However, high does should also be administered with caution.
Due to limitations in the methodological quality, subgroup analysis
revealed better outcomes in studies with low methodological quality
than in those with high methodological quality. In the subgroup
analysis on adverse effects, the only significant difference was
obtained between the oral and inhalation subgroups, with the latter
being associated with more adverse effects. However, the adverse
effects caused by inhalation were mild to moderate and the patients
were able to tolerate them. Taken together, the present
meta-analysis demonstrated the efficacy and safety of NAC therapy
for IPF.
Of note, the present study has certain limitations.
First, none of the trials included was a randomized controlled
trial, and certain studies were case-control or retrospective
studies. Consequently, a certain bias associated with flaws in the
methodology was inevitable. Furthermore, various important
end-point variables were not evaluated, as certain outcomes were
reported in a different format, e.g., delta FVC without FVC. In
addition, no differentiation was made in terms of disease stage and
genotypes, which may influence the efficacy of NAC therapy. With
regard to adverse effects and mortality, it was not possible to
determine whether all these reported adverse effects and mortality
were directly associated with NAC. In the PANTHER trial, a regimen
involving prednisone, azathioprine and NAC increased the rates of
mortality, hospitalization and serious adverse events, while the
result of the follow-up study showed that there were no significant
differences between NAC and placebo in terms of mortality and acute
exacerbation (45). A recent study
indicated that an immunosuppressive agent affecting the telomere
length in leukocytes resulted in adverse events in the patients
with IPF in the PANTHER trial (46).
This suggests that the safety of NAC therapy for IPF may be
severely limited by the addition of azathioprine. Finally, the
search performed for the present study did not include any ongoing
trials and sensitivity analysis was not performed in the current
meta-analysis.
In conclusion, the results of the present
meta-analysis suggested that NAC therapy is a safe and effective
modality that may reduce the decline in the lung function of
patients with IPF. Furthermore, combined therapy including NAC and
oral administration of NAC may be more effective than monotherapy
and administration via inhalation. However, these results require
to be interpreted with caution due to possible methodological flaws
of the studies included. More well-designed, high-quality,
multicenter randomized controlled trials with larger sample sizes
are urgently required to clarify these results.
Acknowledgements
The authors would like to thank Dr Hailang He
(Department of Respiratory Medicine, Affiliated Hospital of Nanjing
University of Chinese Medicine, Nanjing, Jiangsu, China) for
assistance with this updated review and Editage [www.editage.cn] for English language editing.
Funding
This study was finically supported by the National
Nature Science Foundation of China (grant no. 81673936) and the
Postgraduate Research & Practice Innovation Program of Jiangsu
Province (grant no. KYCX17_1311).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
XM and QW conceived and designed the study. FC and
JR performed the review. FC JR and ZC analyzed the data and wrote
the manuscript. XM was responsible for quality control. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lederer DJ and Martinez FJ: Idiopathic
pulmonary fibrosis. N Engl J Med. 378:1811–1823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hutchinson J, Fogarty A, Hubbard R and
McKeever T: Global incidence and mortality of idiopathic pulmonary
fibrosis: A systematic review. Eur Respir J. 46:795–806. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strand MJ, Sprunger D, Cosgrove GP,
Fernandez-Perez ER, Frankel SK, Huie TJ, Olson AL, Solomon J, Brown
KK and Swigris JJ: Pulmonary function and survival in idiopathic vs
secondary usual interstitial pneumonia. Chest. 146:775–785. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YM, Nepali K and Liou JP: Idiopathic
pulmonary fibrosis: Current status, recent progress, and emerging
targets. J Med Chem. 60:527–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kliment CR and Oury TD: Oxidative stress,
extracellular matrix targets, and idiopathic pulmonary fibrosis.
Free Radic Biol Med. 49:707–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinnula VL, Fattman CL, Tan RJ and Oury
TD: Oxidative stress in pulmonary fibrosis: A possible role for
redox modulatory therapy. Am J Respir Crit Care Med. 172:417–422.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paliogiannis P, Fois AG, Collu C, Bandinu
A, Zinellu E, Carru C, Pirina P, Mangoni AA and Zinellu A:
Oxidative stress-linked biomarkers in idiopathic pulmonary
fibrosis: A systematic review and meta-analysis. Biomark Med.
12:1175–1184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borok Z, Buhl R, Grimes GJ, Bokser AD,
Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM and Crystal
RG: Effect of glutathione aerosol on oxidant-antioxidant imbalance
in idiopathic pulmonary fibrosis. Lancet. 338:215–216. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hagiwara SI, Ishii Y and Kitamura S:
Aerosolized administration of N-acetylcysteine attenuates lung
fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med.
162:225–231. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demedts M, Behr J, Buhl R, Costabel U,
Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent
F, et al: High-dose acetylcysteine in idiopathic pulmonary
fibrosis. N Engl J Med. 353:2229–2242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Homma S, Azuma A, Taniguchi H, Ogura T,
Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M and
Kudoh S; Japan NAC Clinical Study Group, : Efficacy of inhaled
N-acetylcysteine monotherapy in patients with early stage
idiopathic pulmonary fibrosis. Respirology. 17:467–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakamoto S, Itoh T, Muramatsu Y, Satoh K,
Ishida F, Sugino K, Isobe K and Homma S: Efficacy of pirfenidone in
patients with advanced-stage idiopathic pulmonary fibrosis. Intern
Med. 52:2495–2501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto S, Muramatsu Y, Satoh K, Ishida
F, Kikuchi N, Sano G, Sugino K, Isobe K, Takai Y and Homma S:
Effectiveness of combined therapy with pirfenidone and inhaled
N-acetylcysteine for advanced idiopathic pulmonary fibrosis: A
case-control study. Respirology. 20:445–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomioka H, Kuwata Y, Imanaka K, Hashimoto
K, Ohnishi H, Tada K, Sakamoto H and Iwasaki H: A pilot study of
aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis.
Respirology. 10:449–455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bando M, Hosono T, Mato N, Nakaya T,
Yamasawa H, Ohno S and Sugiyama Y: Long-term efficacy of inhaled
N-acetylcysteine in patients with idiopathic pulmonary fibrosis.
Intern Med. 49:2289–2296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Behr J, Bendstrup E, Crestani B, Günther
A, Olschewski H, Sköld CM, Wells A, Wuyts W, Koschel D, Kreuter M,
et al: Safety and tolerability of acetylcysteine and pirfenidone
combination therapy in idiopathic pulmonary fibrosis: A randomised,
double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med.
4:445–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez FJ, de Andrade JA, Anstrom KJ,
King TE Jr and Raghu G: Randomized trial of acetylcysteine in
idiopathic pulmonary fibrosis. N Engl J Med. 370:2093–2101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun T, Liu J and Zhao de W: Efficacy of
N-Acetylcysteine in idiopathic pulmonary fibrosis: A systematic
review and meta-analysis. Medicine (Baltimore). 95:e36292016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kandhare AD, Mukherjee A, Ghosh P and
Bodhankar SL: Efficacy of antioxidant in idiopathic pulmonary
fibrosis: A systematic review and meta-analysis. EXCLI J.
15:636–651. 2016.PubMed/NCBI
|
|
23
|
Birmingham CL: Total parenteral nutrition
in the critically ill patient. Lancet. 353:1116–1117. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Langlois PL, Manzanares W, Adhikari NKJ,
Lamontagne F, Stoppe C, Hill A and Heyland DK: Vitamin C
administration in the critically ill: A systematic review and
meta-analysis. JPEN J Parenter Enteral Nutr. 43:335–346. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manzanares W, Lemieux M, Elke G, Langlois
PL, Bloos F and Heyland DK: High-dose intravenous selenium does not
improve clinical outcomes in the critically ill: A systematic
review and meta-analysis. Crit Care. 20:3562016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu SQ, Wang J, Yan YN and Fu SB: Effects
of acetylcysteine plus prednisone for idiopathic pulmonary fibrosis
patients. Clin Focus. 30:38–40. 2015.(In Chinese).
|
|
27
|
Huang QY: Therapeutic effect of
N-acetylcysteine combined with glucocorticoid for idiopathic
pulmonary fibrosis. Chin J Clin Ration Drug Use. 8:52–53. 2015.(In
Chinese).
|
|
28
|
Jiang AG, Lu HY and Duan DJ: Therapeutic
effect of N-acetylcysteine combined with glucocorticoid for
idiopathic pulmonary fibrosis. J Clin Med Pract. 13:69–70. 2009.(In
Chinese).
|
|
29
|
Jiang YQ and Jiang H: Therapeutic effect
of N-acetylcysteine 20 idiopathic pulmonary fibrosis patients.
Chinese Community Doctors. 10:43–44. 2008.(In Chinese).
|
|
30
|
Liu XJ: Glucocorticoid combined with
acetylcysteine for treating idiopathic pulmonary fibrosis in 58
cases. China Pharmaceuticals. 25:99–101. 2016.(In Chinese).
|
|
31
|
Liu Y: Clinical effect of budesonide
atomization inhalation combined with N-Acetylcysteine on idiopathic
pulmonary fibrosis. Pract J Cardiac Cereb Pneumal Vasc Dis.
23:74–77. 2015.(In Chinese).
|
|
32
|
Long QZ, Du J, Zhang XM, Ma W and Hui K:
Curative effect of combination of interferon-γ, n-acetylcysteine
and low dose prednisone on idiopathic pulmonary interstitial
fibrosis. J Guiyang Med Coll. 36:465–469. 2011.(In Chinese).
|
|
33
|
Lu JH and Gao JZ: Therapeutic effect of
N-acetylcysteine combined glucocorticoid on nitric oxide in
idiopathic pulmonary fibrosis patients. Chin J Lab Diagn.
17:1692–1693. 2013.(In Chinese).
|
|
34
|
Luo HJ: Effect of N-acetylcysteine on
pulmonary function of idiopathic pulmonary fibrosis patients. Chin
J Clin Pract Med. 7:60–61. 2006.(In Chinese).
|
|
35
|
Nan QQ: Influence of N-acetylcysteine on
serum interlukin-13 for idiopathic pulmonary fibrosis patients.
Chin J Prim Med Pharm. 14:1513–1514. 2007.(In Chinese).
|
|
36
|
Wang CH, Li CH and Kong B: Therapeutic
effect of N-acetylcysteine on patients with idiopathic pulmonary
fibrosis. Chin J Diffic and Compl Cas. 14:129–136. 2015.(In
Chinese).
|
|
37
|
Yang ZG, Ma XT and Wang SQ: Observation on
treating effect of N-acetylcystein on idiopathic pulmonary
fibrosis. J Med Forum. 29:18–20. 2008.(In Chinese).
|
|
38
|
Zhu JY, Zeng YQ, Yuan LJ, Chen G, Wang YL
and Hu K: Study on the treatment of idiopathic pulmonary fibrosis
with prednisone, N-acetylcysteine combined Captopril. J Yunyang Med
Coll. 28:582–584. 2009.(In Chinese).
|
|
39
|
Noble PW, Barkauskas CE and Jiang D:
Pulmonary fibrosis: Patterns and perpetrators. J Clin Invest.
122:2756–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lancaster LH, de Andrade JA, Zibrak JD,
Padilla ML, Albera C, Nathan SD, Wijsenbeek MS, Stauffer JL,
Kirchgaessler KU and Costabel U: Pirfenidone safety and adverse
event management in idiopathic pulmonary fibrosis. Eur Respir Rev.
26:1700572017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Richeldi L, du Bois RM, Raghu G, Azuma A,
Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y,
et al: Efficacy and safety of nintedanib in idiopathic pulmonary
fibrosis. N Engl J Med. 370:2071–2082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Duecker R, Baer P, Eickmeier O, Strecker
M, Kurz J, Schaible A, Henrich D, Zielen S and Schubert R:
Oxidative stress-driven pulmonary inflammation and fibrosis in a
mouse model of human ataxia-telangiectasia. Redox Biol. 14:645–655.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hosseinzadeh A, Javad-Moosavi SA, Reiter
RJ, Yarahmadi R, Ghaznavi H and Mehrzadi S: Oxidative/nitrosative
stress, autophagy and apoptosis as therapeutic targets of melatonin
in idiopathic pulmonary fibrosis. Expert Opin Ther Targets.
22:1049–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ,
Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al:
TOLLIP, MUC5B, and the response to N-acetylcysteine among
individuals with idiopathic pulmonary fibrosis. Am J Respir Crit
Care Med. 192:1475–1482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Idiopathic Pulmonary Fibrosis Clinical
Research Network, ; Raghu G, Anstrom KJ, King TE Jr, Lasky JA and
Martinez FJ: Prednisone, azathioprine, and N-acetylcysteine for
pulmonary fibrosis. N Engl J Med. 366:1968–1977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Newton CA, Zhang D, Oldham JM, Kozlitina
J, Ma SF, Martinez FJ, Raghu G, Noth I and Garcia CK: Telomere
length and use of immunosuppressive medications in idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. Dec 19–2018.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|