Introduction
Colon cancer is one of the most common malignant
tumors and the third leading cause of cancer-associated mortality
throughout the world (1). The
primary treatment of colon cancer usually involves surgical
resection and chemotherapy using cytotoxic drugs. Oxaliplatin
(L-OHP) is one of the first-line drugs used for the treatment of
metastatic colorectal cancer (CRC) (2). However, many cancer patients develop
chemotherapeutic drug resistance that can lead to colon cancer
treatment failure (3). Therefore,
the search for novel effective agents for overcoming drug
resistance and colon cancer treatment is required.
Ginseng is a medicinal plant with substantial
medicinal effects and has a positive effect in the clinical
treatment of cancer. Ginsenosides are the major active ingredients
of ginseng and have multiple biological activities, including
immunomodulatory effects and anti-inflammatory and antitumor
activities (4,5). A previous study showed that ginsenoside
Rh2 (G-Rh2) is one of the main active components of ginseng with
antitumor activity (6–8). It has been reported that G-Rh2 exerts
anticancer effects in a variety of malignant diseases, including
CRC and breast cancer (9,10). G-Rh2 has been found with a potent
ability to induce cell apoptosis and inhibit cancer cell
proliferation (11).
Apoptosis is a complex process of programmed cell
death (12). Two major
cysteine-aspartate protease (caspase) activation cascades are
involved in cell apoptosis. The first is the extrinsic apoptotic
pathway, mediated by the activation of various cell-surface death
receptors, including Fas, TNF receptor and DR4, which in turn
cleaves and activates three short prodomain caspases, caspase-3, −6
and −7. The other is an intrinsic apoptotic pathway, driven by
Bcl-2 family proteins, which leads to mitochondrial outer membrane
permeabilization and the release of pro-apoptotic factors,
particularly cytochrome c, and leads to the activation of
caspase-9 (13,14). The abnormal regulation of apoptosis
may lead to tumor progression and resistance to chemotherapy
(15). It has been demonstrated that
G-Rh2 induces human hepatoma cell apoptosis via the Bax/Bak-induced
release of cytochrome c and activation of
caspase-9/caspase-8 (16). G-Rh2 can
induce neuroblastoma cell apoptosis through the activation of
caspase-1 and caspase-3 and the upregulation of Bax (17). However, the effect and mechanism of
G-Rh2 on L-OHP-resistant colon cancer cells have not been
clarified.
The purpose of the present study was to investigate
the effect of G-Rh2 on the drug resistance of L-OHP-resistant human
colon cancer cells (LoVo/L-OHP) and to examine its potential
mechanism.
Materials and methods
Reagents and antibodies
G-Rh2 (purity ≥98%) was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and dissolved in
dimethylsulfoxide (DMSO; Sigma-Aldrich; Merck KGaA). Primary
antibodies against Bcl-2, Bax, caspase-3, and P-glycoprotein (P-gp)
were obtained from Abcam (Cambridge, UK). Smad4 and GAPDH were
obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture and treatment
The human CRC cells (LoVo) and human L-OHP-resistant
CRC cells (LoVo/L-OHP) were purchased from American Type Culture
Collection (Manassas, VA, USA) and grown in RPMI-1640 medium (Life
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/ml of penicillin and 100 µg/ml of
streptomycin (Gemini Bio-Products) and maintained at 37°C in a
humidified atmosphere of 5% CO2. The cells were treated
in the following manner: i) cells were treated with a series of
concentrations of G-Rh2 (0, 50, 100, 200 and 250 µg/ml) for 24 h
and then subjected to a 3-(4,5
dimethylthiazol-z-yl)-3,5-diphenyltetrazolium bromide (MTT) assay;
ii) cells were treated with 250 µg/ml G-Rh2 for 24 h and then
subjected to an MTT assay, flow cytometry (FCM), western blotting
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis; iii) cells were treated with 15 µmol/ml L-OHP
for 24 h and then subjected to an MTT assay, FCM, western blotting
and RT-qPCR analysis; iv) cells were treated with 250 µg/ml G-Rh2 +
15 µmol/ml L-OHP for 24 h and then subjected to an MTT assay, FCM,
western blotting and RT-qPCR analysis. An equal volume of DMSO
(final concentration, <0.1%) was used for controls.
Cell viability assay
Cell viability was measured using an MTT assay
(Sigma-Aldrich; Merck KGaA) in the present study. Briefly,
1×104 cells/well LoVo/L-OHP or LoVo cells were seeded
into 96-well plates and allowed to attach for 24 h prior to the
addition of drugs, and were then treated with the indicated drugs
for 24 h at 37°C with 5% CO2. An equal volume of DMSO
(final concentration, <0.1%) was used as a control. Following
treatment, 200 µl MTT (0.5 mg/ml) was added in each well and
incubated at 37°C for 4 h, following which the supernatant was
removed and the cells were incubated with 150 µl DMSO for an
additional 30 min in dark at 37°C. The absorbance at 570 nm of each
well was recorded using a Synergy HT Multi-Mode microplate reader
(Thermo Fisher Scientific, Inc.).
Cell apoptotic analysis
Annexin V-FITC Apoptosis Detection kit (Besbio,
Shanghai, China) was used to detect the cell apoptosis according to
the manufacturer's instructions. Briefly, the LoVo/L-OHP or LoVo
cells (5×105 cells/ml) were seeded in 6-well plates and
incubated with the gradient concentrations of drugs for 24 h. The
treated cells were then harvested and washed with PBS, incubated in
500 µl Annexin-V binding buffer containing 5 µl Annexin-V-FITC for
15 min and then resuspended with 5 µl PI in the dark at room
temperature for another 5 min. The percentages of early and late
apoptotic cells were quantitatively analyzed using a flow cytometer
(BD FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Following treatment, the cells were lysed in RIPA
buffer (Beyotime Institute of Biotechnology, Shanghai, China)
containing phosphatase and protease inhibitors at room temperature
for 1 min. The protein concentration was quantified using a BCA
protein assay kit (BestBio, Shanghai, China). Equal quantities of
proteins (50 µg/lane) were separated by 12% SDS-PAGE and then
transferred onto PVDF membranes (EMD Millipore, Temecula, CA, USA)
followed by blocking with 5% non-fat milk at room temperature for 1
h. Subsequently, the PVDF membranes were incubated with anti-Bax
(1:1,000; cat. no. ab32503; Abcam, Cambridge, UK), anti-Bcl-2
(1:1,000; cat. no. ab196495; Abcam), anti-caspase-3 (1:1,000; cat.
on. 14220; Cell Signaling Technology, Inc.), anti-P-gp (1:1,000;
cat. no. ab103477; Abcam), anti-Smad4 (1:1,000; cat. no. 38454;
Cell Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no. 5174;
Cell Signaling Technology, Inc.) primary antibodies overnight at
4°C, followed by incubation with horseradish peroxidase-conjugated
secondary antibody (1:2,000; cat. no. 7074, Cell Signaling
Technology, Inc.) at room temperature for 1 h. The immunoreactivity
bands were visualized using an enhanced chemiluminescent system
(Pierce, Thermo Fisher Scientific, Inc.) and the densitometry of
the bands was determined with Gel-Pro Analyzer densitometry
software (version 6.3; Media Cybernetics, Inc., Rockville, MD,
USA).
RT-qPCR analysis
Total RNA was extracted with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. A reverse transcription kit (Takara
Bio, Inc., Tokyo, Japan) was used for the reverse transcription of
RNA to cDNA. Reaction conditions for reverse transcription were:
50°C for 5 min and 80°C for 2 min. qPCR was performed using the
SYBR Green real-time PCR kit (Takara Bio, Inc.) on an Applied
Biosystems 7500 real-time PCR detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Amplification conditions for qPCR
were as follows: 5 min at 95°C; 35 cycles of 95°C for 15 sec, 40
sec at 55°C, and 72 °C for 1 min. The primer sequences for PCR were
as follows: P-gp [multidrug resistant 1 (mdr1)a and mdr1b], mdr1a,
forward 5′-CACCATCCAGAACGCAGACT-3′ and reverse
5′-ACATCTCGCATGGTCACAGTT-3′; mdr1b, forward
5′-AACGCAGACTTGATCGTGGT-3′ and reverse 5′-AGCACCTCAAATACTCCCAGC-3′;
Smad4, forward 5′-GACAGCAGCAGAATGGAT-3′ and reverse,
5′-CAGGAGCAGGATGATTAGAA-3′; Bax forward,
5′-GGGTTTCATCCAAGGATCGAGCAGG-3′ and reverse
5′-ACAAAGATGGTCACGGTCTGCC-3′; Bcl-2, forward
5′-GAGAAATCAAACAGAGGCCG-3′ and reverse 5′-CTGAGTACCTGAACCGGCA-3′;
caspase-3, forward 5′-CTGCCTCTTCCCCCATTCT-3′ and reverse
5′-TCGCTTCCATGTATGATCTTTG-3′; GAPDH, forward
5′-CCTCCAAAATCAAGTGGGGCGATG-3′ and reverse
5′-CATATTTGGCAGGTTTTTCTAGAC-3′. The quantitative analysis of mRNA
levels was normalized to GAPDH and relative gene expression levels
were calculated using the 2−ΔΔCq method (18).
Statistical analysis
Statistical analysis was performed with SPSS 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). Differences
among groups were analyzed using Student's t test or one way-ANOVA
followed by Tukey's post hoc test. Data are presented as the mean ±
SD. P≤0.05 was considered to indicate a statistically significant
difference.
Results
G-Rh2 inhibits the proliferation and
induces the apoptosis of LoVo/L-OHP cells
As shown in Fig. 1A.
compared with the half-maximal inhibitory concentration of L-OHP in
the parental LoVo cells, the LoVo/L-OHP cells were more resistant
to L-OHP.
 | Figure 1.Effect of G-Rh2 on the proliferation
and apoptosis of LoVo/L-OHP cells. (A) IC50 of L-OHP to
LoVo cells and LoVo/L-OHP cells (###P<0.001 vs. LoVo
cells). (B) LoVo/L-OHP cells were treated with various
concentrations of G-Rh2 (0, 50, 100, 200 and 250 µg/ml) for 24 h,
and cell viability was measured using a 3-(4,5
dimethylthiazol-z-yl)-3,5-diphenyltetrazolium bromide assay
(*P<0.05 and **P<0.01 vs. 0 µg/ml treatment group). (C)
LoVo/L-OHP cells were treated with 250 µg/ml G-Rh2 or 15 µmol/ml
L-OHP for 24 h, and cell apoptosis (early and late apoptotic cells)
was determined using flow cytometry (**P<0.01 vs. control
group). Data are expressed as the mean ± SD. G-Rh2, ginsenoside
Rh2; L-OHP, oxaliplatin; IC50, half maximal inhibitory
concentration. |
To examine the effects of G-Rh2 on the proliferation
of LoVo/L-OHP cells, an MTT assay was performed to assess the
viability of LoVo/L-OHP cells treated with or without G-Rh2 (0, 50,
100, 200 and 250 µg/ml). The results showed that LoVo/L-OHP cell
viability was significantly reduced following treatment with G-Rh2
in a dose-dependent manner (Fig.
1B). The results of FCM showed that the number of apoptotic
LoVo/L-OHP cells was significantly increased following treatment
with G-Rh2 compared with that in the untreated control group
(Fig. 1C). However, 15 µmol/ml L-OHP
had no significant effects on cell apoptosis.
The drug-resistance genes P-gp and Smad4, and
apoptosis-related proteins were also determined in the present
study. As shown in Fig. 2A-F,
compared with the untreated control group, treatment with G-Rh2
markedly decreased the protein and mRNA levels of P-gp and Bcl-2,
and increased the protein and mRNA levels of Smad4, Bax and
caspase-3 the in LoVo/L-OHP cells. Treatment with 15 µmol/ml L-OHP
had no significant effect on the expression of P-gp, Smad4, Bcl-2,
Bax or caspase-3.
 | Figure 2.Effect of G-Rh2 on the expression of
P-gp, Smad4, Bcl-2, Bax and caspase-3 in LoVo/L-OHP cells.
LoVo/L-OHP cells were treated with 250 µg/ml G-Rh2 or 15 µmol/ml
L-OHP for 24 h, following which (A) protein levels were measured
using western blotting. mRNA levels of (B) P-gp, (C) Smad4, (D)
Bcl-2, (E) Bax and (F) caspase-3 in LoVo/L-OHP cells were measured
using reverse transcription-quantitative polymerase chain reaction
analysis. Data are expressed as the mean ± SD. **P<0.01 vs.
control group. G-Rh2, ginsenoside Rh2; L-OHP, oxaliplatin; P-gp,
P-glycoprotein. |
Rh2 inhibits the proliferation and
induces the apoptosis of LoVo cells
The present study then determined the effect of
G-Rh2 on the proliferation and apoptosis of LoVo cells using an MTT
assay and FCM. The results showed that 250 µg/ml G-Rh2
significantly inhibited the proliferation (Fig. 3A) of the LoVo cells and induced cell
apoptosis (Fig. 3B). In addition, it
was found that 250 µg/ml G-Rh2 significantly increased the protein
(Fig. 3C) and mRNA (Fig. 3D-H) levels of Smad4, Bax and
caspase-3, whereas the protein and mRNA levels of P-gp and Bcl-2
were reduced.
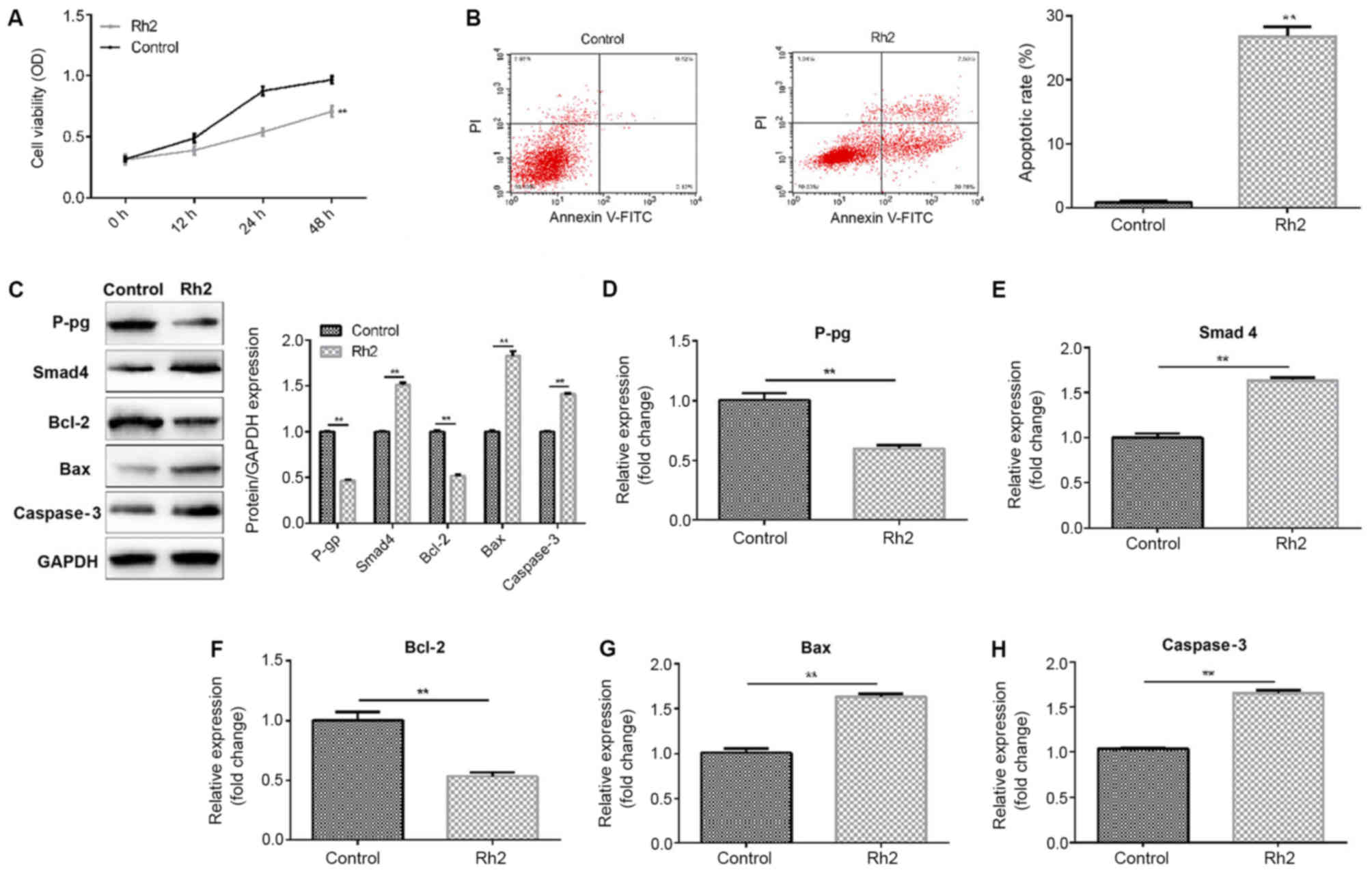 | Figure 3.Effect of G-Rh2 on the proliferation
and apoptosis of LoVo cells. LoVo cells were treated with or
without 250 µg/ml G-Rh2 for 24 h. A 3-(4,5
dimethylthiazol-z-yl)-3,5-diphenyltetrazolium bromide assay was
used to detect (A) cell viability, and (B) flow cytometry was used
to detect cell apoptosis (early and late apoptotic cells). (C)
Protein levels of P-gp, Smad4, Bcl-2, Bax and caspase-3 in LoVo
cells were determined using a western blot assay. mRNA levels of
(D) P-gp, (E) Smad4, (F) Bcl-2, (G) Bax and (H) caspase-3 were
determined by reverse transcription-quantitative polymerase chain
reaction analysis. Data are expressed as the mean ± SD. **P<0.01
vs. control group. G-Rh2, ginsenoside Rh2; P-gp,
P-glycoprotein. |
G-Rh2 reverses L-OHP resistance in
LoVo/L-OHP cells
Treatment with G-Rh2 markedly reversed L-OHP
resistance in the LoVo/L-OHP cells (Fig.
4). How G-Rh2 reversed L-OHP resistance in the LoVo/L-OHP cells
was subsequently investigated, and cell proliferation and apoptosis
were determined. It was found that 15 µmol/ml L-OHP had no
significant effects on LoVo/L-OHP cell proliferation or apoptosis,
whereas G-Rh2 + L-OHP treatment significantly inhibited LoVo/L-OHP
cell proliferation (Fig. 5A) and
induced apoptosis (Fig. 5B). In
addition, to investigate the potential molecular mechanisms
underlying the reversal of drug resistance by G-Rh2 and the
potential mechanism of G-Rh2 on cell proliferation and apoptosis,
the effect of G-Rh2 on drug-resistance genes P-gp and Smad4, and
apoptosis-related genes were examined. The results (Fig. 5C-H) indicated that 15 µmol/ml L-OHP
had no significant effects on the expression of P-gp, Smad4, Bcl-2,
Bax or caspase-3 in LoVo/L-OHP cells. However, treatment with G-Rh2
+ L-OHP significantly reduced the expression of P-gp and Bcl-2, and
increased the expression of Smad4, Bax and caspase-3.
 | Figure 5.Effect of the combination of G-Rh2 and
L-OHP on LoVo/L-OHP cells. LoVo cells were treated with or without
15 µmol/ml L-OHP, or 15 µmol/ml L-OHP + 250 µg/ml G-Rh2 for 24 h.
(A) A 3-(4,5 dimethylthiazol-z-yl)-3,5-diphenyltetrazolium bromide
assay was used to detect cell viability, and (B) flow cytometry was
used to detect cell apoptosis. (C) Protein levels of P-gp, Smad4,
Bcl-2, Bax, and caspase-3 in LoVo cells were determined using a
western blot assay. mRNA levels of (D) P-gp, (E) Smad4, (F) Bcl-2,
(G) Bax and (H) caspase-3 were determined by reverse
transcription-quantitative polymerase chain reaction analysis. Data
are expressed as the mean ± SD. **P<0.01 vs. control group.
G-Rh2, ginsenoside Rh2; L-OHP, oxaliplatin; P-gp,
P-glycoprotein. |
Discussion
The present study indicated that G-Rh2 inhibited the
proliferation and induced the apoptosis of LoVo/L-OHP cells and
LoVo cells, and reversed L-OHP resistance in LoVo/L-OHP cells
through inhibiting cell proliferation and inducing cell apoptosis
via regulating the expression of drug-resistance gene P-gp and
Smad4, and apoptosis-related genes. These findings provide a novel
strategy and theoretical basis for the treatment of CRC.
G-Rh2, one of the main components of ginseng, has
numerous biological activities, and there are no reported side
effects in normal cells. A previous study reported that G-Rh2
reduced cell proliferation and sensitized CRC cells to
5-fluorouracil chemotherapy (19).
In addition, there is evidence that G-Rh2 may be associated with
drug resistance in cancer treatment (20). In the present study, it was
demonstrated that G-Rh2 reversed drug resistance in LoVo/L-OHP
cells and decreased expression of P-gp. In addition, the results
indicated that G-Rh2 had antiproliferative and pro-apoptotic
effects on LoVo/L-OHP and LoVo cells.
P-gp is an ATP-dependent efflux transporter, which
is expressed at high levels in the gastrointestinal tract and
multidrug-resistant tumor cells (21). The inhibition of P-gp can reverse the
multidrug resistance induced by chemotherapeutic agents (22). Previous studies have reported that
G-Rh2 can reverse multidrug resistance in adriamycin-resistant
human breast cancer MCF-7 cells (20) and reverse drug resistance in
5-fuorouracil-resistant LoVo and HCT-8 human CRC cells (19). In a previous study, 20 (S)-Rh2 was
shown to inhibit P-gp in multidrug-resistant cancer cells (21). Consistent with previous reports, the
results of the present study demonstrated that G-Rh2 reversed drug
resistance in LoVo/L-OHP cells and decreased the expression of
P-gp.
Increasing studies have shown that apoptosis is an
important mechanism through which chemotherapeutic agents kill
susceptible cells (23). During the
process of programmed cell death, the Bcl-2 protein interferes with
the activation of caspases by preventing the release of cytochrome
c, which can be induced by Bax (24). A previous study reported that G-Rh2
induces apoptosis via the activation of caspase-1 and −3 and
upregulation of Bax in human neuroblastoma (17). G-Rh2 can inhibit the proliferation of
the A549 human lung adenocarcinoma cell line in a dose-dependent
manner by activating caspase-8/3 to promote apoptosis (25). In the present study, the effects of
G-Rh2 on cell apoptosis-related molecules were investigated. The
results demonstrated that G-Rh2 decreased the expression of Bcl-2
and increased the expression of Bax, caspase-3 and Smad4 in
LoVo/L-OHP and LoVo cells. As reported previously, G-Rh2 inhibits
tumor cell growth and prevents cells from entering the growth
phase, including the G2/M, G1/S and S phases, respectively
(25,26). G-Rh2 can inhibit A172 human glioma
cell proliferation and induce cell cycle arrest status (27). In the present study, it was also
found that G-Rh2 treatment markedly inhibited the proliferation and
induced the apoptosis of LoVo/L-OHP and LoVo cells. In addition,
G-Rh2 + L-OHP treatment significantly inhibited LoVo/L-OHP cell
proliferation and induced apoptosis. To investigate the potential
molecular mechanisms underlying the reversal of drug-resistant by
G-Rh2 and the potential mechanism underlying the effects of G-Rh2
on cell proliferation and apoptosis, the present study also
determined the effect of G-Rh2 on drug-resistance genes P-gp and
Smad4, and apoptosis-related genes. It was found that G-Rh2 + L-OHP
treatment significantly reduced the expression of P-gp and Bcl-2,
whereas the expression levels of Smad4, Bax and caspase-3 were
enhanced. L-OHP alone had no significant effect on the LoVo/L-OHP
cells.
In conclusion, the results of the present study
showed that G-Rh2 effectively reversed drug resistance in
LoVo/L-OHP cells and its potential mechanism involved inhibiting
cell proliferation and promoting apoptosis and changes in drug
resistance genes. These results indicate that G-Rh2 may be a
promising therapeutic approach for drug resistance in CRC
chemotherapy. However, the present study is a preliminary
investigation on the effect of G-Rh2 on oxaliplatin-resistant colon
cancer, and the role of G-Rh2 on L-OHP-resistant colon cancer
requires extensive investigation. For example, whether G-Rh2 has an
effect on other L-OHP-resistant colon cancer cell lines requires
investigation, and in vivo experiments should be performed.
These issues will be investigated in the future.
Acknowledgements
Not applicable.
Funding
This study was supported by the Jiangsu Natural
Science Foundation Project (grant no. SBK20151605 211783) and the
National Natural Youth Project (grant no. 81503574).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM and GG contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. HL, DF, LL, GW and AC contributed to data
interpretation, statistical analysis and literature search. YY, HZ
and JH contributed to statistical analysis and literature search.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abad A, Massutí B, Gallego J, Yuste AL,
Manzano JL, Carrato A, Antón A, Marfa X and Diaz-Rubio E; Spanish
Cooperative Group for Gastrointestinal Tumor Therapy, : Phase I
study of the combination of oxaliplatin, irinotecan and continuous
infusion 5-fluorouracil in digestive tumors. Anticancer Drugs.
15:469–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panczyk M: Pharmacogenetics research on
chemotherapy resistance in colorectal cancer over the last 20
years. World J Gastroenterol. 20:9775–9827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibata S: Chemistry and cancer preventing
activities of ginseng saponins and some related triterpenoid
compounds. J Korean Med Sci. 16 (Suppl):S28–S37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon HY, Kim EH, Kim SW, Kim SN, Park JD
and Rhee DK: Selective toxicity of ginsenoside Rg3 on multidrug
resistant cells by membrane fluidity modulation. Arch Pharm Res.
31:171–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie J, Shao J, Lu Y, Chen J, Wang J, Yu S
and Jia L: Separation of ginseng active ingredients and their roles
in cancer metastasis supplementary therapy. Curr Drug Metab.
14:616–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu FY, Shang WQ, Yu JJ, Sun Q, Li MQ and
Sun JS: The antitumor activity study of ginsenosides and
metabolites in lung cancer cell. Am J Transl Res. 8:1708–1718.
2016.PubMed/NCBI
|
|
8
|
Dong H, Bai LP, Wong VK, Zhou H, Wang JR,
Liu Y, Jiang ZH and Liu L: The in vitro structure-related
anti-cancer activity of ginsenosides and their derivatives.
Molecules. 16:10619–10630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Zhao J, Wang CZ, Searle J, He TC,
Yuan CS and Du W: Ginsenoside Rh2 induces apoptosis and
paraptosis-like cell death in colorectal cancer cells through
activation of p53. Cancer Lett. 301:185–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi S, Kim TW and Singh SV: Ginsenoside
Rh2-mediated G1 phase cell cycle arrest in human breast cancer
cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of
cyclin-dependent kinases. Pharm Res. 26:2280–2288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown JM and Attardi LD: The role of
apoptosis in cancer development and treatment response. Nat Rev
Cancer. 5:231–237. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo XX, Guo Q, Li Y, Lee SK, Wei XN and
Jin YH: Ginsenoside Rh2 induces human hepatoma cell apoptosisvia
bax/bak triggered cytochrome C release and caspase-9/caspase-8
activation. Int J Mol Sci. 13:15523–15535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YS and Jin SH: Ginsenoside Rh2 induces
apoptosis via activation of caspase-1 and −3 and up-regulation of
Bax in human neuroblastoma. Arch Pharm Res. 27:834–839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu GW, Liu YH, Jiang GS and Ren WD: The
reversal effect of Ginsenoside Rh2 on drug resistance in human
colorectal carcinoma cells and its mechanism. Hum Cell. 31:189–198.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou B, Xiao X, Xu L, Zhu L, Tan L, Tang
H, Zhang Y, Xie Q and Yao S: A dynamic study on reversal of
multidrug resistance by ginsenoside Rh2 in
adriamycin-resistant human breast cancer MCF-7 cells. Talanta.
88:345–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Zhou F, Wu X, Gu Y, Ai H, Zheng
Y, Li Y, Zhang X, Hao G, Sun J, et al: 20(S)-ginsenoside Rh2
noncompetitively inhibits P-glycoprotein in vitro and in vivo: A
case for herb-drug interactions. Drug Metab Dispos. 38:2179–2187.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwak JO, Lee SH, Lee GS, Kim MS, Ahn YG,
Lee JH, Kim SW, Kim KH and Lee MG: Selective inhibition of MDR1
(ABCB1) by HM30181 increases oral bioavailability and therapeutic
efficacy of paclitaxel. Eur J Pharmacol. 627:92–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Sun Y, Yue L, Li S, Qi X, Zhao H,
Yang Y, Zhang C and Yu H: JNK pathway and relative transcriptional
factor were involved in ginsenoside Rh2-mediated G1 growth arrest
and apoptosis in human lung adenocarcinoma A549 cells. Genet Mol
Res. 15:2016. View Article : Google Scholar
|
|
26
|
Chen F, Zheng SL, Hu JN, Sun Y, He YM,
Peng H, Zhang B, McClements DJ and Deng ZY: Octyl ester of
ginsenoside Rh2 induces apoptosis and G1 cell cycle arrest in human
HepG2 cells by activating the extrinsic apoptotic pathway and
modulating the Akt/p38 MAPK signaling pathway. J Agric Food Chem.
64:7520–7529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li KF, Kang CM, Yin XF, Li HX, Chen ZY, Li
Y, Zhang Q and Qiu YR: Ginsenoside Rh2 inhibits human A172 glioma
cell proliferation and induces cell cycle arrest status via
modulating Akt signaling pathway. Mol Med Rep. 17:3062–3068.
2018.PubMed/NCBI
|



















