Introduction
Atherosclerosis is the primary cause of
cerebrovascular and cardiovascular diseases, which are the leading
causes of death among adults in China (1). Carotid intima-media thickness (IMT)
measured by ultrasound (US) as a surrogate marker for
atherosclerosis is widely used in basic and clinical research
(2–4). The increase in the maximum IMT
(IMTMax) and mean IMT (IMTMean) is, however,
only part of the information that reflects the atherosclerotic
lesions in the artery (5,6). At times, they also reflect the
physiological aging processes (7).
Atherosclerosis is an inflammatory disease with a
dynamic process (8). Local
inflammation occurs in the formation of plaque (9,10), which
causes roughness of the IM layer. A prospective study has revealed
that the surface granulation of the carotid arterial inner wall is
closely associated with cardiovascular events (11). Carotid intima-media roughness (IMR)
refers to a variation of IMT and measures the changes as
granulations of the IM layer. Due to technical limitations, few
studies have quantitatively evaluated the carotid IMR by using US.
The purpose of the present study was to quantitatively measure the
carotid IMR and investigate its clinical value in cardiovascular
risk stratification.
Materials and methods
Study population
A total of 185 consecutive patients (mean age,
50.1±1.0 years) who underwent carotid US examination at the United
Hospital of Tongji Medical College of Huazhong University of
Science and Technology (Wuhan, China) between November 2013 and
January 2016 were enrolled in the present study. The exclusion
criteria were as follows: i) Stroke; ii) acute myocardial
infarction; iii) peripheral artery disease; iv) acute inflammatory
diseases; v) malignant neoplasms. All participants were screened
for risk factors of cardiovascular disease and were comprehensively
evaluated, including review of medical history, physical
examination and laboratory tests.
Essential hypertension was diagnosed if the systolic
blood pressure (BP) was ≥140 mmHg and/or the diastolic BP was ≥90
mmHg or if subjects were on BP-lowering drugs (12). Hyperlipidemia was diagnosed if the
total cholesterol (TC) to high-density lipoprotein-cholesterol
(HDL-C) ratio was >5 or the patient was undergoing treatment
with lipid-lowering drugs (13).
Diabetes was diagnosed if the fasting plasma glucose level was ≥126
mg/dl on at least two occasions or if subjects were undergoing
treatment with anti-diabetic drugs (14). Obesity was diagnosed if the body mass
index (BMI) was ≥28 kg/m2 (15). The Framingham risk score (FRS) was
used to estimate the 10-year risk of coronary heart disease (CHD)
events (16). The smoking status
included past and present smoking. The FRS was calculated by points
of risk factors: Age, total cholesterol level, HDL-C level,
systolic BP and smoking status.
US examination
Carotid US was performed with a US machine (SSD-α10;
Aloka) using a UST5412 probe with a transmission frequency of 5–13
MHz by a researcher, who was blinded with regard to participant
history. With subjects placed in the supine position in a quiet
air-conditioned room (22–24°C), the extracranial carotid arteries
were visualized in the longitudinal and transverse planes. The
entire length of the common carotid arteries (CCAs) and carotid
bifurcations, including the internal carotid artery as far up as
was possible to observe, was examined for the presence of
atherosclerotic plaques. Plaque was defined as a focal structure
encroaching into the arterial lumen by at least 0.5 mm or 50% of
the surrounding IMT value exhibiting a plaque thickness of >1.5
mm (17). The longitudinal scan
images (acquired while keeping the beam parallel to the vessel
wall) were stored on CD-R disks in DICOM format for off-line
analysis. The left CCA within 1.5 cm of the bifurcation was
visualized in the longitudinal plane by US.
IMT and IMR
Clear longitudinal scan images of the far wall at
the T-wave end frame of the cine-loop recording were selected for
analysis. Measurements were made with computer-assisted analysis
software by using a previously validated snake model-based
segmentation method to detect the IM boundaries automatically
(18).
The IMT and IMR were measured at the far wall of the
CCA and not at the near wall, as the measurement at the near wall
has a larger angle between the US beam and the vessel wall, which
affects the measurement results, and because far-wall measurements
are considered to have a higher validity than near-wall
measurements (19).
IMT was defined as the distance between the
lumen-intima interface and the media-adventitia interface, as
reported previously (20). A
longitudinal region of interest was selected and the IM boundaries
were demarcated. IMT measurement was performed along each vertical
pixel line throughout the entire target vessel in the longitudinal
section (Fig. 1). The
IMTMean/IMTMax was obtained by calculating
the average/max value of these data. IMR was obtained by
calculating the standard deviation of these data.
Reproducibility of IMR
Intra- and interobserver variability of the
measurement of IMR were evaluated through blinded repeated
measurements of the same imaging data set of 30 subjects on two
different occasions 2 weeks apart, with a single researcher for
intraobserver variability and with two different researchers for
interobserver variability.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean or as percentages. Student's t-test and analysis of
variance, with Fisher's least significant difference post-hoc test,
were used to compare the mean values of the measured variables
among the groups. The chi-square test was used for categorical
data. Pearson correlation coefficients were assessed in univariate
analyses comparing IMR and various parameters. Multivariate linear
regression analysis was performed using standard least squares to
assess the independent predictive value of the IMR; odds ratios
(ORs) were calculated to determine the independent contribution of
IMR to carotid atherosclerosis (absence=0, presence=1). The
Bland-Altman test was used to assess variability in the method. The
coefficient of variation was calculated as SD(x-y)/mean(x, y)
×100%. SPSS statistical software version 13.0 for Windows (SPSS,
Inc.) was used for analysis. P<0.05 was considered to indicate
statistical significance.
Results
Population characteristics
Table I presents the
clinical and biochemical characteristics of the patients. Of the
185 subjects, 56 (30.3%) had hypertension, 77 (41.6%) had diabetes,
53 (28.6%) had hyperlipidemia and 52 (28.1%) were smokers. The
prevalence of smokers was significantly higher in males than in
females (chi-square P<0.01), as was the BMI (t-test
P<0.01).
 | Table I.Characteristics of all subjects. |
Table I.
Characteristics of all subjects.
| Parameter | Total (n=185) | Range | Males (n=112) | Females (n=73) |
|---|
| Age (years) | 50.1±1.0 | 19–87 |
48.6±1.3 |
52.3±1.4 |
| Smoking | 52 (28.1) |
| 52 (46.4) | 0 (0)a |
| Hypertension | 56 (30.3) |
| 30 (26.8) | 26 (35.6) |
| Diabetes | 77 (41.6) |
| 52 (46.4) | 25 (34.2) |
| Hyperlipidemia | 53 (28.6) |
| 35 (31.3) | 18 (24.7) |
| Anthropometric
measurements |
|
|
|
|
| SBP
(mmol/l) | 130.3±1.8 |
92–240 | 129.1±2.0 | 132.3±3.4 |
| DBP
(mmol/l) |
82.4±1.0 |
60–140 |
82.1±1.2 |
83.1±1.6 |
| BMI
(kg/m2) |
23.8±0.2 | 16.7–32.3 |
24.3±0.3 |
22.9±0.4b |
| Blood lipids |
|
|
|
|
| FBG
(mmol/l) |
7.0±0.3 |
2.1–33.4 |
6.9±0.3 |
7.1±0.5 |
| TG
(mmol/l) |
1.8±0.1 | 0.4–9.0 |
1.7±0.1 |
1.8±0.2 |
| TC
(mmol/l) |
4.7±0.1 |
0.6–21.5 |
4.6±0.2 |
4.8±0.2 |
| HDL-C
(mmol/l) |
1.4±0.1 | 0.5–9.4 |
1.4±0.1 |
1.5±0.1 |
| LDL-C
(mmol/l) |
2.4±0.1 | 0.4–7.2 |
2.4±0.1 |
2.5±0.1 |
| Ultrasonic
measurements |
|
|
|
|
| IMR
(µm) |
72.0±3.6 |
10.9–230.0 |
74.5±4.8 |
68.1±5.7 |
|
IMTMean (µm) |
675.3±12.8 |
372.0–1354.2 |
685.1±16.4 |
660.4±20.5 |
|
IMTMax (µm) |
822.6±22.2 |
430.6–2217.9 |
843.7±28.5 |
790.2±35.5 |
|
IMTMin (µm) | 565.0±9.9 |
294.0–1018.4 |
573.9±13.4 |
551.3±14.2 |
| Plaque | 57 (30.8) |
| 32 (28.6) | 25 (34.2) |
Correlation between IMR, IMT and
cardiovascular risk factors
A significant positive correlation was determined
between IMR and FRS (r=0.438; P<0.001; Fig. 2A). Patients were then categorized
into three groups according to FRS tertiles (ranges of score: −7-5;
5–11; 11–20); the IMR and IMTMean increased
progressively and significantly with increasing tertiles of CHD
risk factor score (Fig. 2B).
A total of 40 subjects had no major CHD risk
factors, 43 subjects had only one CHD risk factor, 53 subjects had
two CHD risk factors and 49 subjects had three risk factors or
more. Subjects with risk factors had significantly higher values of
IMR and IMTMean than those without risk factors
(Fig. 2C, Table II).
 | Table II.Characteristics of subjects according
to number of risk factors for coronary heart disease. |
Table II.
Characteristics of subjects according
to number of risk factors for coronary heart disease.
|
| Number of risk
factors |
|---|
|
|
|
|---|
| Parameter | 0 | 1 | 2 | ≥3 |
|---|
| Number of
patients | 40 | 43 | 53 | 49 |
| Age (years) |
39.6±1.5 |
51.0±2.2a |
52.2±1.7a |
55.6±1.7a |
| Males | 17 (42.5) | 30
(71.4)d | 31
(58.5)d | 34
(69.4)d |
| SBP | 119.0±1.3 | 120.9±1.5 |
134.2±4.1a,b |
144.3±4.1a–c |
| DBP |
78.5±1.0 |
78.6±0.9 |
85.1±2.3a,b |
86.5±2.2a,b |
| BMI |
22.4±0.4 |
24.1±0.4 | 23.2±0.4 | 25.1±0.5 |
| FBG |
5.2±0.1 |
5.8±0.4 |
7.5±0.7a,b |
9.0±0.5a–c |
| TG |
1.1±0.1 |
1.4±0.2 |
1.9±0.2a |
2.4±0.2a–c |
| TC |
4.5±0.1 |
4.6±0.2 |
4.5±0.2 |
5.0±0.4 |
| HDL |
1.7±0.1 |
1.4±0.1 |
1.3±0.1 |
1.4±0.2 |
| LDL |
2.4±0.1 |
2.5±0.1 |
2.4±0.1 |
2.6±0.2 |
| Smoking (%) | 0 (0) | 12
(27.9)d | 18
(34.0)d | 22
(44.9)d |
| IMR |
32.9±2.7 |
64.5±6.9a |
89.1±7.4a,b |
92.0±6.7a,b |
|
IMTMean |
551.4±19.9 |
672.4±21.5a |
708.8±24.9a |
742.9±25.3a,b |
|
IMTMax |
637.3±30.4 |
810.9±40.0a |
875.4±46.3a |
927.0±43.9a,b |
|
IMTMin |
476.9±18.0 |
580.8±17.2a |
594.3±18.9a |
591.2±19.6a |
| Plaque (%) | 2 (5.0) | 7 (16.3) | 24
(45.3)d,e | 24
(49.0)d,e |
Pearson correlation analysis results are
demonstrated in Table III. The
IMR, similar to the IMTMean, was significantly
correlated with several risk factors for CHD. Multiple regression
analysis was performed with IMR and IMTMean as the
dependent variables and with variables that met statistical
significance in the univariate analysis of IMR and
IMTMean. The analysis indicated that Age, SBP, smoking
status and TC/HDL-C ratio were independently associated with IMR
and IMTMean.
 | Table III.Univariate and multivariate
associations between IMR, IMTMean and cardiometabolic
parameters. |
Table III.
Univariate and multivariate
associations between IMR, IMTMean and cardiometabolic
parameters.
|
| IMR |
IMTMean |
|---|
|
|
|
|
|---|
| Parameter | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
| Age | 0.361b | 0.292b | 0.447b | 0.376b |
| SBP | 0.353b | 0.266b | 0.328b | 0.225b |
| DBP | 0.255b |
| 0.224b |
|
| Smoking | 0.263b | 0.289b | 0.207b | 0.191b |
| FBG | 0.131 |
| 0.102 |
|
| BMI | 0.142 |
| 0.165a |
|
| TC/HDL-C ratio | 0.222b | 0.193b | 0.157b | 0.142b |
Association between IMR and carotid
atherosclerosis
The prevalence of carotid plaque in the whole study
population was 30.8% (Table I) and
increased progressively and significantly between risk 1 (1 risk
factor) and risk 2 (2 risk factors) for CHD (chi-square test
P<0.05; Table II). The subjects
were then stratified based on the presence of carotid plaque
(absence=0, presence=1). IMR was significantly higher in subjects
with carotid plaque than in subjects without carotid plaque
(103.6±7.1 vs. 57.9±3.6 µm; P<0.001; Fig. 2D).
In a further analysis, all subjects were divided
into three groups according to IMR tertiles. There was a
significant increase in the prevalence of carotid plaque across the
tertiles of IMR (4.9, 33.9 and 53.2% in tertiles 1, 2 and 3,
respectively; P<0.05; Table IV).
Compared with those subjects in the lowest tertile of IMR, those in
the intermediate and highest tertiles had a significantly elevated
OR regarding the presence of plaque in the carotid tree
(intermediate tertile, OR=9.90, 95%CI: 2.77–35.41, P<0.001;
highest tertile, OR=22.00, 95%CI: 6.22–77.81, P<0.001). Logistic
regression adjusted for age, sex, smoking status, obesity,
hypertension, diabetes mellitus and hyperlipidemia indicated that
there were still significantly increased ORs for those subjects in
the highest tertile compared with the lowest tertile (OR=10.61,
95%CI: 2.15–52.49, P=0.004; Table
IV).
 | Table IV.ORs for the influence of IMR in
different tertiles on carotid atherosclerosis in all subjects. |
Table IV.
ORs for the influence of IMR in
different tertiles on carotid atherosclerosis in all subjects.
| IMR | n | Plaque | Crude OR
(95%CI) | Crude P-value | Adjusted OR
(95%CI) | Adjusted
P-value |
|---|
| Tertile 1 | 61 | 3 (4.9) | 1.00 | – | 1.00 | – |
| Tertile 2 | 62 | 21
(33.9)a | 9.90
(2.77–35.41) | <0.001 | 3.32
(0.72–15.37) | 0.124 |
| Tertile 3 | 62 | 33
(53.2)a,b | 22.00
(6.22–77.81) | <0.001 | 10.61
(2.15–52.49) | 0.004 |
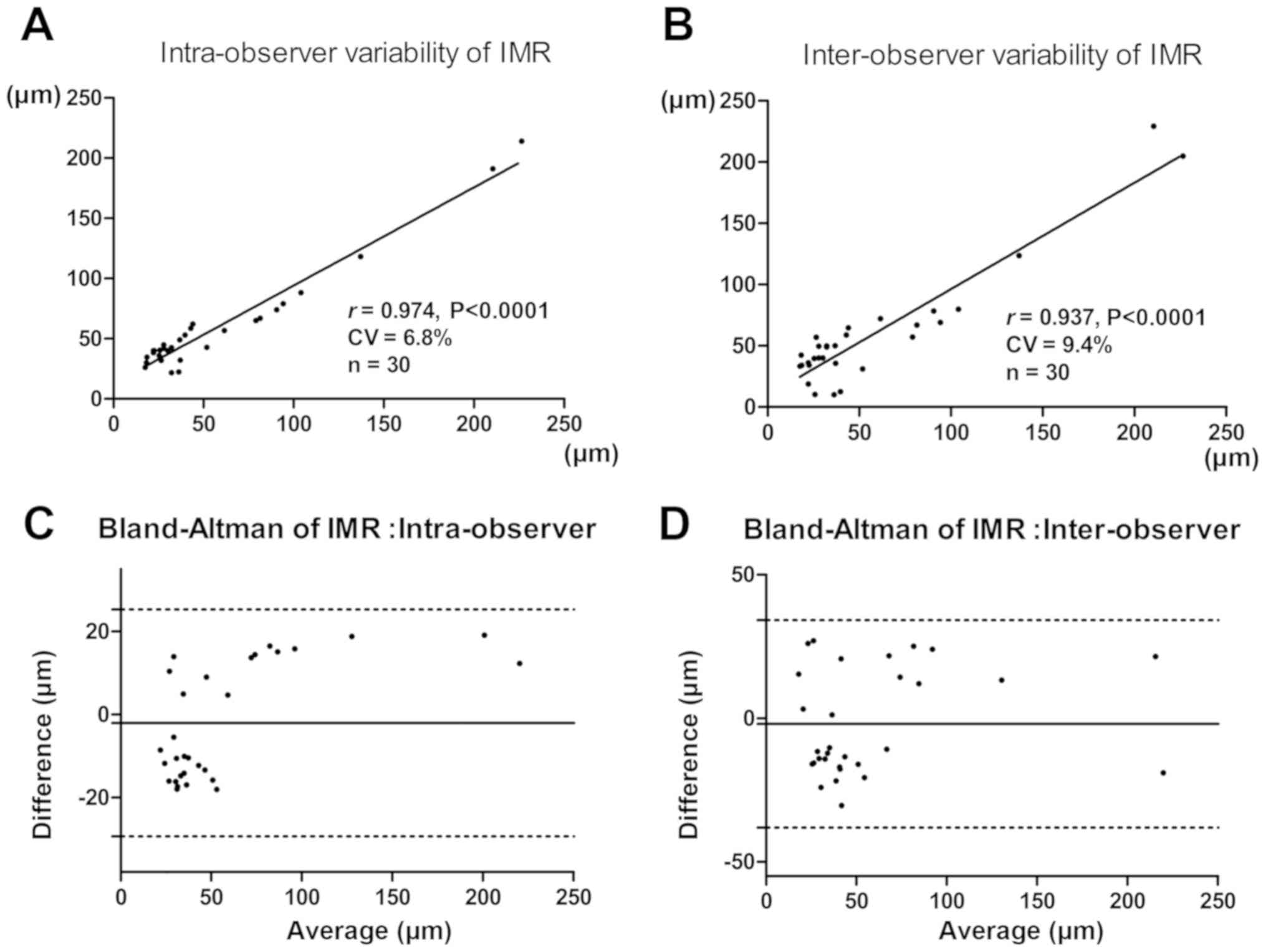
Coefficient of variation of repeated
measurement
The measurements of IMR demonstrated an
intra-observer variability with a coefficient variation of 6.8% and
interobserver variability with a coefficient variation of 9.4%. The
Bland-Altman plots suggested that differences between the two
measurements in inter-observer were similar throughout the range of
IMR, with the difference between the two measurements in
intra-observer also similar (Fig.
3).
Discussion
Atherosclerosis is the primary cause of vascular
disease (1). The ability of
non-invasive methods to detect atherosclerosis is a matter of
clinical interest. Carotid IMT is a surrogate indicator of
atherosclerosis for predicting cardiovascular and cerebrovascular
outcomes (21). US represents a
simple, non-invasive technique for the measurement of IMT, which is
widely used to study the presence and progression of
atherosclerosis (22–24). The increase in the IMTMax
and IMTMean is, however, only a part of the information
that reflects the atherosclerotic lesions in the artery (4–6). IMR,
describing the amount of variation of a set of IMTs, is expected to
be able to quantify the irregularities of the IM layer. Certain
studies have pointed out that besides IMT, a rough intimal surface
is also a typical feature of atherosclerosis (13). Due to the limitation of measurement
technology, research on IMR is rare. In the present study,
computer-assisted analysis software was used to automatically track
the IM layer, obtain the IMT value of each pixel in the region of
interest and calculate its standard deviation to reflect the IMR.
In a previous study by our group and in the present study, the
Bland-Altman test demonstrated that measurement of the IMR was
reproducible and was able to stably reflect the morphologic changes
of the carotid IM (25).
Various risk factors influence IMT (26). In the present study, the IMR was also
increased in association with several CHD risk factors, including
age, hyperlipidemia, hypertension and cigarette smoking, and was
significantly associated with FRS, which is a risk score and an
index of cumulative cardiovascular risk commonly used for assessing
the probability of heart attack or death from heart disease within
10 years (16). The results of Cheng
et al (27) are similar to
those of the present study where the presence of only one risk
factor was associated with a significant increase in IMR and IMT.
Furthermore, the IMR and IMT increased with the number of
cardiovascular risk factors. It is worth noting that when the two
risk factors are combined, only IMR is increased compared to the
low-risk group (1 risk factor), whilst IMT displays no significant
change. This indicates that the IMR is more sensitive to the
influence of risk factors than the IMT. The reason may be that IMR
is more sensitive to atherosclerosis than IMT. Atherosclerosis is
an inflammatory disease with the characteristics of inconsistent
lesion degree of vascular segments (15,28).
Local inflammation occurs during the formation of plaque (29), which leads to roughness of the IM
layer. Therefore, IMR reflects atherosclerotic changes in the
vascular wall. Although the IMT is highly associated with
atherosclerosis, increasing IMT may not always be due to
atherosclerosis. Homma et al (7) indicated that increasing IMT at
plaque-free sites does not indicate atherosclerotic changes and
reflects diffuse physiologic aging processes as diffuse intimal
thickening.
In the present study, IMR was significantly higher
in subjects with carotid plaque than in subjects without carotid
plaque. For cases with IMR >84.8 µm, the prevalence of plaque in
the carotid arteries is 11 times higher than in subjects with IMR
<33.8 µm (cut-off values selected from IMR tertiles), adjusted
for age, sex, smoking status, obesity, hypertension, diabetes
mellitus and hyperlipidemia. This result demonstrates that IMR may
be used as a novel indicator to evaluate the risk of
atherosclerosis, which is consistent with the results of other
studies. Graf et al (30)
revealed that the morphologic characteristics of the CCA (IMT
inhomogeneity) are positively correlated with the degree of carotid
bulb stenosis. Furthermore, as reported by Luijendijk et al
(4) and Ishizu et al
(31), the IMR of healthy subjects
and patients with manifest coronary artery disease are
significantly different. Belcaro et al (11) described these changes as granulations
of the IM layer and developed a morphologic classification system
of arterial wall changes. A 6-year follow-up was performed on 2,322
asymptomatic subjects, revealing that this structural alteration
was a major criterion for future cardiovascular events. Therefore,
it is necessary for subjects with IMR ≥84.8 µm to be followed up
closely, even if these subjects may not have combined carotid
plaque and risk factors.
IMR has a potential application scope in future
clinical practice: i) IMR may indicate atherosclerotic lesions
prior to IM thickening, which may be the earliest morphological
non-invasive index of atherosclerotic lesions that is detectable at
present. ii) IMR as an indicator of cardiovascular risk may help to
develop a clinical treatment strategy. iii) Due to the examination
being non-invasive, simple and easy to repeat, IMR is a suitable
parameter to effectively monitor the therapeutic effect of statins
in carotid atherosclerotic lesions. With more studies supporting
the diagnostic value of IMR, its clinical application prospects
will be broader.
The present study had several limitations. Due to
technical constraints, IMR and IMT were measured only at the
straight artery, while the curved vascular segments and the plaques
were avoided. Thus, the region of interest selected may not have
been that with the most severe atherosclerosis. Furthermore, IMR
and IMT require measurement with post-processing software following
storage of the US image. Furthermore, the quality of the US image
may affect the measurement with this analysis software. As another
limitation, intracranial arteries cannot be examined using IMR
scans. In addition, prospective studies are required to demonstrate
the predictive effect of IMR regarding the risk of cardiovascular
events.
In conclusion, IMR measurement of the CCA quantified
in US images based on this computer-assisted analysis software is
feasible. Carotid IMR, which reflects the morphological changes of
the IM layer, may help estimate the extent of atherosclerosis and
may be used for risk stratification of patients with cardiovascular
risk factors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81601540, 81500109,
81530056, 81671705 and 81401429), Huazhong University of Science
and Technology Interdisciplinary Innovation Team (grant no.
0118530300) and the Fundamental Research Funds for the Central
Universities, HUST (grant nos. 2015LC024 and 2015QN208).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and XYC designed the study. YW, QL, XL and LY
performed data acquisition, analysis, interpretation of the data
and drafting of the manuscript. YW and XL carried out statistical
analysis. YW, XL, LZ, LY, MXX and QL acquired funding. QL, MXX
supervised the study. YW and XYC analyzed data using various
software applications. YW, XL, QL, MXX, LZ, QL checked the
integrity of the data and accuracy of the data analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Union Hospital at Huazhong University of Science
and Technology (Wuhan, China), and the methods were applied in
accordance with the approved guidelines. Informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He J, Gu D, Wu X, Reynolds K, Duan X, Yao
C, Wang J, Chen CS, Chen J, Wildman RP, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Touboul PJ, Labreuche J, Bruckert E,
Schargrodsky H, Prati P, Tosetto A, Hernandez-Hernandez R, Woo KS,
Silva H, Vicaut E and Amarenco P: Hdl-c, triglycerides and carotid
IMT: A meta-analysis of 21,000 patients with automated edge
detection IMT measurement. Atherosclerosis. 232:65–71. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lethen H, Tries HP, Kersting S, Bramlage P
and Lambertz H: Improvement of coronary microvascular function
after angiotensin receptor blocker treatment with irbesartan in
patients with systemic hypertension. J Clin Hypertens (Greenwich).
13:155–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luijendijk P, Lu H, Heynneman FB, Huijgen
R, de Groot EE, Vriend JW, Vliegen HW, Groenink M, Bouma BJ and
Mulder BJ: Increased carotid intima-media thickness predicts
cardiovascular events in aortic coarctation. Int J Cardiol.
176:776–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt-Trucksass A, Sandrock M, Cheng DC,
Müller HM, Baumstark MW, Rauramaa R, Berg A and Huonker M:
Quantitative measurement of carotid intima-media roughness-effect
of age and manifest coronary artery disease. Atherosclerosis.
166:57–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalla Pozza R, Pirzer R, Beyerlein A,
Weberruß H, Oberhoffer R, Schmidt-Trucksäss A, Netz H and Haas N:
Beyond intima-media-thickness: Analysis of the carotid
intima-media-roughness in a paediatric population. Atherosclerosis.
251:164–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Homma S, Hirose N, Ishida H, Ishii T and
Araki G: Carotid plaque and intima-media thickness assessed by
b-mode ultrasonography in subjects ranging from young adults to
centenarians. Stroke. 32:830–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lind L: Circulating markers of
inflammation and atherosclerosis. Atherosclerosis. 169:203–214.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willerson JT: Systemic and local
inflammation in patients with unstable atherosclerotic plaques.
Prog Cardiovasc Dis. 44:469–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toutouzas K, Grassos H, Synetos A,
Drakopoulou M, Tsiamis E, Moldovan C, Agrogiannis G, Patsouris E,
Siores E and Stefanadis C: A new non-invasive method for detection
of local inflammation in atherosclerotic plaques: Experimental
application of microwave radiometry. Atherosclerosis. 215:82–89.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belcaro G, Nicolaides AN, Laurora G,
Cesarone MR, De Sanctis M, Incandela L and Barsotti A: Ultrasound
morphology classification of the arterial wall and cardiovascular
events in a 6-year follow-up study. Arterioscler Thromb Vasc Biol.
16:851–856. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mancia G, Fagard R, Narkiewicz K, Redón J,
Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G,
Dominiczak A, et al: 2013 ESH/ESC guidelines for the management of
arterial hypertension: The task force for the management of
arterial hypertension of the european society of hypertension (ESH)
and of the european society of cardiology (ESC). J Hypertens.
31:1281–1357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gnasso A, Irace C, Mattioli PL and Pujia
A: Carotid intima-media thickness and coronary heart disease risk
factors. Atherosclerosis. 119:7–15. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
American Diabetes Association: Standards
of medical care in diabetes-2011. Diabetes Care. 1 (Suppl
34):S11–S61. 2011. View Article : Google Scholar
|
|
15
|
Zhou B; Coorperative Meta-Analysis Group
Of China Obesity Task Force, : Predictive values of body mass index
and waist circumference to risk factors of related diseases in
Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi.
23:5–10. 2002.(In Chinese). PubMed/NCBI
|
|
16
|
Expert Panel on Detection and Evaluation
and Treatment of High Blood Cholesterol in Adults, . Executive
summary of the third report of the national cholesterol education
program (NCEP) expert panel on detection, evaluation, and treatment
of high blood cholesterol in adults (Adult Treatment Panel III).
JAMA. 285:2486–2497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Touboul PJ, Hennerici M, Meairs S, Adams
H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S,
Hernandez Hernandez R, et al: Mannheim carotid intima-media
thickness and plaque consensus (2004-2006-2011). An update on
behalf of the advisory board of the 3rd, 4th and 5th watching the
risk symposia, at the 13th, 15th and 20th European stroke
conferences, mannheim, Germany, 2004, Brussels, Belgium, 2006, and
hamburg, Germany, 2011. Cerebrovasc Dis. 34:290–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Zhou Y, Cheng X, Song E and Li G:
Ultrasound intima-media segmentation using hough transform and dual
snake model. Comput Med Imaging Graph. 36:248–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wendelhag I, Gustavsson T, Suurküla M,
Berglund G and Wikstrand J: Ultrasound measurement of wall
thickness in the carotid artery: Fundamental principles and
description of a computerized analysing system. Clin Physiol.
11:565–577. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh J, Wunsch R, Turzer M, Bahner M, Raggi
P, Querfeld U, Mehls O and Schaefer F: Advanced coronary and
carotid arteriopathy in young adults with childhood-onset chronic
renal failure. Circulation. 106:100–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polak JF, Pencina MJ, Pencina KM,
O'Donnell CJ, Wolf PA and D'Agostino RB Sr: Carotid-wall
intima-media thickness and cardiovascular events. N Engl J Med.
365:213–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gardin JM, Bartz TM, Polak JF, O'Leary DH
and Wong ND: What do carotid intima-media thickness and plaque add
to the prediction of stroke and cardiovascular disease risk in
older adults? The cardiovascular health study. J Am Soc
Echocardiogr. 27:998–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polak JF, Szklo M and O'Leary DH:
Associations of coronary heart disease with common carotid artery
near and far wall intima-media thickness: The Multi-Ethnic Study of
Atherosclerosis. J Am Soc Echocardiogr. 28:1114–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gigante B, Leander K, Vikström M,
Baldassarre D, Veglia F, Strawbridge RJ, McLeod O, Gertow K,
Sennblad B, Shah S, et al: Low levels of IgM antibodies against
phosphorylcholine are associated with fast carotid intima media
thickness progression and cardiovascular risk in men.
Atherosclerosis. 236:394–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Xie M, Zhang L, Lu X, Cheng X and Lv
Q: Carotid intima-media roughness and elasticity in hypertensive
patients with normal carotid intima-media thickness. J Ultrasound
Med. 2018:(Epub ahead of print).
|
|
26
|
Raitakari OT, Juonala M, Kähönen M,
Taittonen L, Laitinen T, Mäki-Torkko N, Järvisalo MJ, Uhari M,
Jokinen E, Rönnemaa T, et al: Cardiovascular risk factors in
childhood and carotid artery intima-media thickness in adulthood:
The Cardiovascular Risk in Young Finns Study. JAMA. 290:2277–2283.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng X, Zhou Y, Jin Y, Li G, Wang H and
Song E: Intima-medial thickness homogeneity in the common carotid
artery: Measurement method and preliminary clinical study. J Clin
Ultrasound. 40:559–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otsuka F, Kramer MC, Woudstra P, Yahagi K,
Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR,
et al: Natural progression of atherosclerosis from pathologic
intimal thickening to late fibroatheroma in human coronary
arteries: A pathology study. Atherosclerosis. 241:772–782. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bentzon JF, Otsuka F, Virmani R and Falk
E: Mechanisms of plaque formation and rupture. Circ Res.
114:1852–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graf IM, Schreuder FH, Hameleers JM, Mess
WH, Reneman RS and Hoeks AP: Wall irregularity rather than
intima-media thickness is associated with nearby atherosclerosis.
Ultrasound Med Biol. 35:955–961. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishizu T, Ishimitsu T, Kamiya H, Seo Y,
Moriyama N, Obara K, Watanabe S and Yamaguchi I: The correlation of
irregularities in carotid arterial intima-media thickness with
coronary artery disease. Heart Vessels. 17:1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|