Introduction
Caesarean section is an important obstetric surgery
to resolve fetal output of pregnant females who are unable to
deliver due to dystocia or certain obstetric complications
(1). In developing countries, the
proportion of caesarean section in maternal females is exhibiting
annual increases, particularly in China (2). The current caesarean section rate in
obstetrics and gynecology departments of most hospitals in China
has far exceeded the tolerable limit set by the World Health
Organization (3,4). In 2004, 29.1% of neonates were
delivered by caesarean section in the United States, which is the
highest rate ever reported. The overall rate has increased by
>40% since 1996, reflecting two concurrent trends: An increase
in the primary rate (14.6 to 20.6%) and a steep decline in the rate
of vaginal birth after caesarean section (28.3 to 9.2%) (5). Due to the increase in caesarean section
and its demand, anesthesia for caesarean section is of utmost
importance. Anesthesiologists are required to select appropriate
anesthetic drugs, methods and timing to shorten the operation time
and reduce the degree of surgical injury to the maternal patient
(6,7). Commonly used methods for caesarean
section anesthesia mainly include spinal anesthesia, epidural
anesthesia, and spinal and epidural combined anesthesia. Among
them, lumbar anesthesia is a common method of caesarean section
anesthesia (8,9). Lumbar anesthesia has the advantages of
simple operation, rapid onset of anesthesia, good abirritation,
good muscle relaxation and easy control of the anesthesia level.
However, the limitation is short anesthesia time that is not
possible to prolong (10–12).
To achieve optimal anesthesia for caesarean section,
the selection and combination of anesthetic drugs are crucial
factors. Dexmedetomidine belongs to the α2 adrenergic receptor
agonists and is relatively selective (13,14). The
pharmacological effects of dexmedetomidine include anxiolytic,
sedative, analgesic, hypnotic and sympathetic blockade. The US Food
and Drug Administration approved the use of dexmedetomidine in
mechanical ventilation patients in the adult intensive care unit,
as well as in pediatrics, neurosurgery and fiberoptic bronchoscopy
for cardiovascular surgery (15,16). The
application of dexmedetomidine may affect the immune system of the
maternal patient and neonate (17).
A previous study indicated that dexmedetomidine exerts
anti-inflammatory effects through regulation of the type 2 T-helper
cell (Th2)-associated cytokines interleukin (IL)-4 and IL-6
(18). However, whether
dexmedetomidine administered for caesarean section affects the
immune system of maternal patients and neonates has remained
largely elusive. Therefore, the present study aimed to investigate
the effect of dexmedetomidine combined with lumbar anesthesia on
Th1/Th2 cytokines in maternal patients undergoing caesarean section
and their neonates.
Patients and methods
General patient information
A total of 60 full-term maternity patients admitted
to the Department of Obstetrics and Gynecology of Liaocheng
People's Hospital (Liaocheng, China) who were diagnosed as
singleton pregnancies and underwent caesarean section between
January 2017 and September 2017 were selected. The fetus was in the
head position and complete pre-operative preparations were made to
record the relevant clinical data. The patients were all primipara
with a mean age of 26.3±3.5 years (range, 22–37 years), a mean body
weight of 74.7±6.8 kg (range, 57–83) and a mean body height of
162.1±3.4 cm (range, 152–175 cm). The subjects received routine
prenatal-associated examinations prior to the surgery, including
B-ultrasound and electrocardiogram. The inclusion criterion was an
American Society of Anesthesiologists (ASA) grade of I or II
(19). The exclusion criteria were
maternal eclampsia, an ASA grade of III or IV, preterm infants,
multiple pregnancies, contraindications to spinal anesthesia,
diabetes mellitus, infections, pregnancy complications (e.g.
pregnancy-induced hypertension syndrome), heart disease, history of
local anesthetics and opioid allergies, spinal trauma history,
blood system problems including coagulation dysfunction, opioid
application within one week prior to surgery and pregnancy duration
of >38 weeks. The present study was approved by the Ethics
Committee of Liaocheng People's Hospital (Liaocheng, China). All
subjects provided written informed consent.
Major reagents and instruments
TRIzol reagent, RNA extraction kit, PCR primers,
High Capacity cDNA Reverse Transcription kit (cat. no. 4368814) and
QuantiTect SYBR Green RT-PCR kit were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). IL-2, IL-4, TNF-α and IL-10 ELISA
kits were purchased from eBioscience. A Labsystem 1.3.1 microplate
reader was obtained from Bio-Rad Laboratories.
Grouping
The subjects were equally and randomly divided into
a control group treated by lumbar epidural anesthesia and a
combination group treated by dexmedetomidine combined with lumbar
epidural anesthesia. Dexmedetomidine was continuously pumped into
the L3-L4 space for lumbar epidural anesthesia using a micropump at
a dose of 0.8 mg/kg.
Visual analog scale (VAS) score,
adverse reactions, traction response and neonates' Apgar score
The traction response was evaluated using the
standard for assessing the effect of traction response, as follows:
1, No perineal or stomach discomfort, no vomiting, nausea or
meteorism; 2, mild genital or stomach discomfort, no nausea or
vomiting; 3, perineal pain, stomach discomfort, obvious meteorism,
or even nausea and vomiting that requires treatment with drugs. The
VAS score (0, no pain; 10, the greatest pain imaginable) was
evaluated 1 h after surgery and used to assess the pain. After the
neonatal outcomes, the Apgar score was assessed by pediatricians at
the 1st and 5th minute. Adverse reactions, including nausea,
itching, vomiting and respiratory depression, were recorded
intra-operatively and at 1, 2, 6 and 12 h post-surgery.
Blood sample collection and
storage
Blood samples were collected from each group on
post-operative day 1. A total of 2 ml blood was collected from the
portal vein of the neonates. The sample was centrifuged at 2,000 ×
g for 15 min at 4°C. The serum was placed in an Eppendorf tube and
stored at −20°C.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from peripheral blood
mononuclear cells using TRIzol and reverse transcribed to
complementary DNA. The primers were designed by Primer Premier 6.0
(Table I) and synthesized by Sangon
Biotech. The PCR thermocycling conditions were as follows: 52°C for
1 min, and 35 cycles of 92°C for 30 sec, 58°C for 50 sec and 72°C
for 35 sec. The 2−∆∆Cq method (20) was applied to calculate the relative
expression level.
 | Table I.Sequences of primers used for PCR. |
Table I.
Sequences of primers used for PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| GADPH |
AGTGCCAGCCTCGTCTCATAG |
CGTTGAACTTGCCGTGGGTAG |
| IL-2 |
CAGAGATCTAAGCAGCGACTT |
TGGGACCTCATCTCCGTCA |
| TNF-α |
GATCTCCTAAACGGAATAGCG |
GACTCTGGCTCAATCCGTC |
| IL-4 |
AACGGGCCTAAGGATCTCAAT | TGGCTGCACATCGTCA |
| IL-10 |
TCTCAAGAGCGTCAAGATA |
AATCTCTCCGTCAATCCT |
ELISA
The serum levels of IL-2, IL-4, IL-10 and TNF-α in
each group were detected by ELISA. The collected peripheral blood
was centrifuged and the supernatant was obtained. The experimental
procedure was performed according to the ELISA kit instructions.
The 50 µl diluted standard substance and samples were added to a
96-well plate and incubated at 37°C for 30 min. After washing for 5
times, 50 µl reagent A and 50 µl reagent B was added to each well,
followed by incubation at 37°C for 10 min. Finally, 50 µl stop
solution was added to each well and the absorbance was read on a
microplate reader. A standard curve was prepared to determine the
sample concentration based on the optical density value.
Statistical analysis
All data analyses were performed using SPSS 22.0
software (IBM Corp.). Measurement data were expressed as the mean ±
standard deviation and compared by using the unpaired Student's
t-test. Correlations were assessed by Pearson's correlation
analysis. The test level was set at α=0.05. P<0.05 was
considered to indicate a statistically significant difference.
Results
Dexmedetomidine decreases the VAS
score of maternal females after caesarean section under lumbar
anesthesia
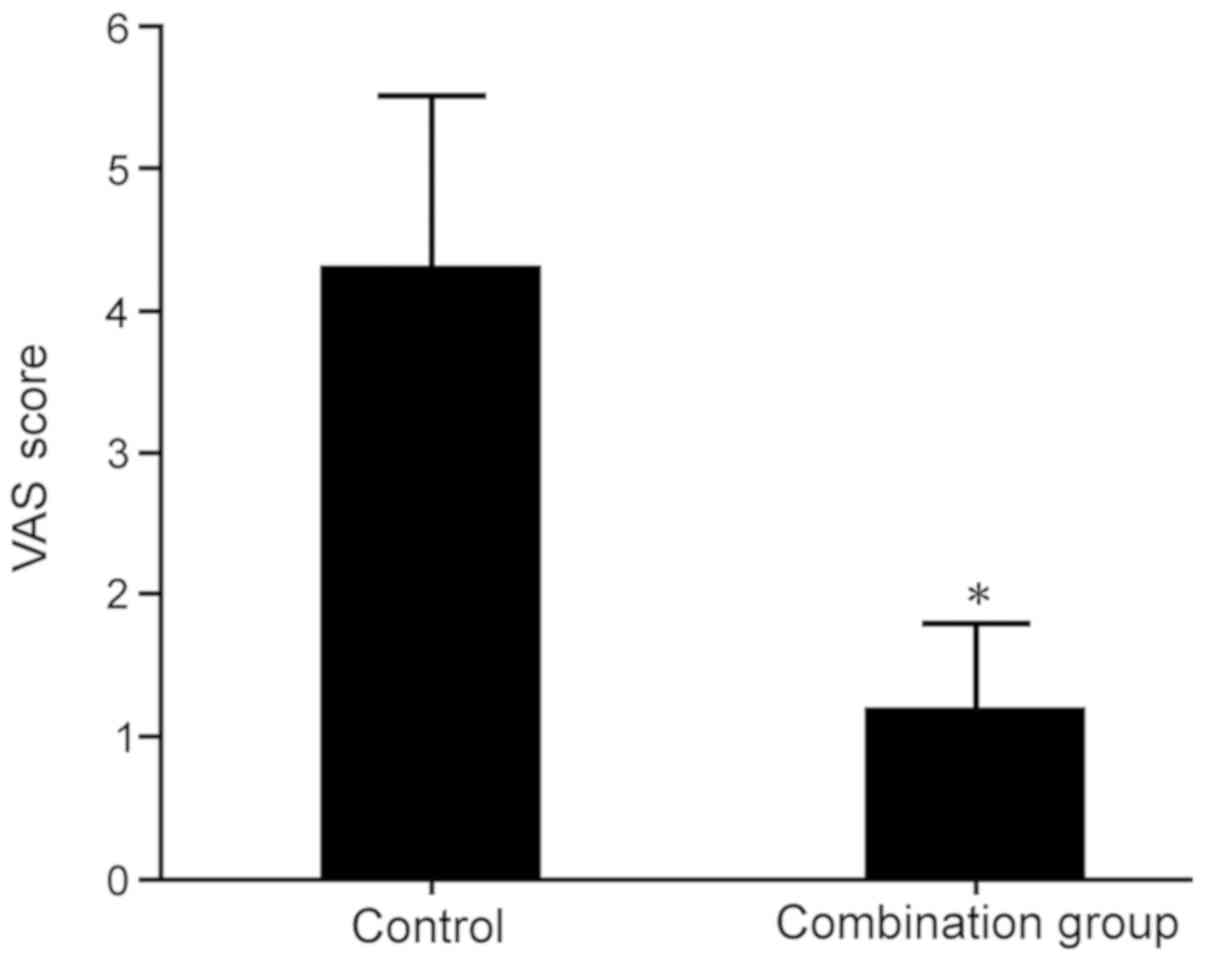
The pain in each group was assessed using the VAS
scoring method. The results indicated that at 1 h after surgery,
the VAS score in the combination group was significantly lower than
that in the lumbar anesthesia control group (P<0.05; Fig. 1).
Dexmedetomidine reduces adverse
reactions in females with caesarean section under lumbar
anesthesia
Adverse reactions in the control group receiving
lumbar anesthesia and in the combination group receiving
dexmedetomidine combined with lumbar anesthesia were observed. It
was indicated that dexmedetomidine in addition to lumbar anesthesia
obviously reduced the adverse reactions, such as nausea, emesis,
shiver and cutaneous pruritus, during caesarean section surgery
compared with those in the lumbar anesthesia control group
(P<0.05; Table II).
 | Table II.Influence of dexmedetomidine combined
with lumbar anesthesia on adverse reactions in females receiving
caesarean section. |
Table II.
Influence of dexmedetomidine combined
with lumbar anesthesia on adverse reactions in females receiving
caesarean section.
| Group | Bradycardia | Itching | Hypotension | Nausea/vomiting | Total |
|---|
| Control (n=30) | 2 (6.6) | 5 (16.6) | 4 (13.3) | 6 (20.0) | 17 (56.6) |
| Combination
(n=30) | 1 (3.3) | 1 (3.3)a | 2 (6.6)a | 1 (3.3)a | 5 (16.6)a |
Dexmedetomidine reduces the traction
response of females with caesarean section under lumbar
anesthesia
The traction response of females with caesarean
section was compared between the two groups. The results
demonstrated that dexmedetomidine combined with lumbar anesthesia
reduced the traction response compared with that in the lumbar
anesthesia control group (P<0.05; Table III).
 | Table III.Impact of dexmedetomidine combined
with lumbar anesthesia on traction response in females with
caesarean section. |
Table III.
Impact of dexmedetomidine combined
with lumbar anesthesia on traction response in females with
caesarean section.
|
| Traction response
score |
|---|
|
|
|
|---|
| Group | 1 | 2 | 3 |
|---|
| Control (n=30) | 15 (50) | 11 (16.7) | 4 (13.3) |
| Combination
(n=30) | 27
(90)a | 2
(6.7)a | 1
(3.3)a |
Effect of dexmedetomidine applied
during caesarean section combined with lumbar anesthesia on the
Apgar score of neonates
No statistically significant difference on the Apgar
score of neonates was identified between the two groups (P>0.05;
Fig. 2).
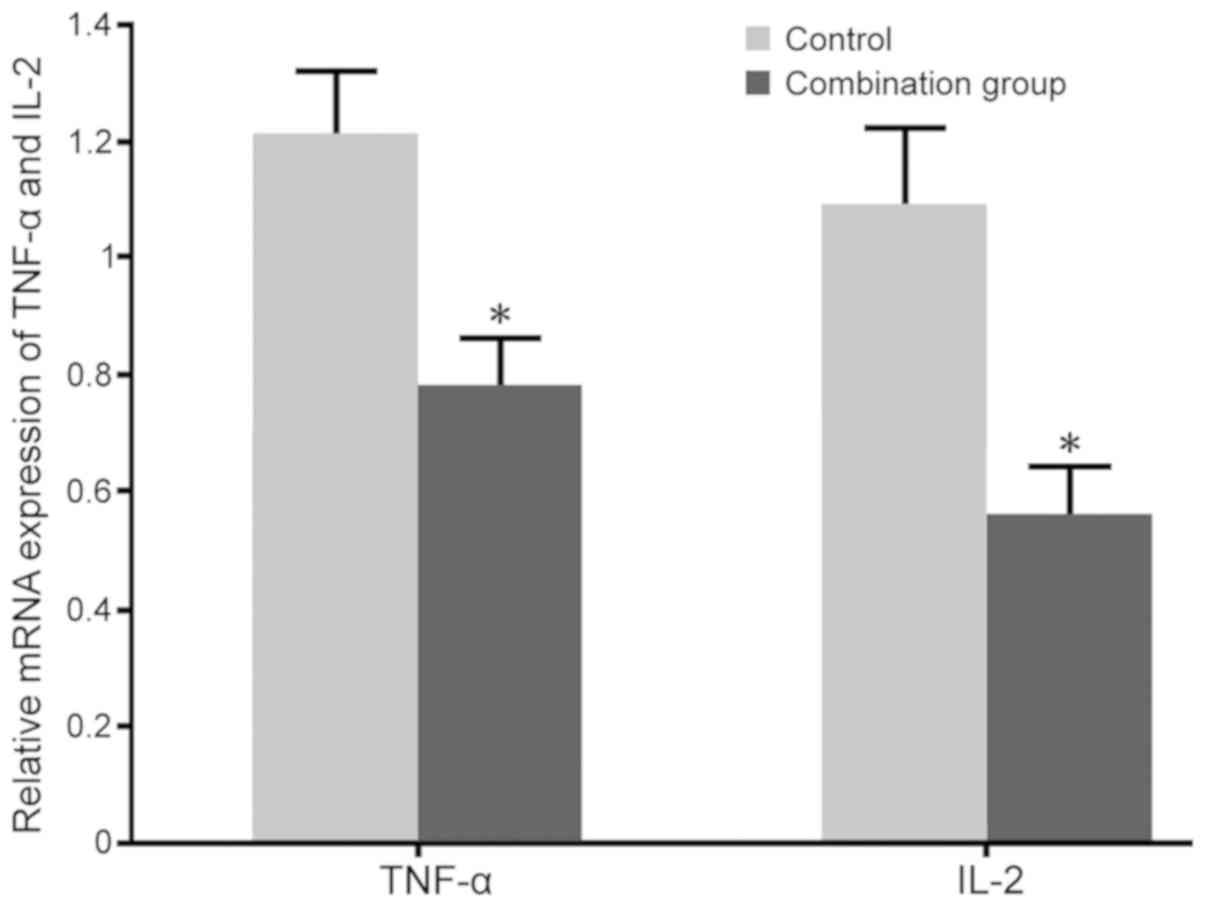
Impact of dexmedetomidine combined
with lumbar anesthesia during caesarean section on Th1 cytokines in
maternal females
No significant differences in the levels of TNF-α
(96.3±6.5 pg/ml in the control and 95.9±5.3 pg/ml in the
combination group) and IL-2 (81.6±4.7 pg/ml in the control and
83.5±4.3 pg/ml in the combination group) were observed between the
two groups prior to surgery. RT-qPCR and ELISA were adopted to test
the impact of dexmedetomidine combined with lumbar anesthesia on
TNF-α and IL-2 mRNA and protein levels in the blood of maternal
females. The results indicated that dexmedetomidine combined with
lumbar anesthesia in females with caesarean section markedly
decreased the TNF-α and IL-2 expression and secretion compared with
those in the control group (P<0.05; Figs. 3 and 4).
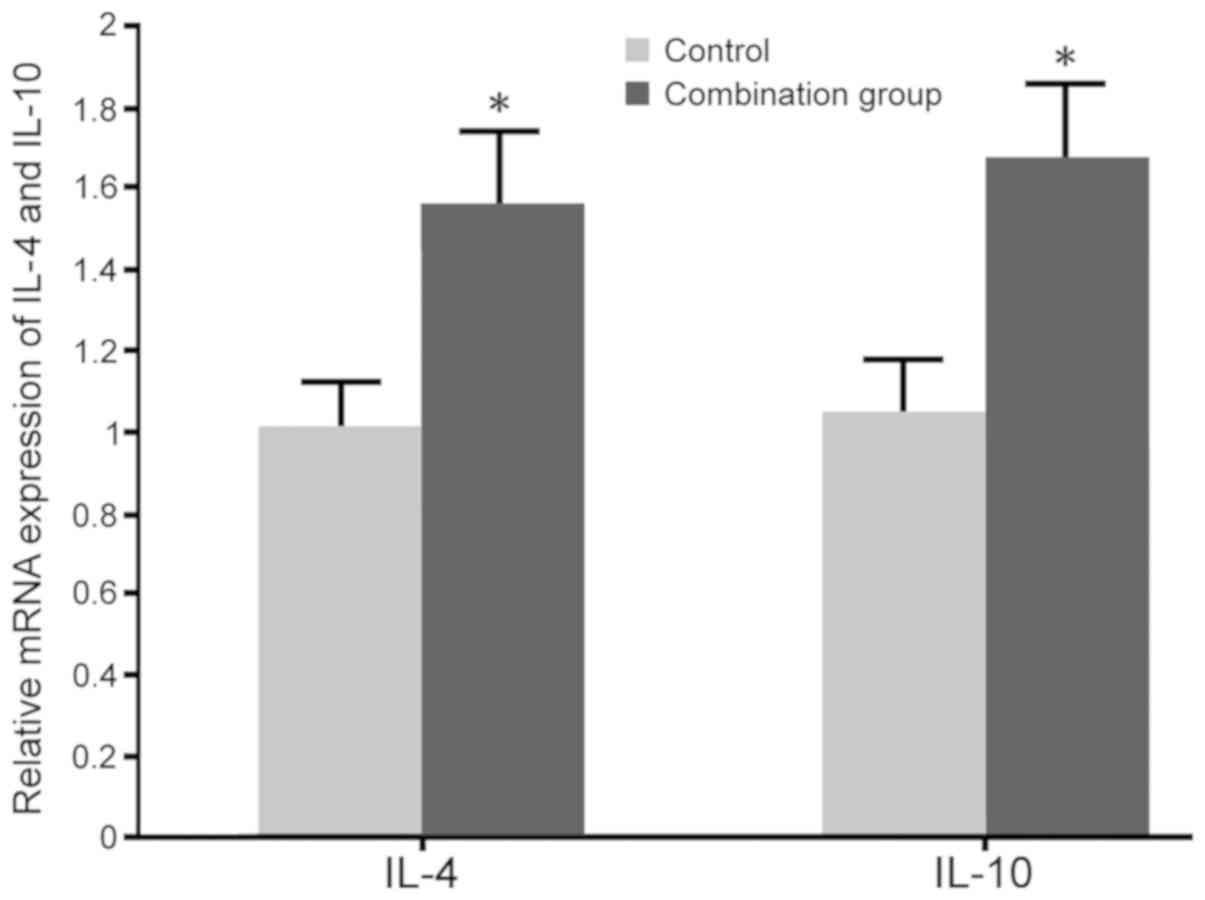
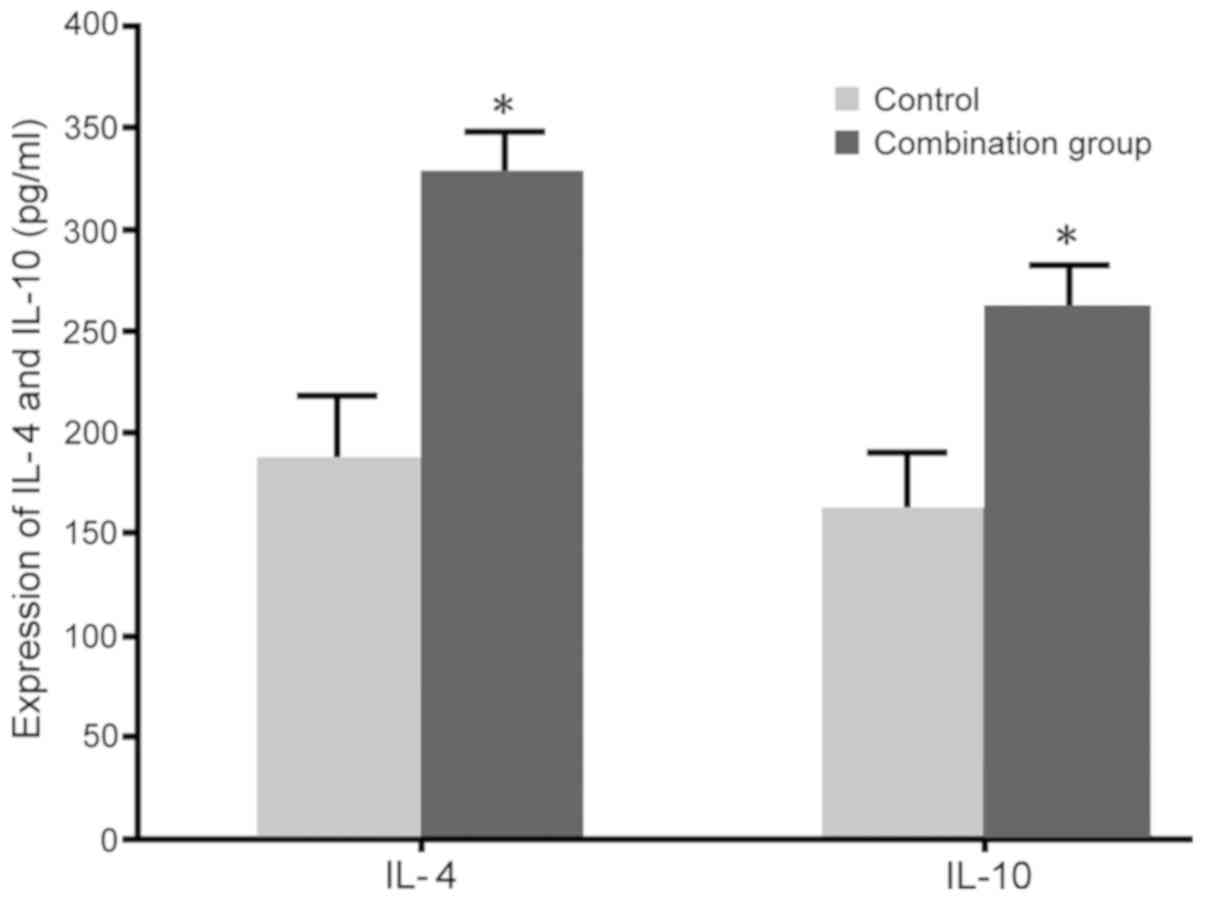
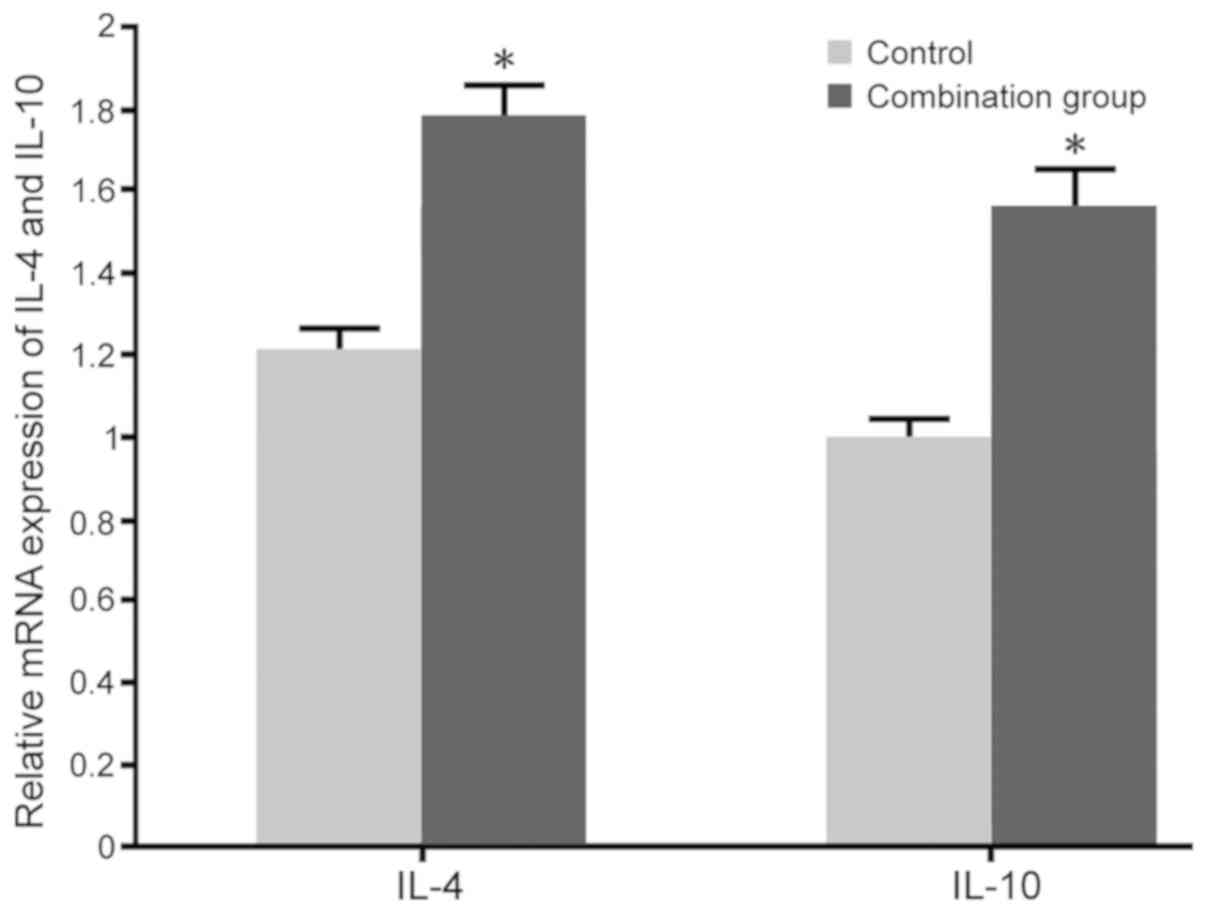
Impact of dexmedetomidine combined
with lumbar anesthesia on Th2 cytokines in maternal females
No significant differences in the levels of IL-4
(60.3±3.2 pg/ml in the control and 64.1±3.9 pg/ml in the
combination group) and IL-10 (139.2±6.1 pg/ml in the control and
145.3±8.1 pg/ml in the combination group) were observed between the
two groups prior to surgery. RT-qPCR and ELISA were adopted to
determine the impact of dexmedetomidine combined with lumbar
anesthesia on IL-4 and IL-10 levels in maternal blood. The results
indicated that dexmedetomidine combined with lumbar anesthesia in
females with caesarean section promoted the IL-4 and IL-10 mRNA
expression and protein secretion compared with those in the control
group (P<0.05; Figs. 5 and
6).
Effect of dexmedetomidine combined
with lumbar anesthesia on Th1 cytokines in neonates
RT-qPCR and ELISA were adopted to test the impact of
dexmedetomidine combined with lumbar anesthesia on TNF-α and IL-2
mRNA and protein levels in the blood of neonates. The results
indicated that dexmedetomidine combined with lumbar anesthesia
caused a significant decline in the TNF-α and IL-2 mRNA expression
and protein secretion in neonates compared with those in the
control group (P<0.05; Figs. 7
and 8).
Impact of dexmedetomidine combined
with lumbar anesthesia on Th2 cytokines in neonates
RT-qPCR and ELISA were adopted to determine the
impact of dexmedetomidine combined with lumbar anesthesia on IL-4
and IL-10 mRNA and protein levels in the blood of neonates. The
results indicated that dexmedetomidine combined with lumbar
anesthesia promoted the IL-4 and IL-10 mRNA expression and
secretion in neonates compared with that in the control group
(P<0.05; Figs. 9 and 10).
Discussion
Dexmedetomidine is used in the clinic for analgesia,
sedation and reduction of anesthetic agents. Reasonable application
of anesthetics may reduce the occurrence of post-operative
complications and rapidly exert anesthetic effects to achieve
timely recovery and promote the improvement and recovery of spinal
cord injury (21). Although
dexmedetomidine is not recommended for pregnant females, a previous
study indicated that appropriate doses of dexmedetomidine are
beneficial for sedation of pregnant subjects (22). In addition, dexmedetomidine was
reported to regulate immune suppression, which is caused by
surgery-associated stress (23). In
the present study, the effects of dexmedetomidine combined with
lumbar anesthesia were compared with those of lumbar anesthesia
alone on maternal females receiving caesarean section and their
neonates. The results indicated that dexmedetomidine combined with
lumbar anesthesia reduced the VAS scores, adverse reactions and
traction responses without affecting neonatal Apgar scores. These
results suggest that dexmedetomidine combined with lumbar
anesthesia enhances the anesthetic effect and promotes recovery of
maternal patients after caesarean section.
Post-operative anesthesia-associated complications
are one of the problems that affect the surgical process and
post-operative recovery of caesarean section patients, mainly
including pruritus, post-operative pain, respiratory depression,
chills and hypotension (24,25). However, in the present study, the use
of dexmedetomidine combined with lumbar anesthesia was indicated to
reduce adverse reactions and traction response. The increased
secretion of the Th1 inflammatory factors IL-2 and TNF-α activates
the inflammatory response and facilitates leukocyte adhesion,
providing conditions for the further development of inflammation
(26,27). The Th2 cytokines IL-4 and IL-10
antagonize Th1 inflammatory cytokines, and suppress the Th1
response and subsequent release of associated factors to enhance
the humoral immune response (28).
Wegmann et al (29) first
postulated the concept that a shift from a Th1 response to a Th2
bias occurs during pregnancy, which functionally induces maternal
immune tolerance and suppression. Several clinical studies have
demonstrated a Th2 bias in the circulating Th cytokine profile in
normal pregnancies, and an increase in the Th1 ratio in cases of
recurrent miscarriage (30) and in
preeclampsia (31). The present
study demonstrated that dexmedetomidine combined with lumbar
anesthesia resulted in decreased expression of IL-2 and TNF-α, and
increased expression of IL-4 and IL-10 in maternal females after
caesarean section and their neonates compared with those who had
received lumbar anesthesia alone. Although caesarean section may
influence the level of IL-2 and IL-4, no significant differences
compared with pregnant women with normal delivery have been
reported (32). In the present
study, all participants received caesarean section; therefore, the
changes of IL-2 and IL-4 are due to the different anesthetic
treatments. The changes in Th1/Th2 cytokines observed in the
present study indicate that dexmedetomidine combined with lumbar
anesthesia modulates the Th1/Th2 balance and inhibits Th1 cell
differentiation, consistent with a previous study demonstrating
that dexmedetomidine inhibited inflammation through upregulation of
the Th2-associated cytokines IL-4 and IL-6 (18). However, the exact mechanisms of how
dexmedetomidine modulates the Th1/Th2 balance in maternal females
receiving caesarean section and their neonates require further
elucidation.
In conclusion, dexmedetomidine in addition to lumbar
epidural anesthesia reduces the maternal VAS score, adverse
reactions and traction response, as well as promotes the conversion
of Th1 cytokines to Th2 cytokines in maternal females receiving
caesarean section and their neonates.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
Not applicable.
Authors' contributions
WS and PZ performed the experiments and analyzed the
data. WS designed the study and wrote the manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Liaocheng People's Hospital (Liaocheng, China). All
subjects provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poli-Neto OB, Campos Martins Chamochumbi
C, Toscano P, Pitanguy Julio M, Marques W Jr, Rosa-E-Silva JC,
Candido-Dos-Reis FJ and Nogueira AA: Electromyographic
characterization of abdominal wall trigger points developed after
caesarean section and response to local anaesthesia: An
observational study. BJOG. 125:1313–1318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng XL, Xu L, Guo Y and Ronsmans C:
Factors influencing rising caesarean section rates in China between
1988 and 2008. Bull World Health Organ. 90:30–39, 39A. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singal S, Bharti R, Dewan R, Divya, Dabral
A, Batra A, Sharma M and Mittal P: Clinical outcome of
postplacental copper T 380A insertion in women delivering by
caesarean section. J Clin Diagn Res. 8:OC01–OC04. 2014.PubMed/NCBI
|
|
4
|
Ozkan Seyhan T, Orhan-Sungur M, Basaran B,
Savran Karadeniz M, Demircan F, Xu Z and Sessler DI: The effect of
intra-abdominal pressure on sensory block level of single-shot
spinal anesthesia for cesarean section: An observational study. Int
J Obstet Anesth. 24:35–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menacker F, Declercq E and Macdorman MF:
Cesarean delivery: Background, trends and epidemiology. Semin
Perinatol. 30:235–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Agwany AS: Considerable observations in
cesarean section surgical technique and proposed steps. Arch
Gynecol Obstet. 297:1075–1077. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Sun W, Wang C, Liu F and Zhou M:
Delivery during extracorporeal membrane oxygenation (ECMO) support
of pregnant woman with severe respiratory distress syndrome caused
by influenza: A case report and review of the literature. J Matern
Fetal Neonatal Med. 32:2570–2574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi YC, Guo H, Chen J, Sun G, Ren RR, Guo
MZ, Peng LH and Yang YS: Initial meconium microbiome in Chinese
neonates delivered naturally or by cesarean section. Sci Rep.
8:32552018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carness JM and Lenart MJ: Spinal
anaesthesia for cesarean section in a patient with vascular type
ehlers-danlos syndrome. Case Rep Anesthesiol.
2018:19247252018.PubMed/NCBI
|
|
10
|
Yamashita A and Irikoma S: Comparison of
inflationary non-invasive blood pressure (iNIBP) monitoring
technology and conventional deflationary non-invasive blood
pressure (dNIBP) measurement in detecting hypotension during
cesarean section. JA Clin Rep. 4:52018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishio Y, Hiraki T, Taniguchi H and
Ushijima K: Anesthetic management during a cesarean section in a
patient with cleidocranial dysplasia: A case report. JA Clin Rep.
4:22018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bard M, Bersot Y, Legros V, Raimond E and
Malinovsky JM: Hemodynamic monitoring by the aortic velocity-time
integral in supra sternal Doppler echocardiography and total
cavo-pulmonary derivation in cesarean delivery. J Clin Anesth.
46:99–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eskandr AM, Metwally AA, Ahmed AA, Elfeky
EM, Eldesoky IM, Obada MA and Abd-Elmegid OA: Dexmedetomidine as a
part of general anaesthesia for caesarean delivery in patients with
pre-eclampsia: A randomised double-blinded trial. Eur J
Anaesthesiol. 35:372–378. 2018.PubMed/NCBI
|
|
14
|
Fan L, Zhang J, Lv Z, Guo H and Zhao Y:
Clinical research on the dexmedetomidine applied for
patient-controlled sedation during the lower limbs operation under
combined spinal-epidural anesthesia. Pak J Pharm Sci. 29:1095–2100.
2016.PubMed/NCBI
|
|
15
|
Sayed E and Yassen KA: Intraoperative
effect of dexmedetomidine infusion during living donor liver
transplantation: A randomized control trial. Saudi J Anaesth.
10:288–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Das A, Chhaule S, Bhattacharya S, Basunia
SR, Mitra T, Halder PS, Chattopadhyay S and Mandal SK: Controlled
hypotension in day care functional endoscopic sinus surgery: A
comparison between esmolol and dexmedetomidine: A prospective,
double-blind and randomized study. Saudi J Anaesth. 10:276–282.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conti G, Ranieri VM, Costa R, Garratt C,
Wighton A, Spinazzola G, Urbino R, Mascia L, Ferrone G, Pohjanjousi
P, et al: Effects of dexmedetomidine and propofol on
patient-ventilator interaction in difficult-to-wean, mechanically
ventilated patients: A prospective, open-label, randomised,
multicentre study. Crit Care. 20:2062016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A
and Gao C: Anti-inflammatory effects of perioperative
dexmedetomidine administered as an adjunct to general anesthesia: A
Meta-analysis. Sci Rep. 5:123422015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doyle DJ and Garmon EH: American Society
of Anesthesiologists Classification (ASA Class). StatPearls
[Internet] Treasure Island (FL): StatPearls Publishing; 2019 Jan
19
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan F, Fu H, Yang P, Sun K, Wu S, Lv M,
Dong Z and Dong T: Dexmedetomidine-fentanyl versus
propofol-fentanyl in flexible bronchoscopy: A randomized study. Exp
Ther Med. 12:506–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nair AS and Sriprakash K: Dexmedetomidine
in pregnancy: Review of literature and possible use. J Obstetric
Anaesthesia Critical Care. 3:3–6. 2013. View Article : Google Scholar
|
|
23
|
Wang L, Zhang A, Liu W, Liu H, Su F and Qi
L: Effects of dexmedetomidine on perioperative stress response,
inflammation and immune function in patients with different degrees
of liver cirrhosis. Exp Ther Med. 16:3869–3874. 2018.PubMed/NCBI
|
|
24
|
Bawdane KD, Magar JS and Tendolkar BA:
Double blind comparison of combination of 0.1% ropivacaine and
fentanyl to combination of 0.1% bupivacaine and fentanyl for
extradural analgesia in labour. J Anaesthesiol Clin Pharmacol.
32:38–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kundra TS, Nagaraja PS, Singh NG,
Dhananjaya M, Sathish N and Manjunatha N: Effect of dexmedetomidine
on diseased coronary vessel diameter and myocardial protection in
percutaneous coronary interventional patients. Ann Card Anaesth.
19:394–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong SI, Shin JA, Cho S, Kim HW, Lee JY,
Kang JL and Park EM: Resveratrol attenuates peripheral and brain
inflammation and reduces ischemic brain injury in aged female mice.
Neurobiol Aging. 44:74–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmoutaz Mahmoud H and Rashwan DAE:
Efficacy of dexmedetomidine versus ketofol for sedation of
postoperative mechanically ventilated patients with obstructive
sleep apnea. Crit Care Res Pract. 2018:10150542018.PubMed/NCBI
|
|
28
|
Lee CH, Park JH, Ahn JH and Won MH:
Effects of melatonin on cognitive impairment and hippocampal
neuronal damage in a rat model of chronic cerebral hypoperfusion.
Exp Ther Med. 11:2240–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wegmann TG, Lin H, Guilbert L and Mosmann
TR: Bidirectional cytokine interactions in the maternal-fetal
relationship: Is successful pregnancy a TH2 phenomenon? Immunol
Today. 14:353–356. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Druckmann R and Druckmann MA: Progesterone
and the immunology of pregnancy. J Steroid Biochem Mol Biol.
97:389–396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Mosmann TR, Guilbert L,
Tuntipopipat S and Wegmann TG: Synthesis of T helper 2-type
cytokines at the maternal-fetal interface. J Immunol.
151:4562–4573. 1993.PubMed/NCBI
|
|
32
|
Werlang ICR, Mueller NT, Pizoni A,
Wisintainer H, Matte U, Costa SHAM, Ramos JGL, Goldani MZ,
Dominguez-Bello MG and Goldani HAS: Associations of birth mode with
cord blood cytokines, white blood cells and newborn intestinal
bifidobacteria. PLoS One. 13:e02059622018. View Article : Google Scholar : PubMed/NCBI
|