Introduction
Changes in dietary habits and an aging population
have led to the gradual increase in the incidence of peripheral
vascular diseases, with a noteworthy rise in lower extremity
arterial occlusive disease (1). The
lower limb blood vessels of patients often exhibit severe
calcification and stenosis, a condition that hampers the
visualization of vascular structures or changes in the vessel
branching (1). Accurate and early
detection of lower extremity arterial stenosis and collateral
circulation can improve disease management by providing patients
with timely and appropriate treatment (2). Currently, clinical diagnosis of lower
extremity arterial disease is reliant on imaging (2). The most commonly used imaging
techniques include lower extremity vascular ultrasound, computed
tomography angiography (CTA), magnetic resonance angiography and
digital subtraction angiography (DSA) (2). The application of DSA is adjudged to be
the ‘gold standard’ for the assessment of lower extremity arterial
pathologies (1). However, DSA-based
analyses are invasive, and it is highly likely that computerized
tomography (CT) lower extremity arterial angiography will become
the procedure of choice. In comparison with conventional CT lower
extremity arterial angiography, CT Gemstone Spectral Imaging (GSI),
which functions by imaging under alternating high and low voltage
conditions, provides higher image quality (3). Therefore, in the present study, the
best single-energy (SE) scanning mode, which was achieved by
optimising the contrast-to-noise ratio (CNR), and multi-slice
spiral CT (MSCT) combined energy-scanning mode were employed to
perform lower extremity arterial angiography. The imaging quality
of the two modes, along with their relative precision in lower
extremity arteriography imaging, were compared and evaluated.
Materials and methods
Patient information
A total of 64 individuals (39 males; 25 females)
were clinically diagnosed with or suspected of lower extremity
arterial occlusive disease based on energy spectrum CT lower
extremity arterial imaging between December 2015 and December 2016
at The Second Affiliated Hospital of Harbin Medical University
(Harbin, China). They were between 41 and 78 years of age with
height ranging between 155 and 182 cm, and the body mass index
(BMI) ranging between 21.8 and 23.6. Patients were randomly
assigned to the experimental group (32 patients) or the control
group (32 patients). Some of the clinical manifestations included
lower limb swelling, low skin temperature, limping, chronic
infections, ulcers, gangrene and trauma. Within 1 week all patients
were assessed using lower extremity arterial digital subtraction
angiography or Doppler ultrasound. This study was designed to be a
randomized, controlled study, with all patients undergoing CTA
lower extremity arterial imaging. The inclusion criteria were: i)
≥18 years of age, clinically diagnosed with or suspected of
peripheral arterial occlusive disease; ii) liver and kidney
function adjudged to be within the normal range; and iii) no
history of allergy to iodinated contrast agents. The exclusion
criteria were: i) BMI ≥30 kg/m2; and ii) inability to
undergo CT examination. All patients were required to sign informed
consent forms. This study was approved by the Ethics Committee of
The Second Affiliated Hospital of Harbin Medical University
(Nangang, China).
Examination method
Patients were examined using the Discovery CT-750HD
model CT GSI machine (GE Healthcare). Scanning parameters and tube
voltage in the experimental group were set to automatic mode.
Images were reconstructed using the best electronic voltage
measured with an optimised CNR. The control group was treated with
mixed energy scanning mode of the MSCT. The scanning tube voltage
was 120 kVp and the tube current was 200 mA. All other parameters
were maintained constant between the two groups, with only slight
variations between individuals: Pitch, 1.375:1; tube rotation
speed, 0.8 sec/turn; scanning layer thickness, 5 mm; the abdominal
aortic level was set as the monitoring point; and 150 HU was set as
the monitoring threshold for the commencement of a trace trigger
scan. Delay was set to 6 sec. Scanning direction was from the head
to the foot. The layer thickness during image reconstruction was
1.25 mm. The contrasting agent (80–100 ml of 350 mg/ml iodohydrin)
was injected at a rate of 4.0 ml/sec, followed by the delivery of
20–40 ml physiological saline.
Image processing
Original data obtained from the CT machine were
first transmitted to the image post-processing workstation. The GSI
image of the experimental group was calculated using a
post-processing software (GSI-view software, volume share 5; GE
Healthcare) to process the SE image of the best electron
perturbation based on the maximum CNR of the blood vessel/muscle.
The best electronic voltage was determined to be in the range of
55–60 keV. The three-dimensional images of the lower extremity
arteries from both experimental and control groups were
reconstructed using Multi-Planner Reformation, Volume Rendering,
Maximum Intensity Projection and Curve Reformation. Two senior
physicians independently examined the images using a double-blinded
method for all directions and angles. In incidences where their
diagnoses were not in agreement, DSA and Doppler ultrasound results
were compared, and further consultations took place until mutual
agreements were reached.
Image quality evaluation
The following parameters of the lower extremity
arteries were included: Lower extremity arterial course,
distribution, shape, width and length of the lesion, with or
without stenosis, occlusion and deformities, the presence of
collateral circulation, vascular wall changes and vascular
artifacts. The iliac arteries, the superficial femoral artery, the
popliteal artery and the anterior tibial artery were evaluated. Two
imaging diagnostic doctors, using a double-blinded method, were
recruited to perform a subjective evaluation of the images.
Evaluation of image quality was performed using a five-point system
(4), with all images fixed in the
same window width and position, applying the following specific
scoring criteria: 5 points, excellent image; 4 points, good image;
3 points, intermediate image; 2 points, poor image and 1 point,
very poor.
Image analysis
In this study, 22 patients in the experimental group
were subjected to CTA and DSA. As DSA may present risks and is
expensive, only the patients with severe clinical symptoms and
those that needed interventional treatment underwent the
procedures; 22 of the 32 patients required this intervention. The
lower extremity arteries were divided into eight anatomical
segments: Common iliac artery, internal iliac artery, external
iliac artery, femoral artery, popliteal artery, anterior tibial
artery, posterior tibial artery and fibular (peroneal) artery. The
degree of blood stenosis in coronary artery stenosis was assessed
using a four-tier assessment system (5): Mild stenosis, <50%; moderate
stenosis and luminal stenosis, 50%-75%; severe stenosis and tube
cavity stenosis, >75%; and complete occlusion, 100%.
Stenosis=[(stenosis near the proximal end of the normal diameter of
the vessel-stenosis of the diameter of the vessel)/stenosis near
the proximal end of the normal vascular diameter] ×100. The
diameter of the vessel was measured was to assess stenosis. In
cases of multiple stenoses observed in the same vascular segment,
the highest level of stenosis was used for comparison. A total of
28 lower extremity arterial CTA and DSA from similar vascular
segments of 22 patients were compared.
Statistical analysis
All data were analyzed using the SPSS20.0 software
package (IBM Corp.). Count index was presented as percentage, and
measurement index was presented as mean ± standard deviation.
χ2 test was used for comparisons of sex, whereas
independent samples t-test was used for comparisons of age, height,
weight and objective image quality. Mann-Whitney U-test was applied
for rating subjective image quality. The sensitivity and
specificity of vascular stenosis were calculated using the 95%
confidence interval. Consistency between the two groups was
analyzed using the κ-test, and P<0.05 was considered to indicate
a statistically significant difference.
Results
General comparison
No significant differences were identified in
relation to sex, age, height and weight between patients in the
experimental and control groups (Table
I).
 | Table I.Comparison of participants from two
groups. |
Table I.
Comparison of participants from two
groups.
| Parameters | Experimental | Control | P-value |
|---|
| Age | 54±5 | 58±4 | >0.05 |
| Sex
(male/female) | 18/14 | 21/11 | >0.05 |
| Height | 164±6.3 | 166±5.5 | >0.05 |
| BMI | 22.5±1.27 | 21.3±2.16 | >0.05 |
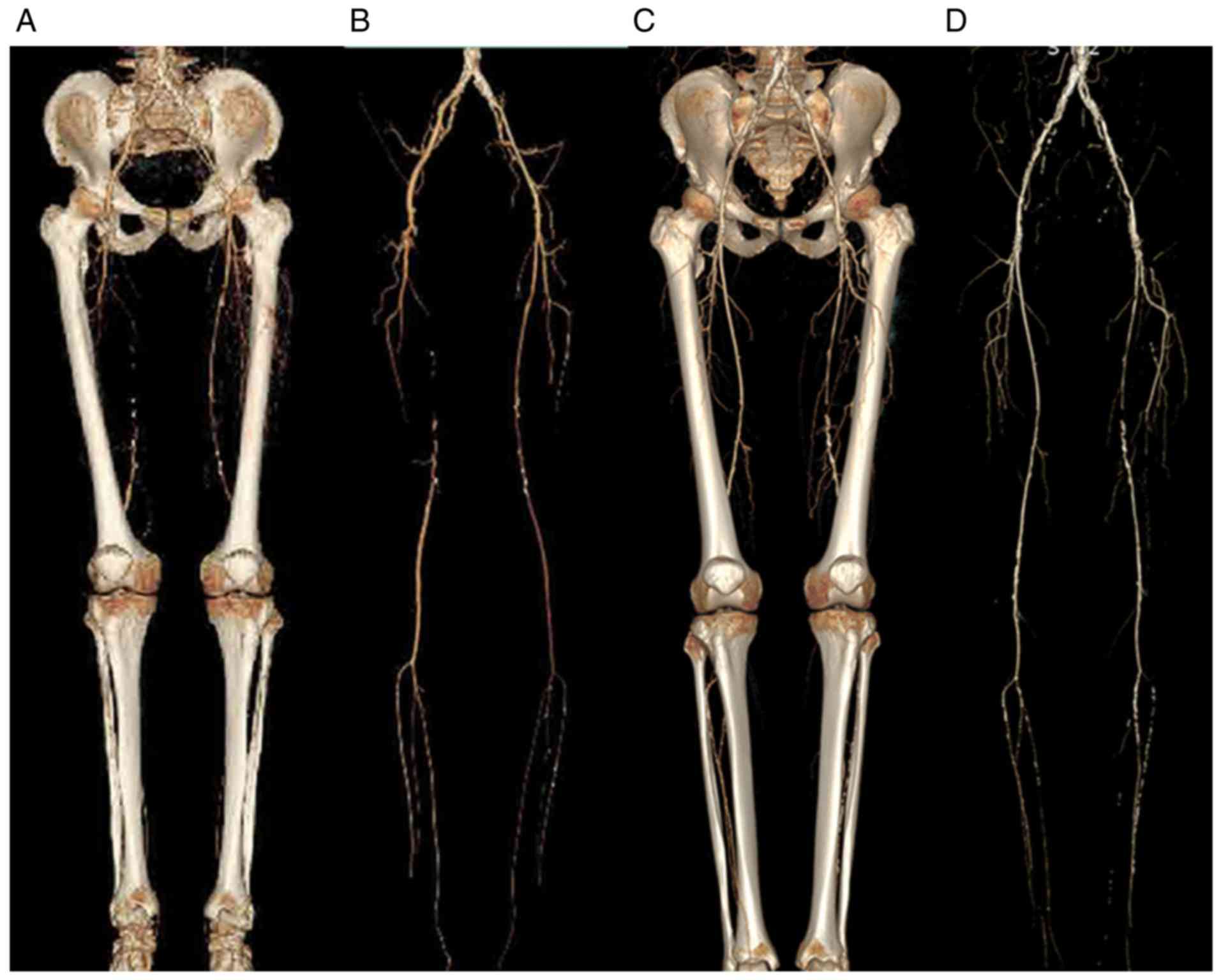
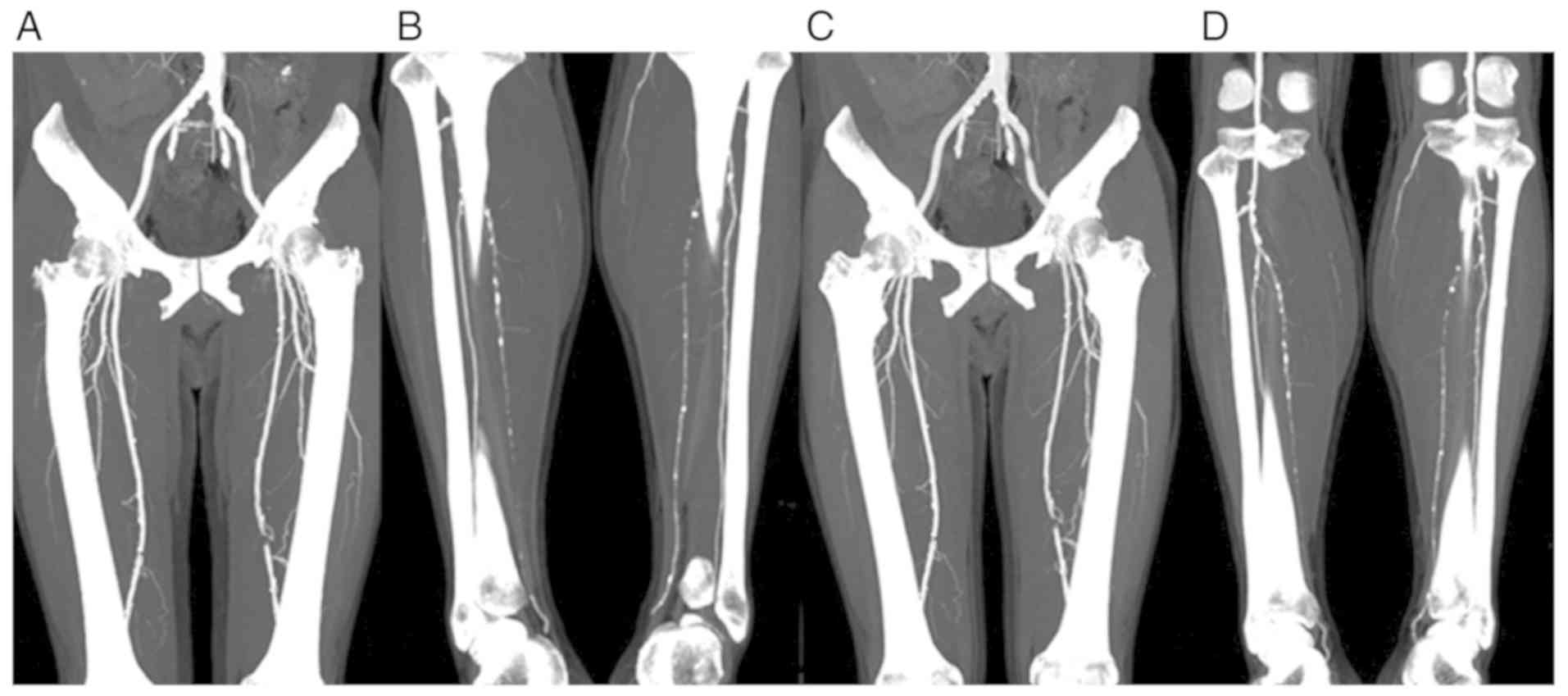
Image quality evaluation
(Objective)
The best electron emission images reconstructed
using GSI were more uniform compared with the conventional MSCT
model. The arterial image was uniform, the edge of the vessel was
smooth, the small branch was well defined, the artifact was
minimal, the background noise (BN) was low and the CNR was greatly
improved. The CT values of the contrast agent in the iliac,
superficial femoral, popliteal and anterior tibial arteries were
better compared with those derived from the MSCT mixed energy
model. The CT value and CNR of the contrast agent were observed to
be significantly higher in the iliac, femoral, popliteal and
anterior tibial arteries of the experimental group compared with
the control group (P<0.05). The CNR of the MSCT hybrid energy
scanning mode was improved, and the image quality was better than
that of the MSCT hybrid energy-scanning model. No significant
difference was identified for BN between the two groups (P>0.05;
Table II).
 | Table II.Comparison of image quality
(objective) from two participant groups. |
Table II.
Comparison of image quality
(objective) from two participant groups.
| Parameters | Experimental | Control | P-value |
|---|
| Iliac Arteries CT
Value (HU) | 525.4±54.1 | 386.2±61.3 | <0.05 |
| Superficial Femoral
arteries CT Value (HU) | 488.6±53.2 | 352.1±41.4 | <0.05 |
| Popliteal Arteries CT
Value (HU) | 410.6±63.7 | 320.4±58.1 | <0.05 |
| Anterior Tibial
arteries CT Value (HU) | 352.2±75.4 | 230.5±68.2 | <0.05 |
| Background Noise | 9.15±1.12 | 10.27±1.24 | >0.05 |
| Contrast Noise
Ratio | 53.8±9.5 | 34.7±8.6 | <0.05 |
Image quality evaluation
(Subjective)
Evaluation of the quality of the scanned images was
independently performed by two imaging diagnostic physicians. The
total image quality score from physician 1 was 155 for the
experimental and 133 for the control groups, whereas physician 2
scored 154 for the experimental and 134 for the control groups
(Table III). There was also a high
degree of consistency between the physicians' evaluations of the
image quality. The evaluation demonstrated that the image quality
in the experimental group was better compared with that of the
control group. The arterial image was uniform, the edge of the
vessel was smooth, the small branch was well defined, the artifact
was minimal, the BN was low and the CNR was greatly improved.
Experimental images showed the vascular wall changes more clearly
and the vascular artifacts were markedly increased (Figs. 1 and 2).
 | Table III.Comparison of image quality
(subjective) from two participant groups. |
Table III.
Comparison of image quality
(subjective) from two participant groups.
|
|
Group |
|---|
|
|
|
|---|
|
| Experimental | Control |
|---|
|
|
|
|
|---|
| Physician | 5 Points | 4 Points | 3 Points | 2 Points | 1 Point | Total points | 5 Points | 4 Points | 3 Points | 2 Points | 1 Point | Total points |
|---|
| #1 | 27 | 5 | 0 | 0 | 0 | 155 | 26 | 6 | 0 | 0 | 0 | 154 |
| #2 | 13 | 11 | 8 | 0 | 0 | 133 | 13 | 12 | 7 | 0 | 0 | 134 |
Comparative analyses of CTA and DSA in
diagnosing lower limb arterial stenosis
CTA and DSA were applied to the lower extremity
arteries of 22 patients in the experimental group. A total of 352
blood vessels were applied in the comparative analyses of CTA and
DSA described in the present study. A summary of the specific
distribution of vascular stenosis levels was presented in Table IV. Of the 352 blood vessels
analyzed, 311 scored consistently for the degree of stenosis in
blood vessels using the two diagnostic techniques applied; with the
diagnostic accuracy rate of 94.03% (331/352). In incidences of
misdiagnosis, three moderate stenoses of the blood vessels were
underestimated by the CTA as mild stenosis, two segments of severe
stenosis of the CTA were underestimated as moderate stenosis, four
mild stenoses of the vascular CTA were overestimated as moderate
stenosis, seven segments of moderate stenosis of the vascular CTA
were overestimated as severe stenosis and five segments of severe
stenosis of the vascular CTA were overestimated as occlusion. The
sensitivity of CTA in the diagnosis of lower extremity arterial
stenosis (≥50%) was 93.18% (205/220) with the specificity at 95.45%
(126/132) compared with the diagnostic ‘gold standard’ DSA. The
application of CTA and DSA for the diagnosis of lower extremity
arterial stenosis was determined to be reliable (κ=0.927), with a
high degree of consistency. Therefore, the analyses performed in
the present study suggest that both CTA imaging and DSA can be used
as a reliable means of diagnosing lower extremity arterial
stenosis.
 | Table IV.Assessment of lower extremity arterial
stenosis from 44 segments using CTA and DSA. |
Table IV.
Assessment of lower extremity arterial
stenosis from 44 segments using CTA and DSA.
|
| DSA (segment) |
|---|
|
|
|
|---|
| CTA (segment) | Healthy | Mild stenosis | Moderate
stenosis | Severe stenosis | Complete
occlusion | Total |
|---|
| Healthy | 73 | 0 | 0 | 0 | 0 | 73 |
| Mild stenosis | 0 | 57 | 3 | 0 | 0 | 60 |
| Moderate
stenosis | 0 | 4 | 79 | 2 | 0 | 85 |
| Severe stenosis | 0 | 0 | 7 | 83 | 0 | 90 |
| Complete
occlusion | 0 | 0 | 0 | 5 | 39 | 44 |
| Total | 73 | 61 | 89 | 90 | 39 | 352 |
Discussion
Atherosclerosis is a systemic vascular disease.
Lower extremity arterial occlusive disease is caused by a number of
conditions, including distal limb ischemia, nutritional disorders,
limb ulceration and necrosis (6).
Arterial CTA has long been applied as the most direct and effective
method for the diagnosis of arteriosclerosis of the lower
extremity; as it can visualize the lower extremity arteries to
accurately display the intraluminal contrast agent through the
vessel wall calcification (6). These
features enable the accurate and reliable diagnosis of lower
extremity arterial disease and the development of treatment
strategies. The ability of CTA to determine the formation of distal
collateral circulation in the lower extremity arterial wall is
superior to that of conventional DSA angiography and
ultrasonography (7,8).
In the MSCT coupled with mixed energy imaging system
currently in clinical use, the tube produced by the X-ray system
has a certain spectral width of a multi-chromatogram (9). It generates photons at a spectrum of
energy levels that can penetrate the human body. Low-energy rays
can be easily absorbed whereas high-energy rays readily pass
through the body (9). Therefore, the
average energy of the rays tends to increase during the
propagation, causing the rays to become harder (9). This beam-hardening effect can result in
severe beam hardening artifacts (BHA) that may compromise image
quality and diagnostic assessment. By contrast, the SECT GSI image
utilizes an SE level of X-ray through the object after image
acquisition. This effectively removes the beam hardening effects,
leading to more accurate CT values, improved image quality and
better detection of small lesions (10,11).
Conventional CT equipment cannot project an SE X-ray. The energy
spectrum CT with an X-ray tube that instantaneously switches
between high- and low-energy imaging modes can generate mixed
energy images. The SE image of 40–140 keV can be reconstructed
using energy spectrum analysis (12). At an SE level, the difference between
the set point of interest and the physical organ can be maximized,
whilst the noise value can be minimized. The clinical application
of energy spectrum CT is based primarily on the following four
technical characteristics: Elimination of hardening artifacts,
optimization of image quality and CNR, quantitative analysis of the
matter and comprehensive analysis of energy spectrum (13,14). In
the present study, the optimized image quality and CNR was first
determined to be used in the energy spectrum synthesis analysis to
reconstruct a single contrast image with the best CNR. Compared
with the images produced using mixed energy CT, those produced
using the best electron micrographs were uniform. The vascular and
branch anatomical structures were imaged with high precision, and
the lesions were revealed to be much more evident.
The aim of the present study was to investigate
whether the GSI is superior to the MSCT in the visualization of the
anatomical details of the lower extremity arteries, the contrast
between blood vessels and surrounding tissues and the optical
amenability of the vascular lesions for diagnosis. The best SE
energy map in the experimental group was in the 55–60 keV range, in
which blood vessels and muscle exhibited the largest CNR. Outside
of this range, the CNR was reduced. The lower the X-ray energy
level, the higher the contrast CT value and hence greater the
difference between blood vessels and the surrounding tissue CT. By
contrast, CNR was lower and BN was higher in the control group.
Higher X-ray energy levels resulted in smaller BN, in turn causing
a sharp drop in the CT value of the contrast agent and a decrease
in CNR. Therefore, the function of the best SE graph was to find a
balance between the CT difference and BN, optimizing the CNR
leading to the best keV (15).
Results from the present study demonstrated that the best electron
emission images reconstructed using GSI were more uniform compared
with the conventional MSCT model. The arterial image was uniform,
the edge of the vessel was smooth, the small branch was well
defined, the artifact was minimal, the BN was low and the CNR was
greatly improved. The CT values of the contrast agent in the iliac,
superficial femoral, popliteal and anterior tibial arteries were
better compared with those derived from the MSCT mixed energy
model. The results demonstrated that the CNR of the MSCT hybrid
energy scanning mode was improved, and the image quality was better
than that of the MSCT hybrid energy-scanning model. This was
consistent with the subjective evaluation results.
As patients with lower extremity arterial occlusive
disease often exhibit additional underlying pathological
conditions, including hypertension and diabetes, the need to
strictly control for the amount of contrast agent is crucial
(16). SECT GSI imaging can alter
the contrast agent CT value and CNR, and can be performed in the
absence of a contrast agent to offer a certain degree of
remediation. Therefore, SECT GSI imaging, without affecting the
diagnostic criteria, uses a lower dosage of contrast agent by
adjusting the energy value of the SE energy map. This has important
clinical consequences, as it reduces the side effects associated
with contrast agents. In addition, SECT GSI imaging has the
potential to reduce metallic artifacts and BHA (17), which is helpful for the clearer
display of stenting in patients undergoing lower extremity arterial
stent implantation and to provide a theoretical basis for assessing
restenosis after stenting.
DSA has long been considered as the ‘gold standard’
for the diagnosis of vascular lesions; it can readily detect the
dynamic vascular changes in addition to the underlying pathological
alterations, all of which are crucial in the treatment of vascular
lesions (1). Although DSA is
invasive and often results in trauma, it is still considered as an
indispensable tool for the diagnosis and treatment of lower limb
vascular disease (7). Patients with
lower extremity arteriosclerosis obliterans often manifest varying
degrees of cardiovascular and cerebrovascular disease in other
peripheral parts of the vascular stenosis, thus increasing the risk
of DSA-based examination and surgery (7). By contrast, CTA is a safe and effective
imaging technique for imaging lower limb vascular lesions (18). It entails a high temporal and spatial
resolution, features that are important for identifying the
location, extent, degree of stenosis and collateral circulation, in
addition to occlusion of the distal arterial trunk (1). For patients with DSA results as part of
the diagnostic criteria, the two methods provided consistent
conclusions on the blood vessels in a total of 311 segments, the
diagnostic accuracy of 94.03% (331/352), CTA diagnosis of lower
extremity arterial stenosis (≥50%) has a sensitivity 93.18%
(205/220) and a specificity of 95.45% (126/132), indicating that
CTA diagnosis of lower extremity arterial stenosis parallels that
of DSA. These results are consistent with the literature (3,18). In
the present study analysis, there were 14 incidences of CTA
overestimation and 5 incidences of underestimation of stenosis.
Further examination of these vascular segments revealed that the
extent of stenosis severity was either too high or too low for a
number of reasons. Examples include overestimated vascular segments
with more severe wall calcification, post-treatment methods
improperly applied in some cases, severe vascular stenosis or
completed occlusion, weak vascular enhancement and the eccentric
stenosis of the blood vessel wall caused by some errors. Therefore,
CTA image post-processing must incorporate three-dimensional images
with the axial image from the original combination of observation
techniques. In addition, appropriate adjustments to the window
width and window position are imperative for reducing errors in
determining vascular stenosis or occlusion lesions.
There are a number of limitations associated with
this study. The sample size was relatively small, so that the
results could have been biased, which can affect the accuracy of
the statistical results to a certain extent. In addition, the
contrast agent dosage and injection rate were not adjusted to body
weight indexes of participants, and this might have an impact on
the degree of vascular enhancement. A subjective selection of
vascular region of interest position, vascular CT value, BN and CNR
might have been chosen in a biased fashion. Lastly, radiation dose
measurements were not performed between the two groups of
participants.
In conclusion, this study demonstrated that CT GSI
lower extremity arteriography is a clinically feasible approach and
that the best SE scanning mode image quality is superior to the
image quality of MSCT mixed energy scanning mode. CTA imaging is of
clinical significance in the screening of patients with clinically
suspected lower extremity arteriosclerosis obliterans, as it
facilitates correct diagnosis and may lead to more effective
therapeutic interventions.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (grant no. 81701772).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
GKW, DLZ and JLZ conceived of and designed the
experiments, and wrote and revised the manuscript. GKW, DLZ, ZSL,
SSY, and JLZ performed the experiments and analysed the data. HBW
also performed the experiments.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Harbin Medical
University (Nangang, China).
Patient consent for publication
All patients were required to sign informed consent
forms.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heijenbrok-Kal MH, Kock MC and Hunink MG:
Lower extremity arterial disease: Multidetector CT angiography
meta-analysis. Radiology. 245:433–439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin XZ, Miao F, Li JY, Dong HP, Shen Y and
Chen KM: High-definition CT Gemstone spectral imaging of the brain:
Initial results of selecting optimal monochromatic image for
beam-hardening artifacts and image noise reduction. J Comput Assist
Tomogr. 35:294–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang MJ, Park CM, Lee CH, Goo JM and Lee
HJ: Focal iodine defects on color-coded iodine perfusion maps of
dual-energy pulmonary CT angiography images: A potential diagnostic
pitfall. AJR Am J Roentgenol. 195:W325–W330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pache G, Krauss B, Strohm P, Saueressig U,
Blanke P, Bulla S, Schäfer O, Helwig P, Kotter E, Langer M and
Baumann T: Dual-energy CT virtual noncalcium technique: Detecting
posttraumatic bone marrow lesions-feasibility study. Radiology.
256:617–624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim S, Shin H, Lee Y, Yoon JW, Kang SM,
Choi SH, Park KS, Jang HC, Choi SI and Chun EJ: Effect of metabolic
syndrome on coronary artery stenosis and plaque characteristics as
assessed with 64-detector row cardiac CT. Radiology. 261:437–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mueller T, Hinterreiter F, Poelz W,
Haltmayer M and Dieplinger B: Mortality rates at 10 years are
higher in diabetic than in non-diabetic patients with chronic lower
extremity peripheral arterial disease. Vasc Med. 21:445–452. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pollak AW, Norton PT and Kramer CM:
Multimodality imaging of lower extremity peripheral arterial
disease: Current role and future directions. Circ Cardiovasc
Imaging. 5:797–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kock MC, Dijkshoorn ML, Pattynama PM and
Myriam Hunink MG: Multi-detector row computed tomography
angiography of peripheral arterial disease. Eur Radiol.
17:3208–3222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bowden RG: Contrast nephropathy: Risk
factors and the role of beta blockers. Anatol J Cardiol.
15:2412015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Danad I, Fayad ZA, Willemink MJ and Min
JK: Recent advances in cardiac computed tomography: Dual energy,
spectral and molecular CT imaging. JACC Cardiovasc Imaging.
8:710–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obaid DR, Calvert PA, Gopalan D, Parker
RA, West NJE, Goddard M, Rudd JHF and Bennett MR: Dual-energy
computed tomography imaging to determine atherosclerotic plaque
composition: A prospective study with tissue validation. J
Cardiovasc Comput Tomogr. 8:230–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Danad I, Hartaigh BÓ and Min JK:
Dual-energy computed tomography for detection of coronary artery
disease. Expert Rev Cardiovasc Ther. 13:1345–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arnoldi E, Lee YS, Ruzsics B, Weininger M,
Spears JR, Rowley CP, Chiaramida SA, Costello P, Reiser MF and
Schoepf UJ: CT detection of myocardial blood volume deficits:
Dual-energy CT compared with single-energy CT spectra. J Cardiovasc
Comput Tomogr. 5:421–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi N, Vrtiska TJ, Kawashima A,
Hartman RP, Primak AN, Fletcher JG and McCollough CH: Detectability
of urinary stones on virtual nonenhanced images generated at
pyelographic-phase dual-energy CT. Radiology. 256:84–190. 2010.
View Article : Google Scholar
|
|
15
|
Yao Y, Ng JM, Megibow AJ and Pelc NJ:
Image quality comparison between single energy and dual energy CT
protocols for hepatic imaging. Med Phys. 43:48772016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He J, Ma X, Wang Q, Fan J and Sun Z:
Spectral CT demonstration of the superior mesenteric artery:
Comparison of monochromatic and polychromatic imaging. Acad Radiol.
21:364–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JS and Chen JC: A single scatter model
for X-ray CT energy spectrum estimation and polychromatic
reconstruction. IEEE Trans Med Imaging. 34:403–1413. 2015.
View Article : Google Scholar
|
|
18
|
Li YH, Peng XG, Jin H, Zhao GF, Ding RR,
Wei HL, Lu ZX and Deng G: The improvement of imaging quality of
lower extremity angiography with optimal single energy image of
spectral CT. J Intervent Radiol. 25:997–1001. 2016.
|