Introduction
A poor understanding of the pathophysiology of the
condition, the heterogeneity among patient populations, the variety
of possible etiologies and the lack of reliable outcome predictors
means that the development of effective treatments for liver
failure remains a major challenge (1). Without effective treatments, liver
failure is associated with substantial physiological disability and
a high mortality (2). Orthotopic
liver transplantation has been demonstrated to be effective for the
treatment of liver failure and the reduction of mortality (3). However, this strategy is greatly
limited by a high cost and the lack of donor organs (4). Even when transplantation is feasible, a
liver support system is compulsory to keep patients alive and
maintain neurologic function until an appropriate liver is
available (5).
The concept of a bioartificial liver (BAL) has
emerged as a potential supportive therapy for patients with liver
failure until transplantation can be performed or until recovery is
achieved (6). A typical BAL system
consists of hepatocytes and either a hollow fiber cartridge or
semi-permeable membrane (bioreactor) (7). The efficacy of a BAL system was
determined in a clinical study involving 24 patients with fulminant
hepatic failure (FHF) (1). After
treatment, 6 patients recovered without need for further
transplantation, and in the other 18 patients, BAL provided an
effective bridge treatment until transplantation (1). Human hepatocytes (autologous or
allogeneic) are the optimal choice for the cellular component of
BAL. However, the shortage of human livers and a lack of stable
functional human hepatocyte lines remain critical obstacles that
hinder the use of these cells in BAL systems (5). Porcine hepatocytes offer the advantage
of being physiologically similar to human hepatocytes, readily
available and easy to isolate (8).
More importantly, porcine hepatocytes tend to form cell aggregates,
which have been revealed to exhibit a beneficial effect on the
maintenance of hepatic function (9).
Demetriou et al (10)
reported improved survival in patients with fulminant hepatic
failure (FHF) or subfulminant hepatic failure who were treated with
an extracorporeal porcine hepatocyte-based BAL. The use of
xenogeneic cells is, however, associated with induced
immunogenicity as well as the transmission of zoonoses including as
endogenous porcine retroviruses (11).
Hepatocellular adenoma (HCA) is an uncommon benign
liver tumor, which is linked to the use of hormonal contraceptives
(12). Research has indicated that
the monoclonal properties of HCA may facilitate cell lineage
establishment and storage (13),
making HCA an attractive potential source for the cellular
component of the BAL system. Therefore, the present study was
performed to assess the feasibility of using HCA as a cell source
for BAL development.
Materials and methods
HCA specimen collection
The use of human liver samples for scientific
research was approved by the Medical Ethics Committee of Nanfang
Hospital, Southern Medical University (Guangzhou, China) and all
enrolled subjects provided their written informed consent. HCA
tissues were obtained from 8 patients who underwent liver
hepatectomies at the Department of Hepatobiliary Surgery of Nanfang
Hospital (Guangzhou, China) between September 2008 and March 2013.
Adjacent liver tissue, which was located at least 2 cm away from
the lesion, was collected from the same patients for use as control
tissue. All patients had been diagnosed with adenomatous
hyperplasia based on pathological analysis and did not receive any
other treatment relevant to this study prior to surgery. Patient
characteristics are recorded in Table
I. The tissues used in the present study exhibited
histopathological features that were typical of HCA. The HCA tumors
were soft and well demarcated with little or no fibrous capsule,
and were composed of liver cell plates that were mildly thickened
or irregular. Thin-walled arteries supplied the tumor parenchyma
without other portal tract elements including significant
connective tissue, bile ducts or ductular reaction. Hepatocytes
within the HCA tumors were of normal size with cytoplasm that was
normal, clear or fatty and some lesions were almost entirely
steatotic.
 | Table I.HCA patient characteristics. |
Table I.
HCA patient characteristics.
| Characteristic |
|
|---|
| Mean age, years
(range) | 40 (22–58) |
| Sex, n (%) |
| Male | 2 (25) |
|
Female | 6 (75) |
| Tumor size, n
(%) |
| ≤5
cm | 5 (62.5) |
| >5
cm | 3 (37.5) |
| Number of tumors, n
(%) |
| ≤1 | 3 (37.5) |
|
>1 | 5 (62.5) |
Prior to tissue dissociation, two small tissue
fragments were removed. One fragment was prepared for hematoxylin
and eosin (H&E), Periodic Acid-Schiff (PAS) and
immunohistochemical (IHC) staining by fixation in neutral buffered
formalin (10%) for 24 h at room temperature, paraffin embedding,
sectioning (4 µm thickness), deparaffinization and dehydration via
a series of graded ethanol rinses. The second tissue portion was
immersed in RNA stabilization solution (Ambion; Thermo Fisher
Scientific, Inc.) and cryopreserved at −80°C for RNA
extraction.
H&E staining
Dried sections of HCA tissue samples were processed
according to the standard H&E staining procedure (14). In brief, sections were stained with
hematoxylin (Zhongshan Goldenbridge Biotechnology Co., Ltd.) for 10
min followed by differentiation in a hydrochloride acid-alcohol
mixture. Then the sections were counterstained in eosin (Zhongshan
Goldenbridge Biotechnology Co., Ltd.) for 3 min, dehydrated in
ethanol and cleaned in xylene before examination using a light
microscope (Olympus BX40; magnification, ×400).
PAS staining
Dried sections of HCA tissue were also stained using
a PAS staining kit (Fuzhou Maixin Biotech Co., Ltd.) according to
manufacturer's protocol. The PAS-stained sections were
counterstained with hematoxylin for 6 min at room temperature and
differentiated in a hydrochloride acid-alcohol mixture, followed by
blue color development in PBS, dehydration in ethanol, cleaning in
xylene and examination using light microscopy (magnification,
×200).
ICH and immunocytochemical
staining
Prior to ICH or immunocytochemical staining, dried
sections of HCA tissue were heated in a microwave oven (1000 watts
for 10 min) in 0.01 M sodium citrate buffer (pH 6.0) for 20 min and
washed with PBS for antigen retrieval. Endogenous peroxidase
activity was subsequently terminated by incubation in 3%
H2O2 solution for 20 min at room temperature.
Tissue sections were then incubated at 4°C overnight with the
following specific primary antibodies: Goat anti-human albumin
(ALB) monoclonal antibody (cat. no. sc-46293; 1:100; Santa Cruz
Biotechnology, Inc.), goat anti-human cytokeratin 18 (CK18)
monoclonal antibody (cat. no. sc-31700; 1:50; Santa Cruz
Biotechnology Inc.) and rabbit anti-human cytochrome p450 2E1
(CYP2E1) monoclonal antibody (cat. no. PB0186; 1:50; Wuhan Boster
Biological Technology, Ltd.). Incubation with biotin-conjugated
goat anti-rabbit IgG (cat. no. PV-9001; Zhongshan Goldenbridge
Biotechnology Co., Ltd.) and biotin-conjugated rabbit anti-goat IgG
secondary antibodies (cat. no. PV-9003; 1:500; Zhongshan
Goldenbridge Biotechnology Co., Ltd) was performed after the
samples were maintained at room temperature for 45 min. The
antibody-antigen complexes were visualized with diaminobenzidine
(1:20, Zhongshan Goldenbridge Biotechnology Co., Ltd) and sections
were counterstained with hematoxylin for 3 min at room temperature.
Finally, the sections were dehydrated in ethanol, cleared in xylene
and examined under light microscopy (magnification, ×200) (15).
The IHC slides were analyzed by three
independent investigators
Each sample was given a score according to the
intensity of the staining (0, no staining; 1, weak staining; 2,
moderate staining; and 3, strong staining) and the extent of
stained cells (0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; and 4,
76–100%). The percentage of positive cell staining for each
intensity was multiplied by the corresponding intensity value to
obtain an immunostaining score ranging from 0 to 12. Products of
the positive staining scores and color intensity scores ranging
from 0, 1–4, 5–8, and 9–12 were finally considered as negative
staining (−), slight positive staining (+), medium positive
staining (++), and strong positive staining (+++),
respectively.
Reverse transcription-quantitative
(RT-q) PCR analysis
Cryopreserved HCA tissues were ground in a mortar
and Trizol® reagent (Takara Bio, Inc.) was used to
extract RNA according to the manufacturer's protocol. RT of RNA
generated 60 µl cDNA which contained 1.8 µg RNA, 12 µl 5X
PrimeScript™ buffer, 3 µl PrimeScript™ reverse transcriptase
(RTase), 3 µl random primers, 3 µl dNTP and RNase-free water (added
to achieve a total volume of 60 µl) using a reverse transcription
reagent kit (Takara Bio, Inc.). The reaction was sustained at 37°C
for 15 min. RTase was heat-inactivated at 85°C for 5 sec. Gene
expression was measured using a RT-qPCR system
(LightCycler® Real Time PCR; Roche Diagnostics). The
reaction solution volume was 20 µl and included forward and reverse
primers (each, 0.2 µM; as indicated in Table II), 10 µl SYBR Green, 7.2 µl sterile
distilled water and 2 µl cDNA. The reactions were performed as
follows: 40 cycles of 95°C for 30 sec and 60°C for 20 sec followed
by one cycle of 65°C for 15 sec. Relative gene expression levels
were calculated using the 2−∆∆Cq method with GAPDH
(Invitrogen; Thermo Fisher Scientific, Inc.) as the endogenous
control gene (16).
 | Table II.Sequences of primers used for RT-qPCR
analysis. |
Table II.
Sequences of primers used for RT-qPCR
analysis.
|
| Primer sequences |
|---|
|
|
|
|---|
| Primer | Forward | Reverse |
|---|
| ALB |
5′-GCCTGCTGACTTGCCTTCATTAG-3′ |
5′-TCAGCAGCAGCACGACAGAGTA-3′ |
| TTR |
5′-CAGAAAGGCTGCTGATGACA-3′ |
5′-ATGCCAAGTGCCTTCCAGTA-3′ |
| AAT |
5′-CAACCTGGCTGAGTTCGCCT-3′ |
5′-CTCGCTGAGGAACAGGCCAT-3′ |
| TF |
5′-CCCTTAACCAATACTTCGGCTAC-3′ |
5′-TTTGCCAAGTTCTCAAATATAGTCG-3′ |
| CYP2E1 |
5′-CTACAAGGCGGTGAAGGAA-3′ |
5′-TCTCATTGCCCTGTTTCCC-3′ |
| G-6-P |
5′-GCTGCTCATTTTCCTCATCAA-3′ |
5′-TTCTGTAACAGCAATGCCTGA-3′ |
| GST-π |
5′-CCTGTACCAGTCCAATACCATCCT-3′ |
5′-TCCTGCTGGTCCTTCCCATA-3′ |
| CPS-1 |
5′-TGAGGGATGCTGACCCCATT-3′ |
5′-CATTGTTGGCGTTGAGCCAG-3′ |
| TAT |
5′-AGGCCAGGTGGTCTGTGAGG-3′ |
5′-AGGGGTGCCTCAGGACAGTG-3′ |
| CYP3A5 |
5′-CCTTACCCCAGTTTTTGAAGCA-3′ |
5′-TCCAGATCAGACAGAGCTTTGTG-3′ |
| CYP3A4 |
5′-CAGGAGGAAATTGATGCAGTTTT-3′ |
5′-GTCAAGATACTCCATCTGTAGCACAGT-3′ |
| GST-α |
5′-TGGCAGAGAAGCCCAAGCTC-3′ |
5′-TGCACCAGCTTCATCCCATC-3′ |
| AFP |
5′-GCTGACATTATTATCGGACAC-3′ |
5′-GAACTTGTCATCAGAGAATGC-3′ |
| CK-18 |
5′-GAGACGTACAGTCCAGTCCTTGG-3′ |
5′-CCACCTCCCTCAGGCTGTT-3′ |
| HNF-1α |
5′-TACACCACTCTGGCAGCCACACT-3′ |
5′-CGGTGGGTACATTGGTGACAGAAC-3′ |
| GAPDH |
5′-GAAGGTGAAGGTCGGAGT-3′ |
5′-GAAGATGGTGATGGGATTTC-3′ |
Isolation and culture of human HCA
cells
Human HCA cells were isolated from the fresh
surgically resected specimens by collagenase digestion as described
previously (17), with minor
modifications in collagenase type and centrifugation time. A HCA
tumor fragment was perfused through vascular orifices on cut
surfaces with Hank's Balanced Salt Solution (HBSS; Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for 15 min to remove blood cells.
The fragment was subsequently perfused with a collagenase type IV
solution (diluted in HBSS to a concentration of 200 U/ml; Gibco;
Thermo Fisher Scientific, Inc.) for a further 15 min, and HCA cells
were released by mincing the tissue and repeatedly pipetting the
solution with a large-bore pipette. The cell suspension was
filtered through a sterile 100 µm nylon mesh, sedimented by
centrifugation at 50 × g for 5 min at room temperature, resuspended
and washed twice with cold HBSS solution. The isolated HCA cells
were seeded at a density of 1×105/cm2 of viable cells
(80% were determined to be viable following Trypan blue staining
for 5 min) in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) on
collagen-coated culture plates (Invitrogen) supplemented with 10%
fetal calf serum (HyClone; GE Healthcare Life Sciences), insulin
(10-8 M; Sigma-Aldrich; Merck KGaA) and dexamethasone (10-8 M;
Sigma-Aldrich; Merck KGaA) for culture at 37°C in 5%
CO2. Growing cell colonies were individually picked and
amplified. The medium was changed twice a week and cell passages
were performed with 0.25% trypsin.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM Corp.). The difference of clinical diagnostic
scores between adjacent liver tissues and hepatocellular adenoma,
based on ICH staining, was analyzed using Wilcoxon rank sum test.
The difference of gene expression between the two independent
groups was calculated using a Student's t test. P<0.05 was
determined to indicate a statistically significant result.
Results
Characteristics of HCA lesions
A total of 13 tumors were observed in 8 patients.
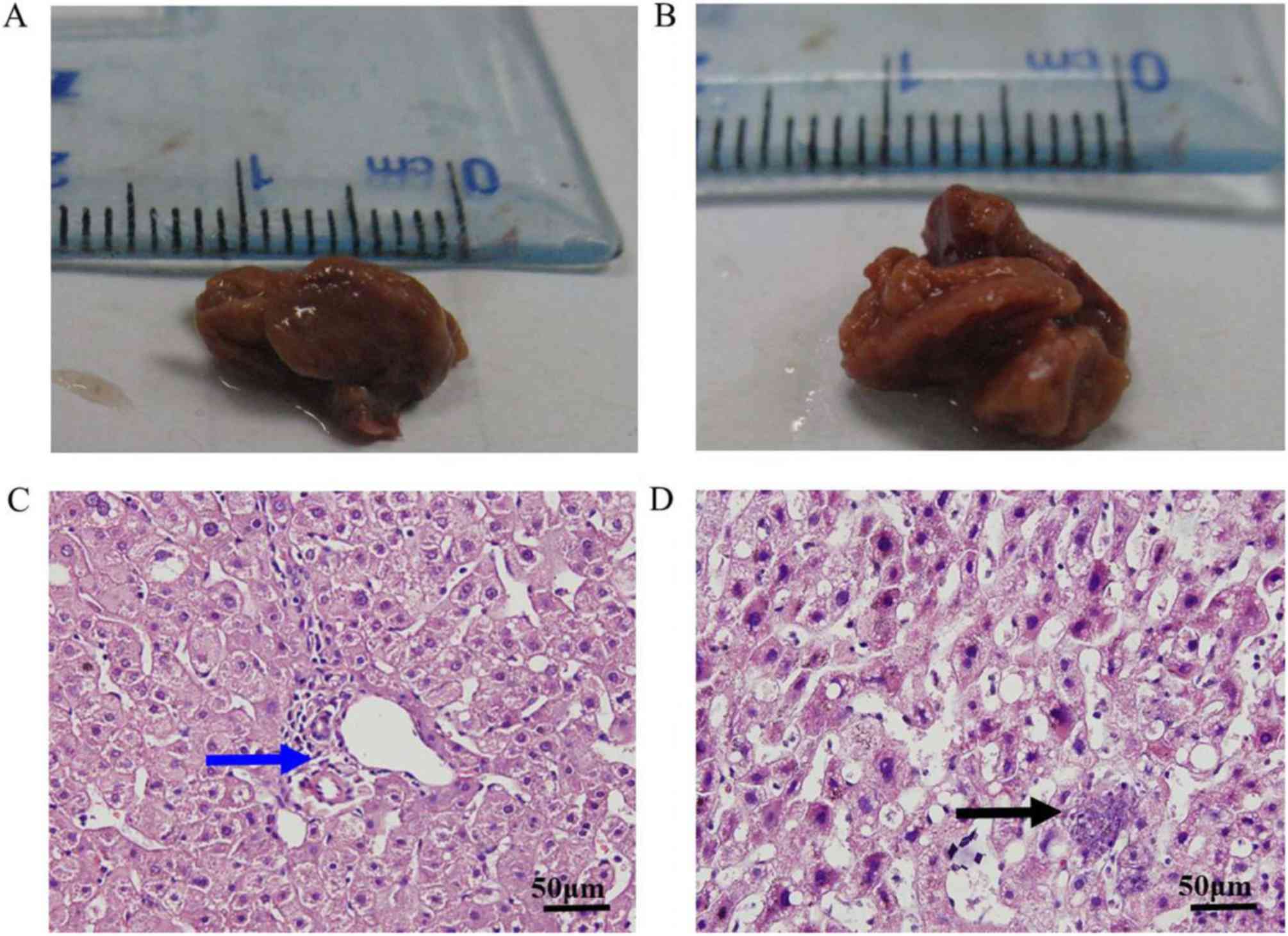
The mean tumor size was 4.36±1.38 cm. Images of the HCA and
adjacent liver tissues are presented in Fig. 1A and B. HCA tissues collected from
patients with adenomatous hyperplasia exhibited reddish brown
coloring similar to that of the adjacent liver tissue. Histological
examination of the HCA specimens revealed cords of hepatocytes,
similar in appearance to normal hepatic parenchyma but lacking
portal tracts, bile ducts and hepatic veins. Hepatocytes in the HCA
samples exhibited a polygonal shape, similar to that of hepatocytes
in adjacent liver tissues (Fig. 1C).
Excessive lipid accumulation and inflammatory cell infiltration was
observed in only the HCA tissues (Fig.
1D).
As presented in Fig. 2A
and B, HCA and adjacent liver tissues stained positively with
PAS, indicating the presence of glycogen. Stained hepatocytes in
adjacent liver tissues revealed homogeneous intensity, whereas in
HCA tissues, the distribution of stained cells was heterogeneous
with varying intensity.
Hepatic function of HCA tissue
To assess the hepatic function of HCA tissue, the
expression of ALB, CK18 and CYP2E1 was examined by IHC and compared
with normal liver tissues (Fig. 3).
HCA and adjacent liver tissues exhibited homogeneous cytoplasmic
staining for ALB, CK18 and CYP2E1. The quantitative results in
Table III reveal that resected
specimens from four of the eight HCA cases (50%) exhibited strong
ALB staining and the other four cases exhibited slight-to-medium
ALB staining. All HCA specimens exhibited medium-to-strong CK18
staining. Following CYP2E1 staining, four of eight HCA specimens
(50%) exhibited medium positive staining, whereas the other half
exhibited slight positive staining. Statistical analysis revealed
that the expression of ALB, CK18 and CYP2E1 in HCA samples did not
differ significantly from that of the normal liver tissues
(P=0.574; 0.442; 0.721, respectively).
 | Table III.Clinical diagnostic scores based on
immunohistochemical staining. |
Table III.
Clinical diagnostic scores based on
immunohistochemical staining.
| Protein | Tissues | − | + | ++ | +++ | P-value |
|---|
| ALB | Adjacent liver
tissues | 0 | 0 | 3 | 5 | 0.574 |
|
| Hepatocellular
adenoma | 0 | 1 | 3 | 4 |
|
| P450 | Adjacent liver
tissues | 0 | 2 | 6 | 0 | 0.442 |
|
| Hepatocellular
adenoma | 0 | 4 | 4 | 0 |
|
| CK18 | Adjacent liver
tissues | 0 | 0 | 3 | 5 | 0.721 |
|
| Hepatocellular
adenoma | 0 | 0 | 2 | 6 |
|
Based on RT-qPCR analysis, the expression of
synthetic function-specific genes [ALB, transthyretin (TTR),
α-1-antitrypsin (AAT) and transferrin (TF)] were similar or higher
in the HCA group compared with those in adjacent liver tissues
(Fig. 4). Similar results were
observed for genes associated with detoxification including CYP2E1,
CYP3A5 and CYP3A4. In addition, the expressions of genes related to
metabolism and conversion function including glutathione
S-transferase-α (GST-α), carbamoyl-phosphate synthase 1 (CPS-1),
tyrosine aminotransferase (TAT) and glutathione S-transferase-π
(GST-π) demonstrated relatively large differences between groups.
However, only the expression of GST-π demonstrated statistical
difference between groups, and was decreased in HCA tissues
compared with adjacent liver tissues (0.38 vs. 0.53; P=0.032),
likely due to the limited sample quantity. For liver phenotype
markers, CK18 and hepatocyte nuclear factor 1α were expressed at
similar levels in HCA and normal tissues, whereas α fetoprotein
(AFP) expression was increased in HCA tissues compared with the
control tissues (0.013 vs. 0.008; P=0.047). These results are in
agreement with those of IHC staining and the subsequent scoring
analysis.
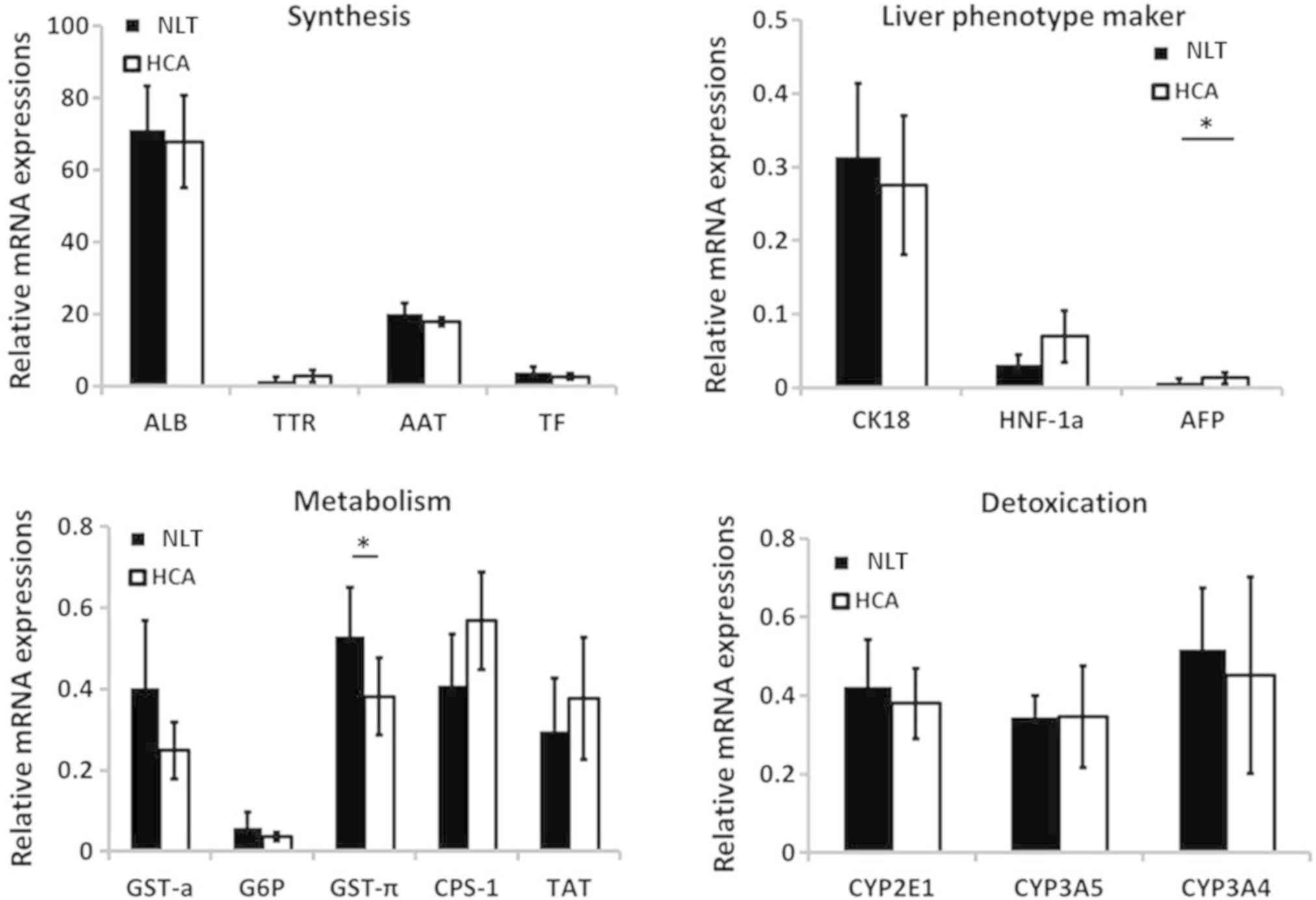 | Figure 4.Comparison of hepatic
function-specific gene expression between HCA and adjacent liver
tissues. Most gene expression levels in the HCA group were
comparable with those in the control group except the expression of
GST-π and AFP. *P<0.05. HCA, hepatocellular adenoma; ALB, α
fetoprotein; NLT, normal liver tissue; TTR, transthyretin; AAT,
α-1-antitrypsin; TF, transferrin; HNF-1α, hepatocyte nuclear factor
1 homeobox A; G6P, glucose-6-phosphate; CPS-1, carbamoyl-phosphate
synthase 1; TAT, tyrosine aminotransferase; CYP, cytochrome. |
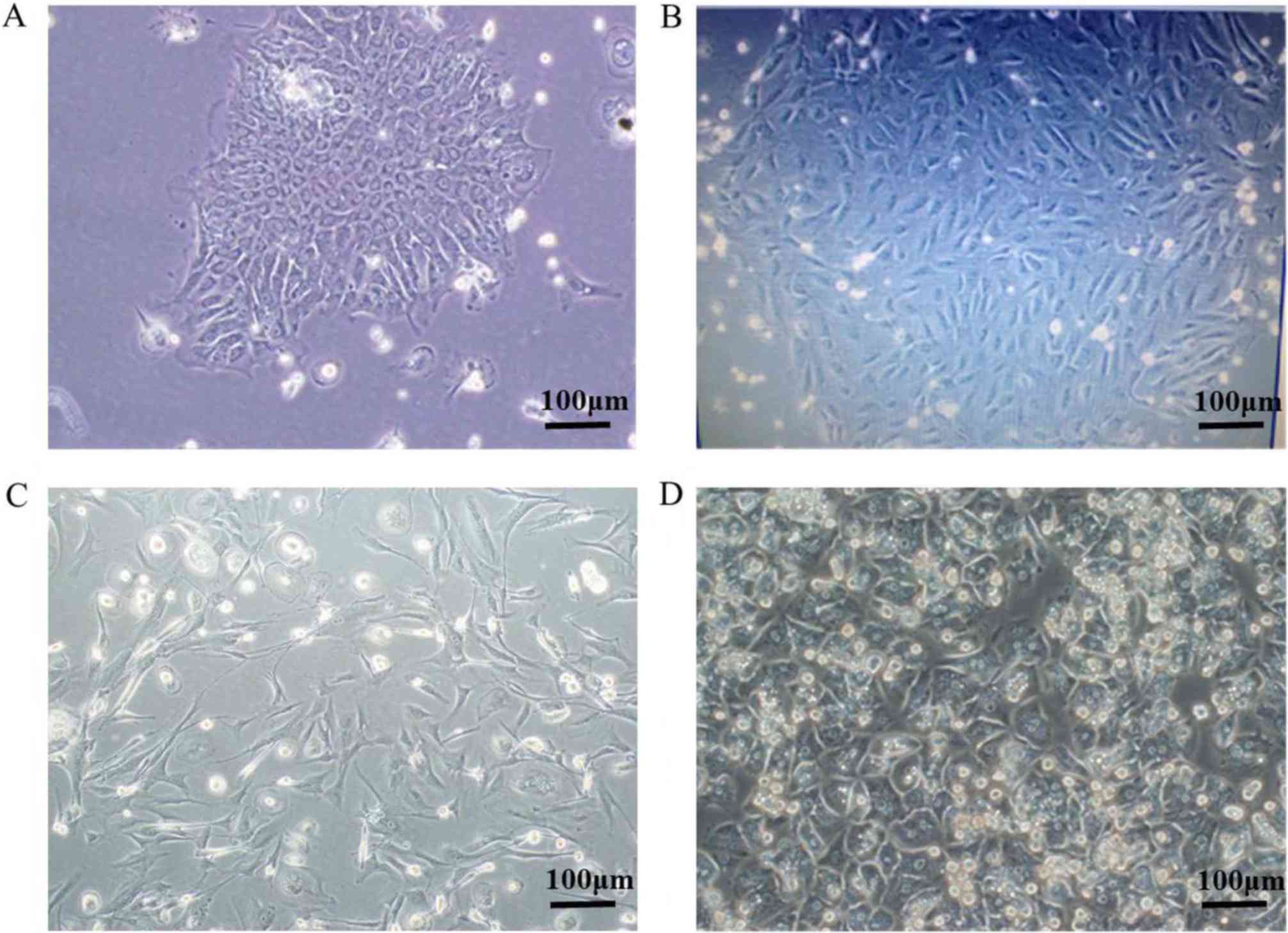
Isolation and culture of primary HCA
cells
Based on the aforementioned function tests, the
current study attempted to isolate primary cells from HCA via
two-step collagenase perfusion. Most of the collected tissues,
including adjacent normal and HCA tissues, were subjected to cell
isolation. All normal cells and most of the isolated HCA primary
cells did not proliferate in vitro and underwent apoptosis
within several weeks (data not shown). A few HCA primary cells
could be passaged 2–5 times. As a result, the current study
hypothesizes that HCA primary cells may be more proliferative than
normal cells. In Fig. 5, micrographs
demonstrate the most proliferative isolated HCA primary cells
following >10 passages that were isolated from a 22-year-old
female patient pathologically diagnosed with HCA. Initially, the
isolated primary hepatocytes grew slowly (Fig. 5A and B). After several weeks, most
primary cells underwent apoptosis, while a continuously
proliferating cell colony was observed (data not shown). The cells
individually grew into cell colonies and the colonies were further
picked by trypsin digestion for amplification due to the higher
proliferative potential. The isolated HCA cells gradually gained
active proliferative capacity and exhibited spindle cell morphology
(Fig. 5C). These cells appeared to
be smaller in size than normal hepatocytes after adhesion in
vitro (Fig. 5D).
Discussion
HCA is a rare monoclonal benign liver tumor, which
originates in the epithelium and usually develops within a healthy
liver (18). HCA is more prevalent
in females than in males and the use of oral contraceptives and
anabolic steroids are established risk factors for the disease
(19). Recent research has focused
on the etiology, clinical manifestations and diagnosis of HCA
(19). However, its functionality as
a liver-derived tumor is rarely characterized. In the present
study, the hepatic function of resected HCA tissues was assessed
and the feasibility of HCA-derived cells as cellular components of
BAL systems was determined.
The liver is a vital organ with a variety of
important functions in the human body including detoxification,
amino acid and lipid metabolism, protein and urea synthesis, and
waste removal (20). Liver failure
(acute or chronic) significantly compromises the aforementioned
functions, resulting in a high morbidity and mortality. BAL systems
aim to serve as a potential supportive therapy for patients with
liver failure that are waiting for or recovering from
transplantation (7). However, the
development of a BAL has been impeded by the scarcity of suitable
biological material (21). In the
present study, the feasibility of HCA tissue as a cellular source
for BAL systems was assessed. The results demonstrated a greater
prevalence of HCA in females compared with males, which is
consistent with results of previous reports (22). Histological examination demonstrated
that hepatocytes in HCA tissue exhibited no obvious atypia and
possessed similar morphology to that of hepatocytes in healthy
liver tissues. Histological analysis therefore revealed that HCA
tissues may function in a similar manner to healthy liver
tissues.
As a crucial component of BAL, many tumor-derived
human cell lines have been studied as potential biological material
for BAL models. The C3A cell line, as a clonal derivative of HepG2
cells, has been used in the extracorporeal liver assist device
system to treat patients with ALF (23). However, no significant effect on
survival was observed due to poor ammonia detoxification and mixed
function oxidase activity (24). The
HepaRG cell line has been reported to be a suitable model for
primary human hepatocytes with respect to retaining various hepatic
functions following culture for a 14-day proliferation phase in
HepaRG medium then a 14-day differentiation phase with 2%
dimethylsulfoxide (DMSO). In 2011, Hoekstra and Chamuleau (25) alluded to the potential of these cells
in BAL, based on their high metabolic and synthetic functionality,
but no further clinical research involving these cells has been
reported. Until now, to the best of our knowledge, no human cell
lines have been demonstrated to be suitable for application in BAL,
primarily due to insufficient functionality.
HCA is a benign liver tumor and its monoclonal
properties may facilitate cell lineage establishment and storage.
In the current study, experiments were performed to determine
whether HCA has potential to be a cell source for BAL development.
The expression of genes involved in various hepatic functions were
analyzed using PAS staining, IHC and RT-qPCR in HCA tissues and
corresponding adjacent liver tissues. Importantly, the expression
of synthetic function-specific genes (ALB, TTR, AAT and TF),
detoxification-related genes (CYT2E1, CYT3A5 and CYT3A4), as well
as the genes related to metabolism and conversion (GST-α, CPS-1,
TAT) demonstrated no statistical difference between HCA and
adjacent liver tissues, although a larger sample size is needed for
confirmation of results. In addition, according to RT-qPCR data,
AFP expression was increased in HCA compared with healthy liver
tissues. Notably, the expression of AFP in HCA and normal tissues
were very low and considerably less than that observed in HCC
tissues in a previous study by the current authors (data not
published). Exploration of the functionality of HCA tissues
provides early evidence of this potential, indicating that further
research into the possibility of using these tissues as a source
for the biological component of BAL is warranted. However, the
current study is preliminary and future research should focus on
the establishment of an HCA cell line and confirmation of its
functional abilities.
In addition to functionality, the proliferative
capability of cells is also crucial for their application in a BAL
system in clinical practice. For BAL development, primary human
hepatocytes are an ideal source of biological material due to their
high level of liver function. However, 10 billion fully functional
primary hepatocytes are required for each BAL and primary cells are
scarce and cannot be efficiently expanded in vitro (26). Therefore, a cell line with the
functionality of primary hepatocytes is desired. In the present
study, isolating primary cells from HCA tissues proliferated for
weeks in vitro. Given the monoclonal characteristics of HCA,
the superiority in the proliferative ability of HCA-derived cells
and the greater potential for cell line preservation compared with
normal hepatocytes, HCA tissues may be a promising alternative to
human hepatocytes as a source of the cellular component of BAL
systems (13). It is possible that
cells with higher proliferative potential were deliberately
selected and that these cells exhibited more pronounced
characteristics of cancer than the non-selected cells. Mutation of
proliferation-associated genes may have occurred in the active cell
colony. Thus, the safety issues involved in the use of HCA remain
unclear which is the limitation of the current study. As a result,
safety evaluation should be performed in future study after the
cell lineage is established.
With their superior proliferative ability and
comparable liver-specific function relative to primary hepatocytes,
HCA-derived cells represent a potentially ideal cell source for a
BAL. Future work will focus on cell lineage establishment.
Furthermore, the safety issues regarding the use of HCA remain
unclear and require more extensive and specific investigation.
Acknowledgments
Not applicable.
Funding
The current study was supported by the Science and
Technology Planning Project of Guangdong Province (grant no.
2014A050503041), the Science and Technology Planning Project of
Guangzhou (grant no. 2014J4500013), and the Guangdong Provincial
Bioengineering Research Center for Gastroenterology Diseases (grant
no. 2017B02029003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AL conceived the current study and provided
technical support. QY and XZ performed the experiments. QY and LD
collected the specimens. YF collected the data and performed
statistical analysis. QY and JL drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The Medical Ethics Committee of Nanfang Hospital,
Southern Medical University (Guangzhou, China) approved all of the
procedures performed in this study. All enrolled subjects provided
their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Demetriou AA: Support of the acutely
failing liver: State of the art. Ann Surg. 228:14–15. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leventhal TM and Liu KD: What a
nephrologist needs to know about acute liver failure. Adv Chronic
Kidney Dis. 22:376–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernal W, Hyyrylainen A, Gera A,
Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O'Grady
JG, Wendon J and Williams R: Lessons from look-back in acute liver
failure? A single centre experience of 3300 patients. J Hepatol.
59:74–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu X, Yang T, Li C, Zhang L, Li M, Huang W
and Zhou P: Human fetal hepatocyte line, L-02, exhibits good liver
function in vitro and in an acute liver failure model. Transplant
Proc. 45:695–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guoliang L, Anye Z, Lifu Z, Xiaoping P,
Yimin Z, Chengbo Y, Yuemei C and Lanjuan L: Effects of plasma from
acute-on-chronic liver failure patients on immortalized human
hepatocytes in vitro. Hepatogastroenterology. 58:1328–1333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Punzalan CS and Barry CT: Acute liver
failure: Diagnosis and management. J Intensive Care Med.
31:642–653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wu Q, Wang Y, Weng C, He Y, Gao M,
Yang G, Li L, Chen F, Shi Y, et al: Novel spheroid reservoir
bioartificial liver improves survival of nonhuman primates in a
toxin-induced model of acute liver failure. Theranostics.
8:5562–5574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Filippi C, Keatch SA, Rangar D, Nelson LJ,
Hayes PC and Plevris JN: Improvement of C3A cell metabolism for
usage in bioartificial liver support systems. J Hepatol.
41:599–605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonavita AG, Quaresma K, Cotta-de-Almeida
V, Pinto MA, Saraiva RM and Alves LA: Hepatocyte
xenotransplantation for treating liver disease.
Xenotransplantation. 17:181–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demetriou AA, Brown RS Jr, Busuttil RW,
Fair J, McGuire BM, Rosenthal P, Am Esch JS II, Lerut J, Nyberg SL,
Salizzoni M, et al: Prospective, randomized, multicenter,
controlled trial of a bioartificial liver in treating acute liver
failure. Ann Surg. 239:660–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi XL, Han B, Tan JJ, Yuan X, Zhang Y,
Xiao JQ, Gu ZZ and Ding YT: Factors influencing the transfer of
porcine endogenous retroviruses across the membrane in
bioartificial livers. Int J Artif Organs. 35:385–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farges O, Ferreira N, Dokmak S, Belghiti
J, Bedossa P and Paradis V: Changing trends in malignant
transformation of hepatocellular adenoma. Gut. 60:85–89. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gouw AS, Zeng W, Buiskool M, Platteel I,
van den Heuvel MC, Poppema S, de Jong KP and Molema G: Molecular
characterization of the vascular features of focal nodular
hyperplasia and hepatocellular adenoma: A role for angiopoietin-1.
Hepatology. 52:540–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji C, Annabi N, Khademhosseini A and
Dehghani F: Fabrication of porous chitosan scaffolds for soft
tissue engineering using dense gas CO2. Acta Biomater. 7:1653–1664.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunstman JW, Korah R, Healy JM, Prasad M
and Carling T: Quantitative assessment of RASSF1A methylation as a
putative molecular marker in papillary thyroid carcinoma. Surgery.
154:1255–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Selden C, Chalmers SA, Jones C, Standish
R, Quaglia A, Rolando N, Burroughs AK, Rolles K, Dhillon A and
Hodgson HJ: Epithelial colonies cultured from human explanted liver
in subacute hepatic failure exhibit hepatocyte, biliary epithelial,
and stem cell phenotypic markers. Stem Cells. 21:624–631. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tonorezos ES, Barnea D, Abou-Alfa GK,
Bromberg J, D'Angelica M, Sklar CA, Shia J and Oeffinger KC:
Hepatocellular adenoma among adult survivors of childhood and young
adult cancer. Pediatr Blood Cancer. 64:e262942017. View Article : Google Scholar
|
|
19
|
Vijay A, Elaffandi A and Khalaf H:
Hepatocellular adenoma: An update. World J Hepatol. 7:2603–2609.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SY, Kim HJ and Choi D: Cell sources,
liver support systems and liver tissue engineering: Alternatives to
liver transplantation. Int J Stem Cells. 8:36–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kandiah PA and Subramanian RM:
Extracorporeal devices. Crit Care Clin. 35:135–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khanna M, Ramanathan S, Fasih N, Schieda
N, Virmani V and McInnes MD: Current updates on the molecular
genetics and magnetic resonance imaging of focal nodular
hyperplasia and hepatocellular adenoma. Insights Imaging.
6:347–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellis AJ, Hughes RD, Wendon JA, Dunne J,
Langley PG, Kelly JH, Gislason GT, Sussman NL and Williams R:
Pilot-controlled trial of the extracorporeal liver assist device in
acute liver failure. Hepatology. 24:1446–1451. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chamuleau RA, Deurholt T and Hoekstra R:
Which are the right cells to be used in a bioartificial liver?
Metab Brain Dis. 20:327–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoekstra R and Chamuleau RA: Recent
developments on human cell lines for the bioartificial liver. Int J
Artif Organs. 25:182–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoekstra R, Nibourg GA, van der Hoeven TV,
Ackermans MT, Hakvoort TB, van Gulik TM, Lamers WH, Elferink RP and
Chamuleau RA: The HepaRG cell line is suitable for bioartificial
liver application. Int J Biochem Cell Biol. 43:1483–1489. 2011.
View Article : Google Scholar : PubMed/NCBI
|