Introduction
According to Chinese epidemiological studies in
2012, the morbidity of chronic kidney disease (CKD) in China is
10.0% and ~36.4% of the population have been diagnosed with
diabetes (1). Research indicates
that diabetes is an independent risk factor of CKD (1). According to the World Health
Organization evaluation data, the population in China includes a
total of 0.114 billion diabetics and 0.493 pre-diabetics (2). Importantly, the diabetic population is
estimated to increase to 0.13 billion by 2025 (2). Diabetic nephropathy (DN) is a major
complication of diabetes, which is also a leading cause of
end-stage renal disease (ESRD) and dialysis in Western countries
(3). The national dialysis
registration quality control data (2012) in China indicate that
18.4% of new hemodialysis patients have DN. Furthermore, a total of
17.5% of the new peritoneal dialysis patients have DN. Notably, DN
has become the second leading cause of ESRD and dialysis in China
(3).
Numerous clinical studies have indicated that
peroxisome proliferator-activated receptor (PPAR)-γ receptor
agonist has favorable renal protective effects (4). PPAR-γ is extensively applied during the
treatment of diabetes. Notably, PPARs are nuclear hormone receptor
superfamily members (5) and nuclear
transcription factors, which are activated by ligands. There are
three major subtypes, namely, α, β and γ. Evidence indicates that
PPAR-γ is widely expressed in glomerulus, renal proximal convoluted
tubule, renal fibroblast and renal collecting tubule (6). Furthermore, research on the role of
PPAR-γ in DN has attracted much attention in previous years
(6).
Trigonelline is a major alkaloid isolated from the
dry seed of Trigonella foenum-graecum L (7). It has been demonstrated to possess
hypoglycemic, cholesterol-lowering, neural regeneration-promoting,
anticancer and sedation effects (8).
Notably, trigonelline can suppress β-amyloid protein in a
dose-dependent manner (9).
Furthermore, trigonelline has been demonstrated to induce neuron
dendrite and axon atrophy, promote cerebral cortical neuron
dendrite and axon regeneration (9),
therefore protecting the central nervous system neuron (8). Zhou et al (8) suggested that trigonelline inhibits
inflammation to prevent fetal growth restriction during pregnancy
in diabetes. Furthermore, Ghule et al (10) indicated that trigonelline ameliorates
diabetic hypertensive nephropathy. These findings suggest that
trigonelline may be able to protect the central nervous system
neurons.
Materials and methods
Experimental animals and induction of
diabetes in rats
A total of 22 male Sprague-Dawley rats aged 5–6
weeks old (150–170 g) were housed in a temperature-controlled
environment (temperature, 22–2°C; humidity, 55–5%) with a 12-h
light/dark cycle and ad libitum access to food and water.
All rats were randomly divided into three groups: Control (n=6),
type 2 diabetes mellitus (T2DM) model (n=8) and trigonelline (n=8)
groups. In T2DM model or trigonelline groups, rats were induced
with T2DM. T2DM was induced in rats by feeding a high-fat diet
(HFD, TP 28708, Trophi Feed High-tech Co., Ltd., Nantong, China)
for 4 weeks. The T2DM model was confirmed when serum glucose were
>16.7 mM. Subsequently, rats were intraperitoneally injected
with 35 mg/kg of streptozotocin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 4 weeks and were also fed a HFD during
these additional 4 weeks. In the control group, rats were fed a
standard diet for the total 8 weeks. The present study was approved
by the Clinical Research and Experimental Animal Ethics Committee
of the First Affiliated Hospital of China Medical University
(Shenyang, China). In the trigonelline group, rats were received
oral administration of 40 mg/kg/day of trigonelline (Sigma-Aldrich;
Merck KGaA) for 8-weeks.
Histopathological analysis of
animals
Following the administration of 35 mg/kg
pentobarbital sodium (IV), rats were sacrificed by decapitation and
the kidneys were collected. Samples were then washed with
phosphate-buffered saline (PBS) and fixed in 10% neutral-buffered
formalin for 24 h at room temperature. Kidney samples were
processed using a routine paraffin embedding technique and 5-µm
sections were prepared and stained with hematoxylin and eosin at
room temperature for 15 min. Sections were observed using an
inverted fluorescence microscope (magnification, ×100; Zeiss Axio
Observer A1; Carl Zeiss AG, Oberkochen, Germany).
ELISA assay
Blood urea nitrogen (BUN; cat. no. C013-2),
creatinine, albumin, interleukin (IL)-1β (cat. no. H002), IL-6
(cat. no. H007), IL-10 (cat. no. H009), IL-18 (cat. no. H015),
malondialdehyde (MDA; cat. no. A003-1), superoxide dismutase (SOD;
cat. no. A001-1-1), glutathione (GSH; cat. no. A006-2) and
glutathione peroxidase (GSH-Px; cat. no. A005) levels were measured
using ELISA kits (all from Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) following treatment with trigonelline.
Caspase-3/9 levels were measured using Caspase-3/9 levels kits
(cat. nos. C1116 and C1158, respectively; Beyotime Institute of
Biotechnology, Haimen, China).
Western blot analysis
A total of 50 mg of kidney tissue samples was
collected, washed with PBS and homogenized with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) for 30 min at 4°C. Samples were centrifuged at 8,000
× g for 10 min at 4°C and the protein concentrations in the
supernatants were quantified using a BCA assay. A total of 50 µg of
total protein was separated using 10% SDS-PAGE electrophoresis and
then transferred to nitro-cellulose membranes. Membranes were
blocked with 5% non-fat milk for 1 h at 37°C and probed with Bax
(cat. no. 5023; 1;2,000), p53 (cat. no. 2527; 1;2,000), glucose
transporter 4 (GLUT4) (cat. no. 2213; 1;1,000), PPAR-γ (cat. no.
2443; 1;1,000), leptin (cat. no. 12497; 1;1,000), tumor necrosis
factor (TNF)-α (cat. no. 11948; 1;1,000) and GAPDH antibodies (cat.
no. 51332; 1;5,000; all Cell Signaling Technology, Inc., Danvers,
MA, USA) at 4°C overnight. After washing with Tris-buffered saline
with Tween-20 for 15–20 min, membranes were probed with secondary
antibody labeled with horseradish peroxidase (7074, 7076, 1;5,000;
Cell Signaling Technology, Inc.) for 1 h at 37°C. The protein bands
were detected using enhanced chemiluminescent substrate (cat. no.
P0018A; Beyotime Institute of Biotechnology) and quantified using
Image Lab 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were demonstrated as the mean ± standard
deviation using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical analysis was performed using the Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Trigonelline increases body weight,
inhibits the kidney weight/body weight ratio and blood glucose
levels in T2DM rats
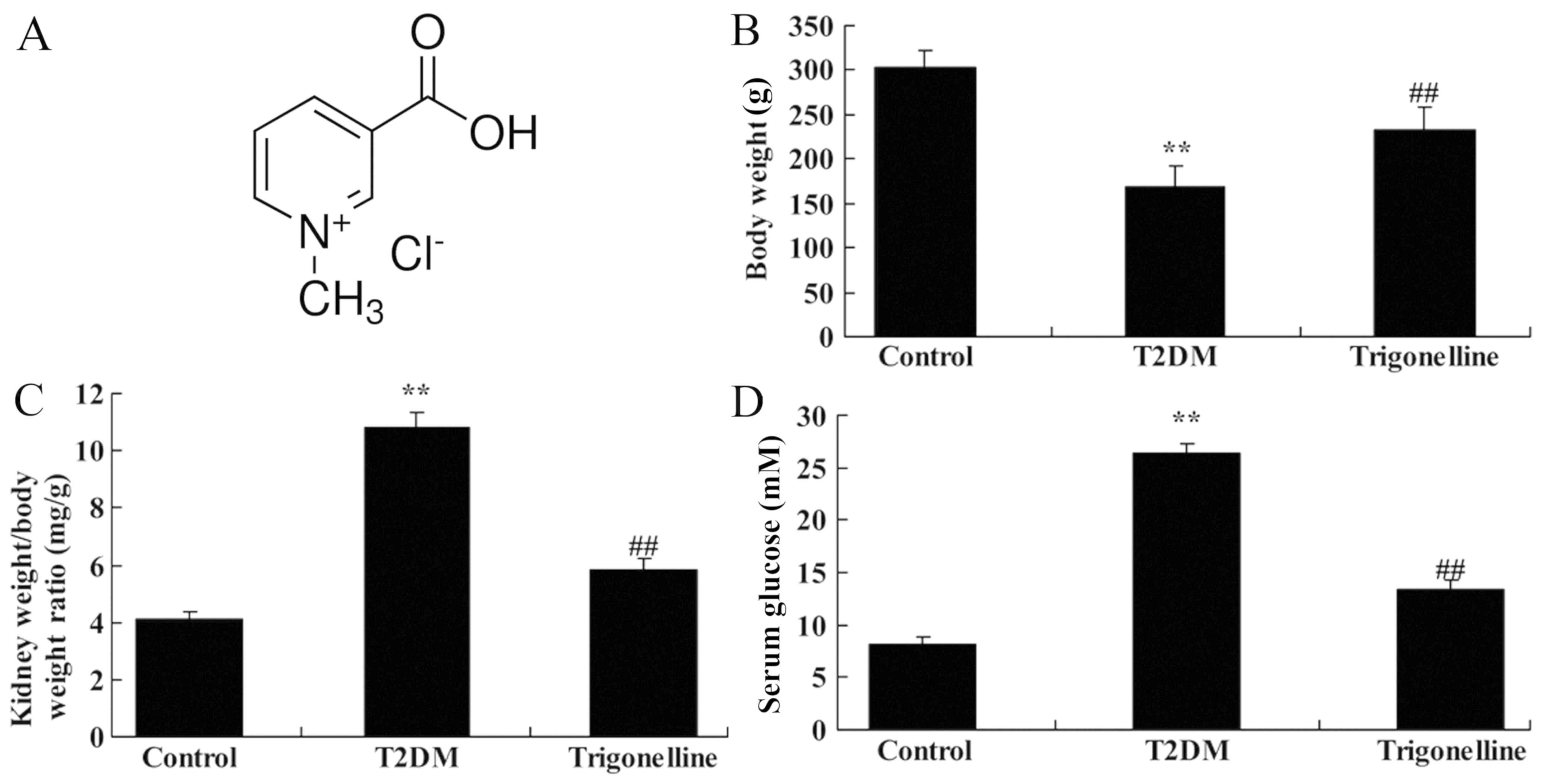
The chemical structure of trigonelline is presented
in Fig. 1A. As indicated in Fig. 1B-D, T2DM resulted in significantly
reduced body weight, elevated kidney weight/body weight ratio and
increased blood glucose compared with the control group. However,
oral administration of 40 mg/kg trigonelline in the trigonelline
group for 60 days led to significantly increased body weight,
suppressed kidney weight/body weight ratio and decreased blood
glucose levels compared with T2DM group (Fig. 1B-D).
Trigonelline reduces the levels of
BUN, creatinine and albumin in T2DM rats
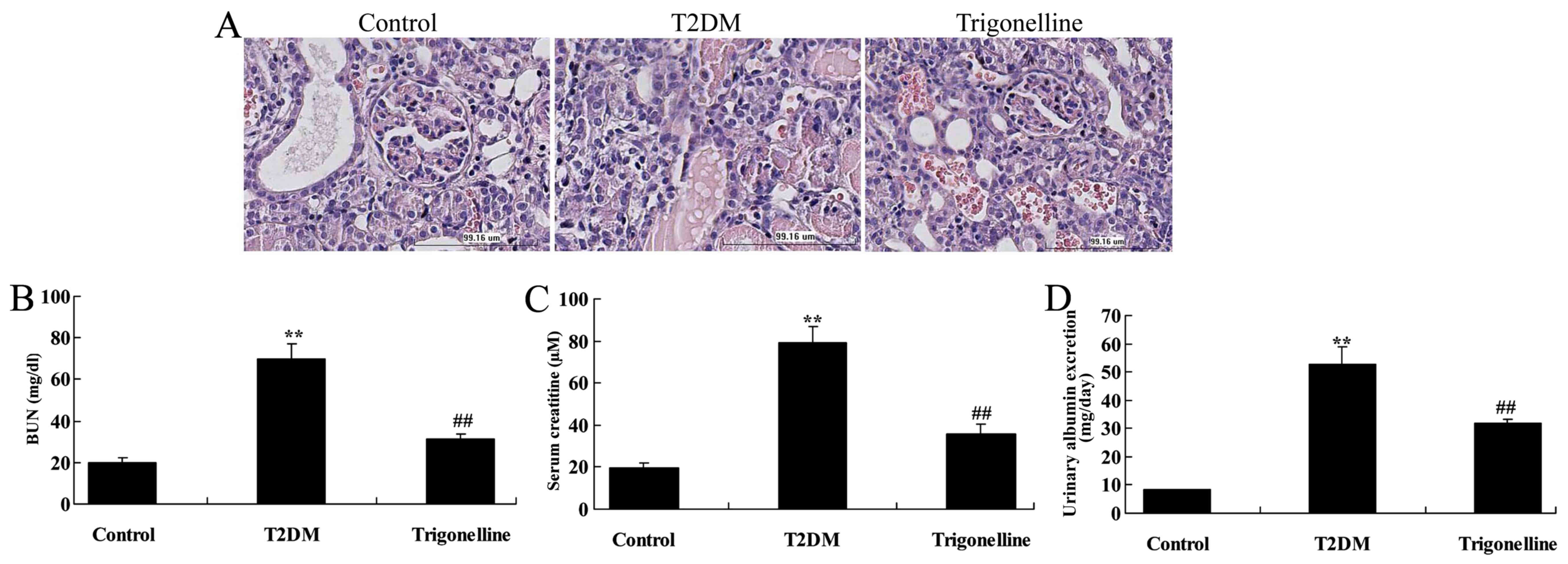
It was indicated that the glomerulus, BUN,
creatinine and albumin levels were increased in T2DM rats in
comparison with control rats (Fig.
2). However, treatment with trigonelline recovered the
glomerulus and reduced the levels of BUN, creatinine and albumin in
T2DM rats (Fig. 2).
Trigonelline reduces inflammation in
T2DM rats
Results indicated significantly increased levels of
IL-1β, IL-6 and IL-18 but suppressed IL-10 levels in T2DM rats
compared with control rats (Fig. 3).
However, trigonelline treatment resulted in significantly decreased
levels of IL-1β, IL-6 and IL-18 but increased IL-10 levels in T2DM
rats (Fig. 3).
Trigonelline reduces oxidative stress
in T2DM rats
Further analysis indicated significantly increased
MDA levels, decreased levels of SOD, GSH and GSH-Px in T2DM rats
compared with control rats (Fig. 4).
Trigonelline administration significantly reduced MDA levels and
promoted SOD, GSH and GSH-Px levels in T2DM rats (Fig. 4).
Trigonelline reduces cell apoptosis in
T2DM rats
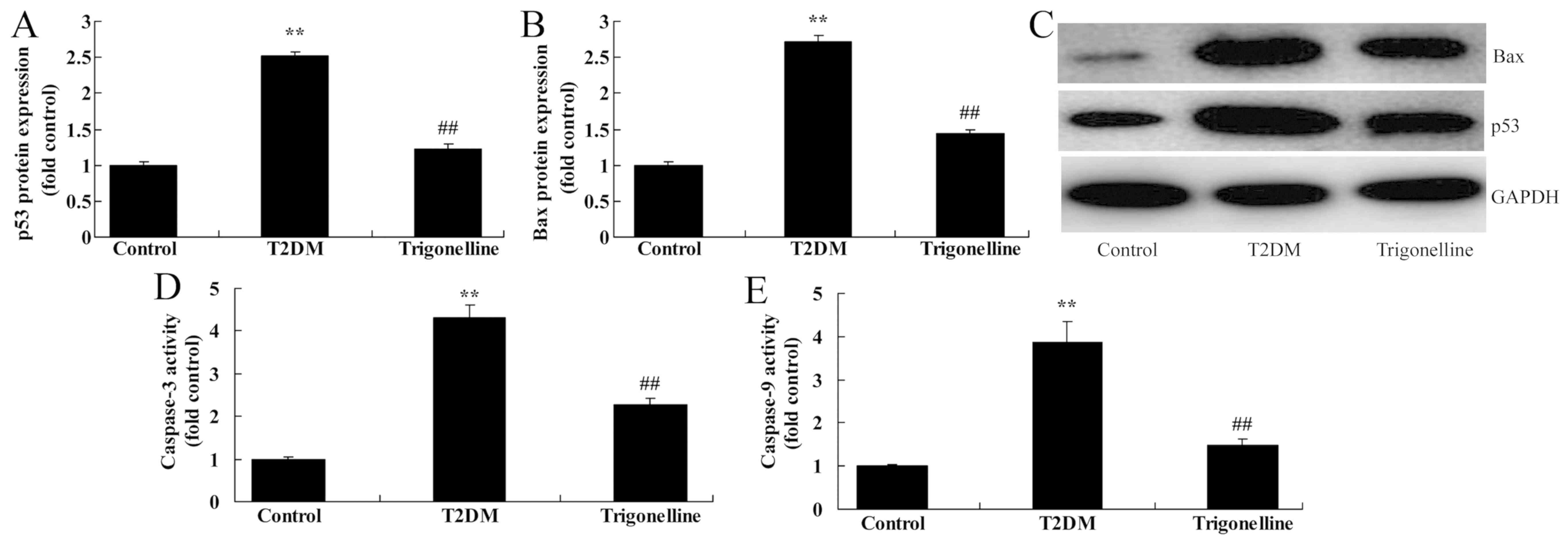
In order to investigate the mechanism of
trigonelline in T2DM rats, the protein expression levels of Bax and
p53 and the activity levels of caspase-3 and caspase-9 were
analyzed. As indicated in Fig. 5,
the protein expression levels of p53 and Bax and the activity
levels of caspase-3 and caspase-9 were significantly increased in
T2DM rats compared with control rats. Conversely, trigonelline
administration significantly suppressed the protein expression
levels of p53 and Bax and reduced the activity levels of caspase-3
and caspase-9 in T2DM rats (Fig.
5).
Trigonelline reduces insulin
resistance in T2DM rats
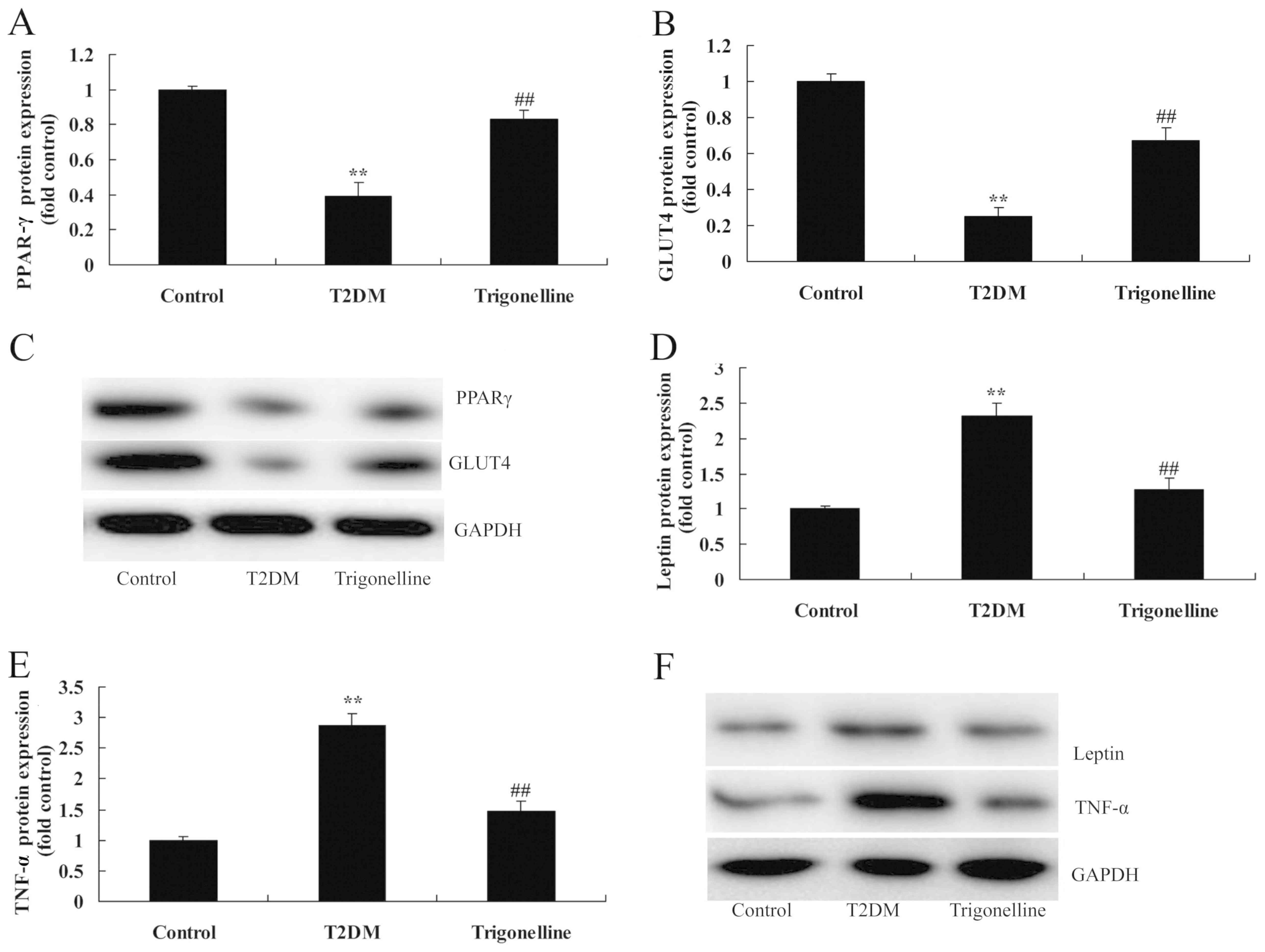
To further explore the mechanism of trigonelline in
T2DM rats, relevant proteins for insulin resistance were analyzed
in T2DM rats. As indicated in Fig.
6, the protein expression levels of GLUT4 and PPAR-γ were
significantly suppressed, whereas the protein expression levels of
leptin and TNF-α were increased in T2DM rats in comparison with
control rats (Fig. 6). However,
trigonelline significantly induced GLUT4 and PPAR-γ protein
expression, and suppressed leptin and TNF-α protein expression in
T2DM rats, compared with the T2DM model group (Fig. 6).
Discussion
Diabetes is a metabolic disease that severely
threatens human health. It can involve the whole body, leading to
lesions in all organs, including the retina and kidneys (11). In recent years, changes in lifestyle
have been witnessed (11).
Consequently, the morbidity of T2DM characterized by insulin
resistance is surging (11). DN has
become the most common cause of ESRD in developed countries,
including Japan and European and American countries (12). The results of the present study
demonstrated that trigonelline significantly increased the body
weight but decreased the kidney weight/body weight ratio and blood
glucose levels in T2DM rats.
Various studies suggest that insulin resistance
serves a vital role in the pathogenesis of diabetes (13,14). The
role of local insulin resistance at a cellular level in target
organ damage has received increased attention (13). Apart from classical insulin reactive
cells, including adipocytes, skeletal muscle cells and hepatocytes
(13), responses of cardiomyocytes,
vascular endothelial cells and other types of cells in the kidney
to insulin, as well as their influence, have become research
hotspots (14). Notably, T2DM has
also been indicated to be a chronic inflammatory disease (14). Numerous inflammatory factors,
including cytokines and adhesion factors, directly participate in
the development of insulin resistance and diabetic complications
(14). Therefore, determining the
effect and interaction mechanism of the two in DN damage is of
importance to clinical work. Additionally, studying the possible
mechanism of suppressing chronic inflammation to improve insulin
resistance and relieve renal damage is also of important scientific
and clinical value (14). The
results of the present study suggested that trigonelline
significantly reduced BUN, creatinine and albumin levels,
indicating reduced inflammation in T2DM rats. Furthermore,
Chowdhury et al (15)
demonstrated that trigonelline protects against oxidative stress
and proinflammatory cytokines in lipopolysaccharide-induced
cognitive impairment in adult mice.
The high glucose status in diabetics typically
induces glucose metabolic disorders, which results in the mass
production of reactive oxygen species (ROS) (16). In this way, ROS can promote
cross-linking polymerization of protein and nucleic acid. SOD, GSH
and GSH-PX are important antioxidases, which can effectively
eliminate ROS from the body to maintain the balance between
oxidation and anti-oxidation (17).
Thus, they can protect the cell from damage (16,17). In
the present study, it was demonstrated that trigonelline reduced
oxidative stress and cell apoptosis in T2DM rats. Notably, Afifi
et al (18) concluded that
trigonelline attenuates oxidative stress biomarkers in high-fat,
high-fructose diet-induced insulin resistance in rats.
Previous studies have demonstrated that PPAR-γ and
GLUT4 protein expression levels were downregulated at the time of
diabetes (19). Additionally,
upregulated PPAR-γ and GLUT4 protein expression levels are
associated with protective effects against DN (20). GLUT4 is a transmembrane transport
protein that promotes glucose transport to insulin-sensitive
tissues for intracellular utilization (19). In this way, it can maintain normal
physiological function of cells. PPAR-γ is a type of
ligand-activated nuclear transcription factor (21) that is associated with fat
differentiation, obesity and insulin resistance (22). Furthermore, PPAR-γ is the target
molecule of euglycemic agents, such as thiazolidnediones (22). The results of the present study
indicated that trigonelline increased GLUT4 and PPARγ protein
expression levels in T2DM rats. Tharaheswari et al (9) suggested that trigonelline and diosgenin
attenuated endoplasmic reticulum stress, oxidative stress-mediated
damage and PPAR-γ activity in T2DM rats.
TNF-α is an early reactive cytokine, which can
induce the downregulation or upregulation of other pro-inflammatory
and anti-inflammatory mediators at the early stage of injury
(23). In this way, TNF-α may induce
an imbalanced inflammatory response (24). Previous findings have indicated that
PPAR-γ participates in regulating the synthesis and secretion of
multiple inflammatory mediators (24). Particularly, PPAR-γ serves a vital
role in adipocyte differentiation, glucolipid metabolism and
insulin resistance (24). PPAR-γ is
a class of ligand-activated nuclear transcription factor that can
regulate the expression of multiple key genes during glucose and
lipid metabolism (25). Notably,
leptin is involved in regulating glucose and lipid metabolism in
the liver (25). Consequently,
leptin may enhance the synthesis of triglycerides and reduce the
production of hepatic glycogen (26). A previous study indicated that
adipocytes can secrete multiple lipid cytokines and protein
factors, among which, leptin, adiponectin, TNF-α and IL-6 are
well-known (27). These components
have been demonstrated to regulate the biological effect of insulin
in target tissues by means of endocrine, paracrine and autocrine
signaling (23). Importantly, they
may serve critical roles in DN (28). TNF-α is a multi-functional
inflammatory cytokine that has been associated with insulin levels,
glucose metabolism and lipid metabolism (29). Furthermore, overexpression of TNF-α
has been indicated in the lipid tissue of obese individuals
(29). Notably, TNF-α acts on the
peripheral target tissue and liver after it is released into the
blood and thereby induces DN (29).
Leptin is the product of the obese gene, which is a hormone
secreted by adipocytes and released into blood (27). Research has indicated that there is a
two-way regulation between leptin and insulin (27). In the present study, the results
indicated that trigonelline suppressed leptin/TNF-α protein
expression in T2DM rats. Zhou et al (8) suggested that trigonelline inhibits
inflammation and protects β cells to prevent fetal growth
restriction during pregnancy through leptin and insulin in
diabetes. Antonisamy et al (7) revealed that trigonelline protected
against indomethacin-induced gastric ulcers in rats through TNF-α.
The present study suggested that trigonelline may regulate
leptin/TNF-α to suppress insulin levels in T2DM rats.
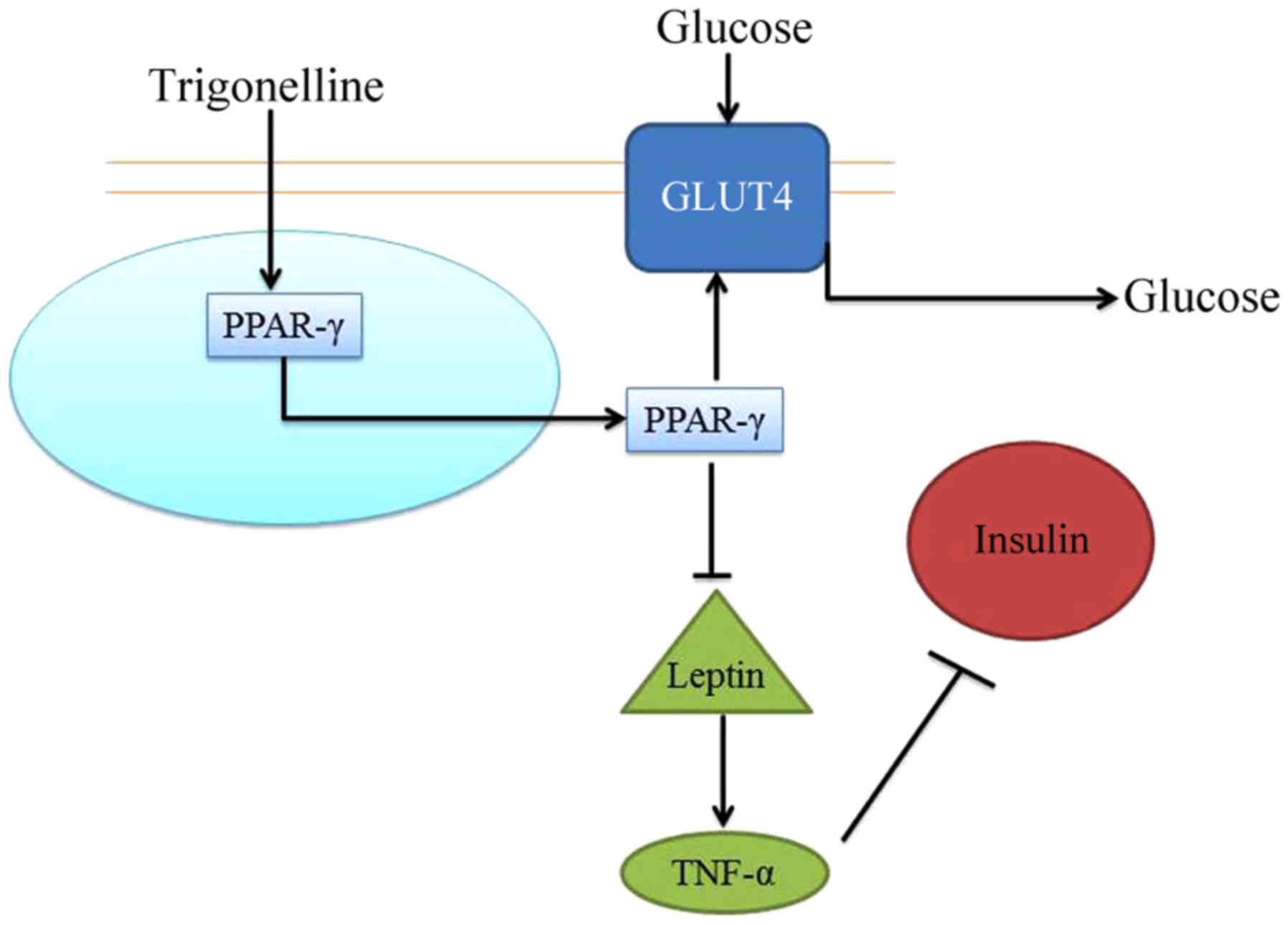
In conclusion, the present study demonstrated that
trigonelline increased the body weight, inhibited kidney
weight/body weight ratio and blood glucose levels in T2DM rats. In
addition, it was indicated that trigonelline may be associated with
antioxidative, anti-inflammatory and anti-apoptotic mechanisms. The
present findings suggest that trigonelline may reduce DN and
insulin resistance in T2DM rats through a PPAR-γ/GLUT4-leptin/TNF-α
signaling pathway (Fig. 7).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QiL designed the experiments; YL, QaL, CW and ZL
performed the experiments; QiL analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research and Experimental Animal Ethics Committee of the First
Affiliated Hospital of China Medical University (Shenyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayer MK, Edwards NC, Slinn G, Moody WE,
Steeds RP, Ferro CJ, Price AM, Andujar C, Dutton M, Webster R, et
al: A randomized, multicenter, open-label, blinded end point trial
comparing the effects of spironolactone to chlorthalidone on left
ventricular mass in patients with early-stage chronic kidney
disease: Rationale and design of the SPIRO-CKD trial. Am Heart J.
191:37–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouchi R, Nakano Y, Fukuda T, Takeuchi T,
Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T and
Ogawa Y: Reduction of visceral fat by liraglutide is associated
with ameliorations of hepatic steatosis, albuminuria, and
micro-inflammation in type 2 diabetic patients with insulin
treatment: A randomized control trial. Endocr J. 64:269–281. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura T, Sato E, Amaha M, Kawagoe Y,
Maeda S and Yamagishi S: Addition of aliskiren to angiotensin II
receptor blockers ameliorates renal tubular injury and reduces
intima media thickness of carotid artery in patients with diabetic
nephropathy. Int J Cardiol. 155:294–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandhi GR, Jothi G, Antony PJ, Balakrishna
K, Paulraj MG, Ignacimuthu S, Stalin A and Al-Dhabi NA: Gallic acid
attenuates high-fat diet fed-streptozotocin-induced insulin
resistance via partial agonism of PPARγ in experimental type 2
diabetic rats and enhances glucose uptake through translocation and
activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J
Pharmacol. 745:201–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukahara R, Haniu H, Matsuda Y and
Tsukahara T: The AGP-PPARγ axis promotes oxidative stress and
diabetic endothelial cell dysfunction. Mol Cell Endocrinol.
473:100–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu H, Desvergne B, Ferrari S and Bonnet N:
Impaired musculoskeletal response to age and exercise in PPARβ(−/-)
diabetic mice. Endocrinology. 155:4686–4696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antonisamy P, Arasu MV, Dhanasekaran M,
Choi KC, Aravinthan A, Kim NS, Kang CW and Kim JH: Protective
effects of trigonelline against indomethacin-induced gastric ulcer
in rats and potential underlying mechanisms. Food Funct. 7:398–408.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou JY, Du XH, Zhang Z and Qian GS:
Trigonelline inhibits inflammation and protects β cells to prevent
fetal growth restriction during pregnancy in a mouse model of
diabetes. Pharmacology. 100:209–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tharaheswari M, Jayachandra Reddy N, Kumar
R, Varshney KC, Kannan M and Sudha Rani S: Trigonelline and
diosgenin attenuate ER stress, oxidative stress-mediated damage in
pancreas and enhance adipose tissue PPARγ activity in type 2
diabetic rats. Mol Cell Biochem. 396:161–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghule AE, Jadhav SS and Bodhankar SL:
Trigonelline ameliorates diabetic hypertensive nephropathy by
suppression of oxidative stress in kidney and reduction in renal
cell apoptosis and fibrosis in streptozotocin induced neonatal
diabetic (nSTZ) rats. Int Immunopharmacol. 14:740–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lindhardt M, Persson F, Currie G, Pontillo
C, Beige J, Delles C, von der Leyen H, Mischak H, Navis G, Noutsou
M, et al: Proteomic prediction and Renin angiotensin aldosterone
system Inhibition prevention Of early diabetic nephRopathy in TYpe
2 diabetic patients with normoalbuminuria (PRIORITY): essential
study design and rationale of a randomised clinical multicentre
trial. BMJ Open. 6:e0103102016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai E, Chan JC, Ito S, Yamasaki T,
Kobayashi F, Haneda M and Makino H; ORIENT study investigators, :
Effects of olmesartan on renal and cardiovascular outcomes in type
2 diabetes with overt nephropathy: A multicentre, randomised,
placebo-controlled study. Diabetologia. 54:2978–2986. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tziastoudi M, Stefanidis I, Hadjigeorgiou
GM, Stravodimos K and Zintzaras E: A systematic review and
meta-analysis of genetic association studies for the role of
inflammation and the immune system in diabetic nephropathy. Clin
Kidney J. 10:293–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Liang Y, Hu T, Wei R, Cai C, Wang
P, Wang L, Qiao W and Feng L: Endogenous Nampt upregulation is
associated with diabetic nephropathy inflammatory-fibrosis through
the NF-κB p65 and Sirt1 pathway; NMN alleviates diabetic
nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt.
Exp Ther Med. 14:4181–4193. 2017.PubMed/NCBI
|
|
15
|
Chowdhury AA, Gawali NB, Munshi R and
Juvekar AR: Trigonelline insulates against oxidative stress,
proinflammatory cytokines and restores BDNF levels in
lipopolysaccharide induced cognitive impairment in adult mice.
Metab Brain Dis. 33:681–691. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suresha BS and Srinivasan K: Fungal
metabolite nigerloxin ameliorates diabetic nephropathy and
gentamicin-induced renal oxidative stress in experimental rats.
Naunyn Schmiedebergs Arch Pharmacol. 387:849–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Volpe CMO, Villar-Delfino PH, Dos Anjos
PMF and Nogueira-Machado JA: Cellular death, reactive oxygen
species (ROS) and diabetic complications. Cell Death Dis.
9:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Afifi NA, Ramadan A, Erian EY, Saleh DO,
Sedik AA, Badawi M and El Hotaby W: Trigonelline attenuates hepatic
complications and molecular alterations in high-fat high-fructose
diet-induced insulin resistance in rats. Can J Physiol Pharmacol.
95:427–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irudayaraj SS, Stalin A, Sunil C,
Duraipandiyan V, Al-Dhabi NA and Ignacimuthu S: Antioxidant,
antilipidemic and antidiabetic effects of ficusin with their
effects on GLUT4 translocation and PPARγ expression in type 2
diabetic rats. Chem Biol Interact. 256:85–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Z, Wan J, Hou X, Geng J, Li X and Bai
X: MicroRNA-27a promotes podocyte injury via PPARγ-mediated
β-catenin activation in diabetic nephropathy. Cell Death Dis.
8:e26582017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wasik AA, Dumont V, Tienari J, Nyman TA,
Fogarty CL, Forsblom C, Lehto M, Lehtonen E, Groop PH and Lehtonen
S: Septin 7 reduces nonmuscle myosin IIA activity in the SNAP23
complex and hinders GLUT4 storage vesicle docking and fusion. Exp
Cell Res. 350:336–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ge J, Miao JJ, Sun XY and Yu JY: Huangkui
capsule, an extract from Abelmoschus manihot (L.) medic,
improves diabetic nephropathy via activating peroxisome
proliferator-activated receptor (PPAR)-α/γ and attenuating
endoplasmic reticulum stress in rats. J Ethnopharmacol.
189:238–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Umapathy D, Krishnamoorthy E,
Mariappanadar V, Viswanathan V and Ramkumar KM: Increased levels of
circulating (TNF-α) is associated with (−308G/A) promoter
polymorphism of TNF-α gene in Diabetic Nephropathy. Int J Biol
Macromol 107B. 2113–2121. 2018. View Article : Google Scholar
|
|
24
|
Jia Z, Xinhua X, Qian Z, Miao Y, Jianping
X, Zhixin W, Yijing L and Mingmin L: PPARγ links maternal
malnutrition and abnormal glucose and lipid metabolism in the
offspring of mice. Yi Chuan. 37:70–76. 2015.PubMed/NCBI
|
|
25
|
Wang X, Shi L, Joyce S, Wang Y and Feng Y:
MDG-1, a potential regulator of PPARα and PPARγ, ameliorates
dyslipidemia in mice. Int J Mol Sci. 18:182017.
|
|
26
|
Wu J, Ding Y, Zhu C, Shao X, Xie X, Lu K
and Wang R: Urinary TNF-α and NGAL are correlated with the
progression of nephropathy in patients with type 2 diabetes. Exp
Ther Med. 6:1482–1488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
González-Clemente JM, Mauricio D, Richart
C, Broch M, Caixàs A, Megia A, Giménez-Palop O, Simón I,
Martínez-Riquelme A, Giménez-Pérez G, et al: Diabetic neuropathy is
associated with activation of the TNF-alpha system in subjects with
type 1 diabetes mellitus. Clin Endocrinol (Oxf). 63:525–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Wu TT, Chen J and Qiu W: Elevated
expression levels of serum insulin-like growth factor-1, tumor
necrosis factor-α and vascular endothelial growth factor 165 might
exacerbate type 2 diabetic nephropathy. J Diabetes Investig.
8:108–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kozłowska L, Rydzewski A, Fiderkiewicz B,
Wasińska-Krawczyk A, Grzechnik A and Rosołowska-Huszcz D:
Adiponectin, resistin and leptin response to dietary intervention
in diabetic nephropathy. J Ren Nutr. 20:255–262. 2010. View Article : Google Scholar : PubMed/NCBI
|