Introduction
Diabetes mellitus (types I and II) affect multiple
organ systems and result in numerous complications, including
myocardial fibrosis (1). Cardiac
fibrosis is a significant factor causing cardiac systolic and
diastolic dysfunction (2). However,
treatment targeting cardiac fibrosis is rare. Cardiac fibrosis has
been identified as the key regulator of cardiac remodeling.
Interstitial fibroblast proliferation and excessive extracellular
matrix are the major characteristics of cardiac fibrosis (3). High glucose (HG) or hyperglycemia, the
major characteristic of diabetes, has been demonstrated to induce
collagen secretion and proliferation of cardiac fibroblasts (CFs)
and to increase oxidative stress (4,5).
Connective tissue growth factor (CTGF) is a
pro-adhesive matricellular protein associated with numerous
diabetic complications (6),
including diabetic cardiomyopathy. The role of CTGF in the
pathogenesis of fibrosis in tissue has been the major focus of
studies on diabetes by our and other groups (7–9). The
induction of CTGF by oxidative stress has been demonstrated in
various studies (10–12).
Trimetazidine (TMZ), an anti-anginal agent,
selectively inhibits the activity of mitochondrial long-chain
3-ketoacyl-CoA thiolase, resulting in inhibition of free fatty acid
(FFA) oxidation and promotion of glucose oxidation (13). In addition to metabolic effects,
studies have indicated that TMZ exerts cardioprotective effects by
reducing oxidative damage, inhibiting inflammation and apoptosis,
and improving endothelial function (14–16).
Thus, it was hypothesized that TMZ reduces oxidative
stress and downregulates the expression of CTGF in CFs, leading to
improvement of hyperglycemia-induced cardiac fibrosis. The present
study was conducted to assess whether TMZ treatment can reduce
collagen secretion and induce changes in CTGF expression in
vitro and in vivo by western blotting and pathological
test experiments.
Materials and methods
Animal model preparation
Male Sprague Dawley (SD) rats (age, 6 weeks; weight,
160–200 g) were obtained from the Animal Department of Sun Yat-Sen
University. A total of 40 rats were randomly subdivided into two
groups: A normal control group (N), consisting of normal rats
(n=10), and a diabetic group, consisting of streptozotocin
(STZ)-treated rats (n=30). Diabetes was induced by a single
intraperitoneal (i.p.) injection of STZ at a dose of 45 mg/kg in
0.1 M citrate-buffered saline (pH 4.4), while the normal rats (N
group) were injected with an equal volume of 0.1 M citrate buffer.
Fasting blood glucose was tested at 1 week after the injection
using a glucometer (Accu-chek® Performa; Roche
Diagnostics), and rats with a blood glucose level of 16.7 mM or
higher were considered diabetic. A total of four STZ-treated rats
died due to the toxicity of STZ, whereas three STZ-treated rats
failed to develop diabetes. The dose of STZ used and mortality rate
observed in the present study were comparable with that of a
previous study (17). Rats that
successfully developed diabetes were randomly subdivided into two
groups: A diabetic control group (C group, n=12), which received
normal saline (NS) by oral gavage, and a diabetes + TMZ group (TMZ
group), which received NS + TMZ (15 mg/kg/day) by oral gavage for
16 weeks (n=11). Rats in the N group (n=10) received NS by oral
gavage in the same timeframe. At the end of the experiment, the
final body weight of these rats was recorded, and the rats were
subjected to the tests described below. A total of 5 rats died
while under anesthesia for hemodynamic testing (1 in the N group, 2
in the C group and 2 in the TMZ group). In the present study, none
of the rats lost weight by >20%. The left ventricular (LV) free
wall was fixed with 4% paraformaldehyde, and the remaining samples
were stored in liquid nitrogen. The total heart (TH) and LV weights
(LVW) were recorded.
Echocardiography (ECG)
measurements
The device used was a high-resolution small animal
ultrasonic imaging system (Visual Sonics) with a frequency of 30
MHz. The test was performed after drug treatment for 16 weeks.
Prior to testing, each rat was anesthetized with a diethyl ether
mask and had its chest fur shaved. The data were collected in the
middle short section of the papillary muscle by two-dimensional ECG
and M-type ECG. The parameters included systolic LV posterior wall
thickness (LVPW-s), diastolic LV posterior wall thickness (LVPW-d),
LV ejection fraction (LVEF), LV fractional shortening (LVFS) and
early diastolic mitral valve blood flow velocity E peak/late
diastolic mitral valve blood flow velocity A peak (E/A).
Hemodynamic measurements
The rats were anesthetized with 1.25%
tribromoethanol (250 mg/kg i.p.). Surgery was performed to assess
the hemodynamic data as described previously (18,19). A
microtip pressure transducer catheter (3.5 Fr; Millar Instruments,
Inc.) was introduced via the right carotid artery into the LV. The
heart rate, LV end-systolic pressure (LVESP), LV end-diastolic
pressure (LVEDP), and the maximum rates of increase and decrease in
LV pressure (±dp/dt) were measured using a commercially available
analog-to-digital converter and analyzed using the AcqKnowledge
software (version 4.2.0; BIOPAC Systems, Inc.).
Exhaustion swimming exercise test
The exhaustion swimming exercise test was performed
to assess exercise capacity according to a method previously
described (20).
Histological analysis
After the hemodynamic measurements were performed,
all rats were euthanatized by cardiac exsanguination under
anesthesia. Euthanasia was confirmed by removing the heart. Hearts
were washed in cold (4°C) saline solution (NaCl 0.9%). The LVs of
the rats were fixed with 4% paraformaldehyde for 24 h and were then
embedded in paraffin. Sections (6 mm) were stained with Masson's
trichrome to detect collagen. To examine the degree of cardiac
fibrosis, 5 fields were randomly selected and the cardiac collagen
volume fraction (CVF) was computed as the ratio of the Masson's
trichrome-stained fibrosis area to the total area of the myocardium
using Image-Pro-plus 5.0 software (Media Cybernetics). Sections
stained with H&E were also analyzed under a microscope (Axio
Imager.Z2; Zeiss AG).
Hydroxyproline and malondialdehyde
(MDA) measurement
The concentrations of hydroxyproline (cat. no.
A030-2-1) and MDA (cat. no. A003-1-2) in myocardial tissue were
detected using commercial kits (Nanjing Jiancheng Bioengineering
Institute) in accordance with the manufacturer's protocols.
Cell culture
CFs were isolated and cultured from 1–3 day old
neonatal SD rats obtained from the Sun Yat-Sen University
Experimental Animal Center (12).
The cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The cells were
cultured in a 5% CO2 incubator under a humidified
atmosphere at 37°C. All cell experiments were performed with CFs at
passages 2–3. CF phenotype was verified using immunofluorescence
staining in a protocol previously described by Fan et al
(21). Briefly, cells were incubated
with FBS, aforementioned, followed by incubation with primary
antibodies against vimentin (1:200; cat. no. v6389; Sigma-Aldrich;
Merck KGaA) and von Willebrand factor (1:200; cat. no. HPA001815;
Sigma-Aldrich; Merck KGaA) overnight at 4°C in humidified chamber.
Following further washing with PBS, cells were incubated with a
mixture of two secondary antibodies (fluorescein-conjugated
anti-mouse IgG; 1:500; sc516140; Santa Cruz Biotechnology, Inc. and
Texas Red®-conjugated goat anti-rabbit IgG, 1:1,000;
ab6719; Abcam) for 1 h at room temperature in the dark. Following
another wash with PBS, cells were incubated with 300 nM DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) for 1 min at room
temperature. Cells were subsequently rinsed again with PBS and
mounted with an aqueous mounting medium. The stained cells were
visualized using a fluorescence microscope (magnification, ×200).
For the experiments, CFs that were grown to 80% confluence and that
were serum-starved in serum-free medium for 24 h prior to treatment
were used. To detect the direct effects of TMZ on myocardial
collagen formation, CFs were subjected to the following treatment
regimens: DMEM with 5.6 mM glucose, designated thereafter as the
normal glucose (NG) group; DMEM with 25 mM glucose, designated as
the high glucose (HG) group and HG + varying concentrations of TMZ
(0.1, 1 and 10 µM) for up to 24 h. Collagen synthesis was assayed
using western blot analysis.
Western blot analysis
Total cell proteins and LV tissues were prepared in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) in accordance with the manufacturer's protocol.
Protein concentrations were determined using Bicinchoninic Acid
assay. Equal amounts of protein (40 µg) were separated by 10%
SDS-PAGE and then transferred to a polyvinylidene difluoride
membrane (Thermo Fisher Scientific, Inc.). The membrane was blocked
in 5% nonfat milk for 1 h at room temperature and was then
incubated with following antibodies: CTGF (1:1,000; cat. no.
ab6992; Abcam), collagen III (Col III; 1:8,000; cat. no. ab7778;
Abcam), collagen I (Col I; 1:1,000; cat. no. 84336; Cell Signaling
Technology, Inc.) and superoxide dismutase 2 (SOD2; 1:5,000; cat.
no. ab13533; Abcam) at 4°C overnight. The membranes were incubated
with horseradish peroxidase-conjugated mouse anti-rabbit IgG
secondary antibody (1:5,000; cat. no. sc-2357; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Protein
expression was determined with enhanced chemiluminescence (EMD
Millipore; Merck KGaA). The bands were quantitatively evaluated by
densitometry using ImageJ software (version 2; National Institutes
of Health).
Analysis of intracellular reactive
oxygen species (ROS) generation
Cellular ROS accumulation in CFs was measured by
using fluorescent probe, dichloro-dihydro-fluorescein diacetate
(DCFH-DA; Beyotime Institute of Biotechnology). Cells were
incubated with 5 µM DCFH-DA for 20 min at 37°C. DCF fluorescence
was detected by flow cytometry (BD FACSCalibur™; emission, 480 nm;
bandpass filter, 530 nm; BD Biosciences). For each sample, 10,000
events were collected. ROS production was calculated as the mean
fluorescence intensity using FlowJo software (version 7.6; FlowJo
Software LLC).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical analysis of the data was performed by
one-way analysis of variance with a Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All calculations were performed with the SPSS software
(version 15.0; SPSS, Inc.).
Results
Effect of TMZ on cardiac structure,
cardiac function and exercise capacity in STZ-induced diabetic
rats
To observe the influence of hyperglycemia on cardiac
structure and function, the dimensions of the LV, cardiac function
and hemodynamic parameters we measured by ECG and Millar
Instruments. The results of the ECG examination suggested that the
LVEF, LVFS and LVPW-d in the C group were lower than those in the N
group (P<0.05). However, these parameters, were not
significantly different between the rats in the C group and the TMZ
group (Table I). Furthermore, the
results of the gross pathological analysis revealed that the ratios
of LV/TH and LVW/BW were higher in the rats in the C group than
those in the rats in the N group (P<0.05). The LV/TH was lower
in the TMZ group than that in the C group (P<0.05). However,
there was no significant difference in LVW/BW between the TMZ group
and the C group (Table II). As
indicated in Table III, the LVEDP
was higher and the dp/dtmax was lower in the C group
than those in the N group (P<0.001 and P<0.05, respectively).
TMZ treatment decreased the LVEDP of diabetic rats (P<0.05). The
exhaustive swimming test revealed that diabetic rats exhibited an
impaired exercise capacity compared with their non-diabetic
counterparts (1273±170.80 vs. 673.5±131.50; P<0.05). However,
TMZ treatment did not improve the exercise capacity of diabetic
rats (608.50±170.80 vs. 673.50±131.50, P>0.05; Fig. S1). In conclusion, the 16 weeks of
hyperglycemia caused significant changes in cardiac structure,
cardiac function and exercise capacity; and subsequent TMZ
treatment improved cardiac fibrosis and LV diastolic function in
diabetic rats.
 | Table I.Effect of TMZ on cardiac function
measured by color Doppler ultrasound. |
Table I.
Effect of TMZ on cardiac function
measured by color Doppler ultrasound.
| Parameter | N (n=9) | C (n=8) | TMZ (n=8) |
|---|
| HR (bpm) | 388±43 | 366±40 | 355±53 |
| E/A | 1.78±0.34 | 1.57±0.33 | 1.65±0.29 |
| LVEF (%) | 66.6±4.8 |
59.4±4.3a |
58.5±6.2a |
| LVFS (%) | 39.1±4.2 |
33.5±4.2a |
32.8±4.4a |
| LVPW-d (mm) | 2.07±0.30 |
1.76±0.13a |
1.73±0.19a |
| LVPW-s (mm) | 2.75±0.26 |
2.33±0.29a |
2.37±0.24a |
| IVS-d (mm) | 1.74±0.28 | 1.51±0.19 | 1.54±0.15 |
| IVS-s (mm) | 2.74±0.46 | 2.41±0.31 |
2.28±0.20a |
 | Table II.Effect of TMZ on left ventricle mass
indexes. |
Table II.
Effect of TMZ on left ventricle mass
indexes.
| Parameter | N (n=9) | C (n=8) | TMZ (n=8) |
|---|
| LVW/TH (mg/mg) | 0.719±0.025 |
0.745±0.035a |
0.704±0.019b |
| LVW/BW (mg/g) | 2.0±0.31 |
2.8±0.36c |
2.7±0.18c |
| BW (g) | 433±52 | 248±50c | 232±57c |
 | Table III.Effect of TMZ on hemodynamics
parameters. |
Table III.
Effect of TMZ on hemodynamics
parameters.
| Parameter | N (n=7) | C (n=8) | TMZ (n=7) |
|---|
| LVSP (mmHg) | 110±18 | 87±15 | 82±16 |
| LVEDP (mmHg) | 0.1±2.9 |
11.5±4.4a |
4.6±5.4b |
|
+dp/dtmax (KPa/sec) | 2425±701 | 1892±427 | 1937±695 |
|
-dp/dtmax (KPa/sec) | 2379±546 |
1481±533c | 1648±501 |
TMZ inhibits hyperglycemia-induced
cardiac fibrosis and CTGF expression in myocardial tissue
Heart sections were stained with Masson's trichrome
to determine the extent of interstitial fibrosis after 16 weeks.
Morphologically, collagen deposition was increased in the C group
but was attenuated in the TMZ group (Fig. 1A). Quantitative evaluation of
interstitial fibrosis in the heart by CVF indicated that TMZ
markedly reduced intermuscular interstitial fibrosis by 23% in the
diabetic rats compared with that in the diabetic rats treated with
vehicle (Fig. 1A). Consistent with
CVF, the LV hydroxyproline content was increased in the C group
compared with the N group (13.4±3.0 vs. 7.2±2.3, P<0.01;
Fig. 1B), whereas the content was
significantly reduced in the TMZ group compared with the C group
(8.5±3.2 vs. 13.4±3.0, P<0.01; Fig.
1B). The protein expression of Col I and Col III was
upregulated in the C group when compared with that in the N group
(Col I: 2.2±0.37 vs. 1.00±0.23, P<0.01; Col III: 1.85±0.15 vs.
1.00±0.20, P<0.01; Fig. 1C).
However, TMZ treatment downregulated the protein expression of Col
I and Col III in diabetic rats compared with the C group (Col I:
1.5±0.41 vs. 2.2±0.41, P<0.05; Col III: 1.29±0.31 vs. 1.84±0.15,
P<0.05; Fig. 1C). The protein
expression of CTGF was increased in the C group compared with that
in the N group (1.37±0.23 vs. 1.00±0.22, P<0.05). Compared with
that in the C group, the protein expression of CTGF was
downregulated in the TMZ group by 27.7% (0.99±0.20 vs. 1.37±0.23,
P<0.05; Fig. 2A).
Effect of TMZ on the protein
expression of superoxide dismutase (SOD-2) and MDA levels in
myocardial tissue
The protein expression of SOD-2 was downregulated in
the C group compared with that in the N group (0.40±0.12 vs. 1.00;
P<0.001; Fig. 2B). Compared with
that in the C group, the protein expression of SOD-2 was
upregulated in the TMZ group (0.64±0.18 vs. 0.40±0.12, P<0.05;
Fig. 2B). MDA levels were increased
in the C group compared with the N group (9.8±2.8 vs. 4.9±2.2,
P<0.01), whereas it was decreased in the TMZ rats compared with
the C group (6.6±2.3 vs. 9.8±2.8, P<0.05; Fig. 2B).TMZ inhibits HG-induced cardiac
collagen synthesis in neonatal rat CFs. Immunofluorescence
staining revealed that the purity of the isolated CFs was >95%
(Fig. 3A). The protein expression of
Col I and Col III was significantly upregulated in the presence of
HG (1.92±0.20 and 2.03±0.30-fold of NG, respectively) and
downregulated by TMZ intervention (Fig.
3A). TMZ (0.1 µM) decreased the protein levels of Col I by
44.3% (1.07±0.17 vs. 1.92±0.10, P<0.01), and TMZ (10 µM)
decreased the protein levels of Col III by 48.5% (1.03±0.26 vs.
2.03±0.30, P<0.05; Fig. 3B). The
hydroxyproline content of the supernatant was increased in the HG
group (1.2±0.04 vs. 1.00±0.03, P<0.01), but was significantly
decreased in the TMZ (1 µM) group (1.05±0.03 vs. 1.2±0.04,
P<0.01; Fig. 3C).
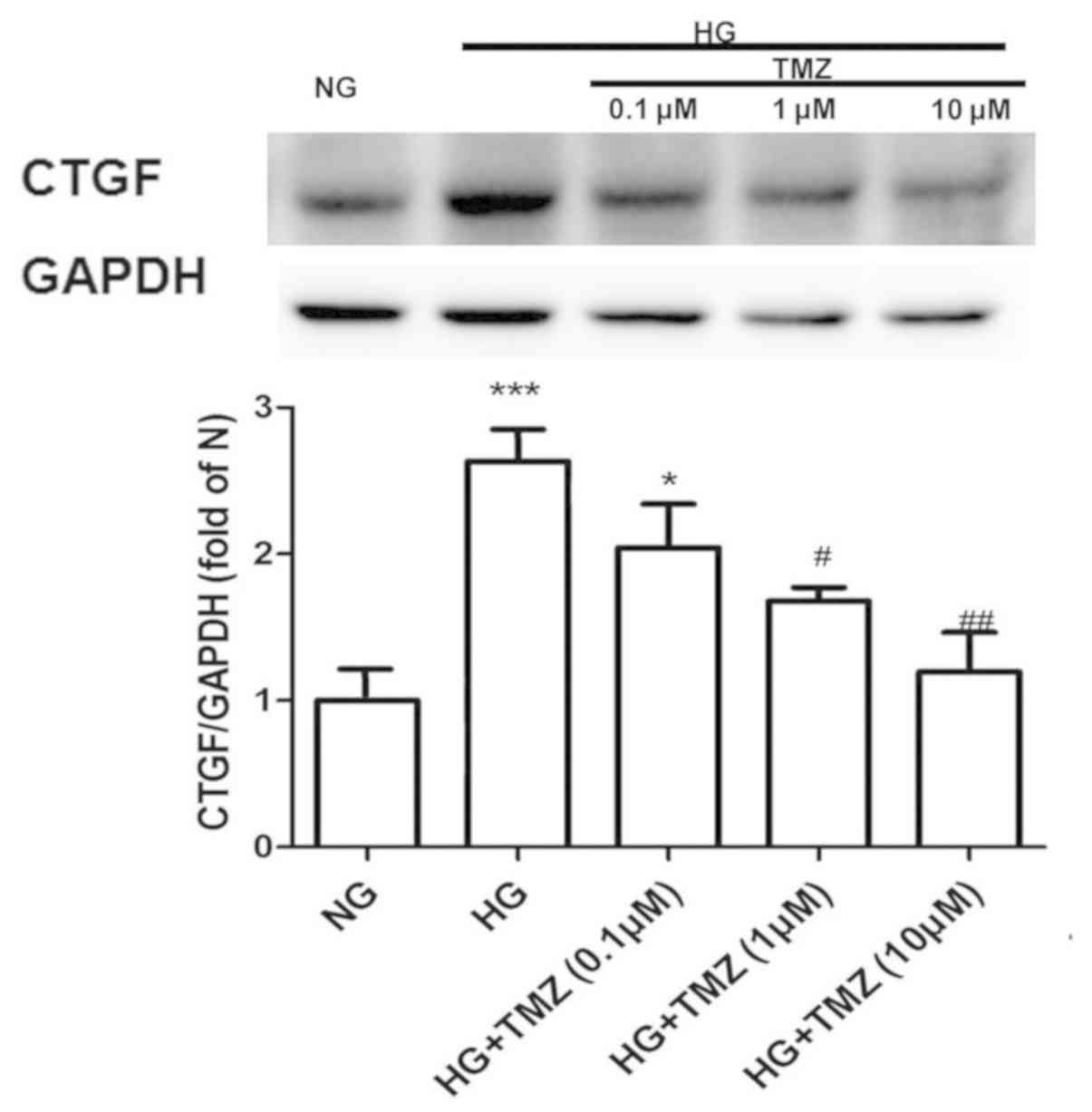
TMZ inhibits HG-induced CTGF
expression in neonatal rat CFs
The protein expression of CTGF increased 2.67-fold
in the HG group compared with that in the NG group. However, TMZ (1
µM) decreased the protein expression of CTGF by 37% in the diabetic
rats (Fig. 4).
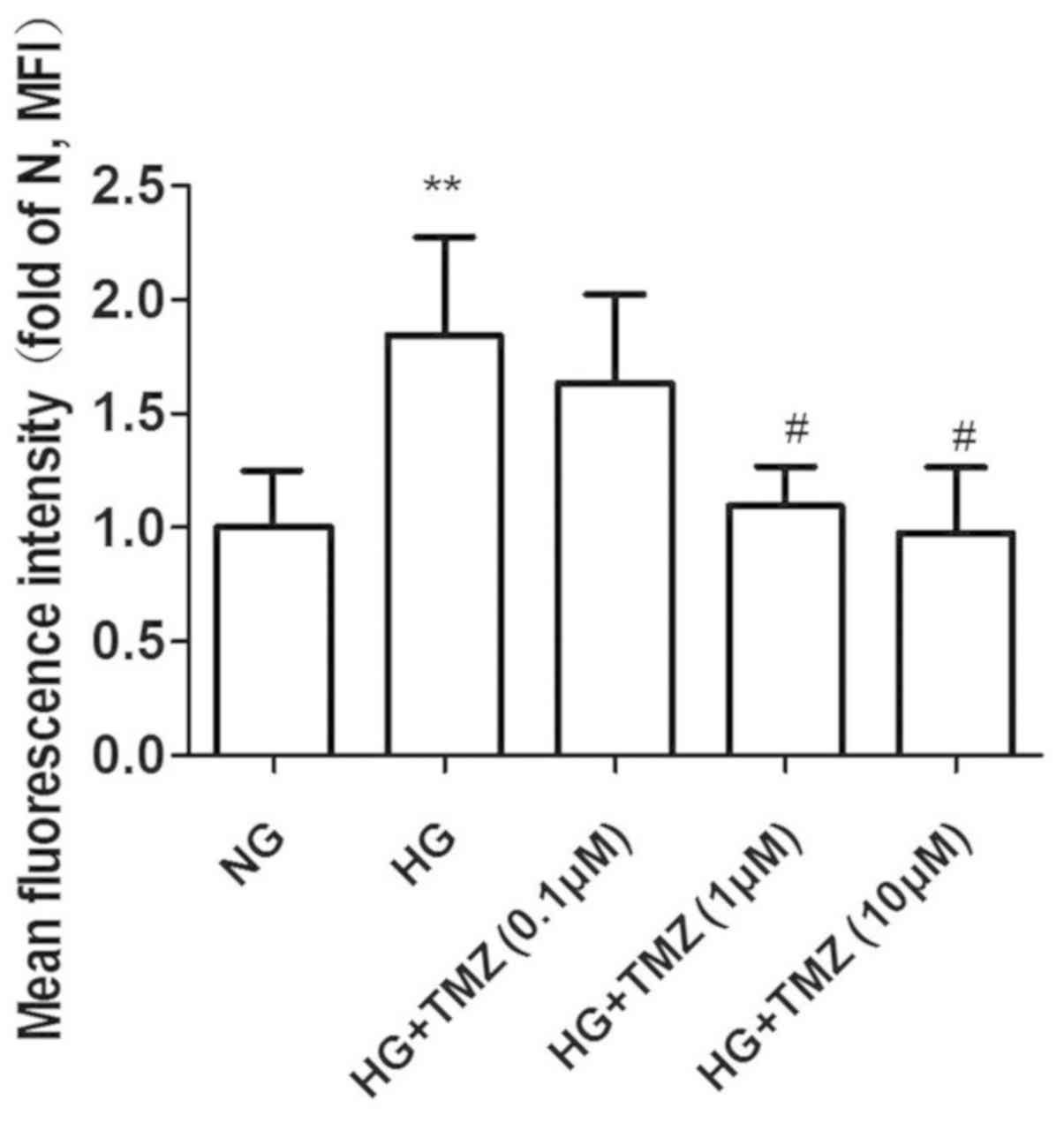
Effect of TMZ on ROS formation in
neonatal rat CFs
ROS levels were evaluated using the ROS fluorescent
dye DCFH-DA. CFs were exposed to HG (25 mM) and normal glucose (5.6
mM) for 24 h. HG induced 1.84-fold increase of ROS, while ROS
production decreased with TMZ treatment at 1 and 10 µM (1.09±0.17
and 0.97±0.29 vs. 1.84±0.43, P<0.05; Fig. 5).
Discussion
It is widely known that hyperglycemia increases the
prevalence of ischemic heart disease (22). In recent years, the effect of
hyperglycemia on non-ischemic heart disease has also been a major
research hotspot. In the present study, STZ-induced diabetic rats
presented with symptoms similar to those in humans with diabetes,
including weight loss, polydipsia, polyuria and hyperglycemia in
the first week of the study. In addition, they exhibited a
reduction in LV systolic and diastolic function and a decrease in
LV walls compared with those of normal rats after 4 months of the
study, suggesting that STZ-induced diabetic rats have symptoms
similar to those of diabetic cardiomyopathy. This rat model may
therefore be used to simulate the heart conditions of diabetic
patients.
Diabetes is a cardiac disease worth studying due to
its association with metabolic abnormalities, which are independent
of diabetic vascular complications. Diabetes leads to changes in
carbohydrate metabolism, including impaired glucose uptake and
reduced glycolysis and pyruvate oxidation (23). In contrast to glucose, fatty acid
uptake is insulin-independent, which allows for an increase in
fatty acids for myocardial oxidation in diabetes (23). In addition, the increase of ROS
induced by hyperglycemia and excessive fatty acid oxidation leads
to myocardial apoptosis and fibrosis, followed by eventual systolic
and diastolic dysfunction of the LV (4,24,25).
Metabolic therapies in diabetes may alleviate myocardial fibrosis
and improve LV function.
TMZ, a long-chain fatty acid β-oxidative inhibitor,
is thought to switch cardiac myocyte metabolism from FFA metabolism
to glucose metabolism, thereby improving the myocardial oxidative
metabolism effect (13). At the same
time, numerous clinical trials have indicated that TMZ may improve
cardiac function in patients with ischemic cardiomyopathy and heart
failure (25–32). It also improves the heart function of
patients with idiopathic dilated diabetes mellitus and it
alleviates the increase in C-reactive protein and B-type
natriuretic peptide to a certain extent (33).
In a study by Belardinelli et al (34), TMZ was able to improve endothelial
function and reduce serum MDA and peroxide in chronic heart
failure. However, studies on the influence of TMZ on diabetic
cardiomyopathy are rare (35). In
the present study, TMZ had no effect on heart rate, EF, FS, LV wall
thickness, body weight, blood glucose, LV mass fraction or exercise
tolerance. LVEDP is a good indicator of LV diastolic function.
Considering that the LVFS and E/A lack sensitivity to the diastolic
function in the rat heart, the hemodynamics of the model were
further examined. TMZ was indicated to decrease LVEDP in patients
with diabetes. In addition, the diabetic rats treated with TMZ had
lower LV/TH ratios. The present results suggest that TMZ has a role
in alleviating ventricular remodeling and improving diastolic
function in rats.
Pathological examination indicated that collagen
deposition was more severe in diabetic rats than in non-diabetic
rats. In addition, TMZ decreased collagen deposition in diabetic
rats. Western blot analysis of myocardial tissue revealed that TMZ
reduced the levels of Col I and Col III in the myocardium of
diabetic rats. It was also demonstrated that TMZ reduced collagen
secretion in vitro. This result indicates that TMZ may
reduce myocardial fibrosis in diabetic rats as one of the
mechanisms to improve diastolic function.
Increases in ROS may lead to activation of multiple
signaling pathways, resulting in cell fibrosis and death. Diabetes
may cause myocardial fibrosis by increasing the levels of oxidative
stress (36). Aragno et al
(37) suggested that
dehydroisoandrosterone improves cardiac fibrosis by reducing
oxidative damage induced by high glycemia. In the present study,
TMZ decreased the MDA level of myocardial tissue of the diabetic
rat model in vivo and isolated rat CFs in vitro, and
reduced the secretion of ROS in myocardial fibroblasts induced by
hyperglycemia. This result is similar to that of McLennan et
al (9), who reported that TMZ
reduced the secretion of ROS in myocardial fibroblasts induced by
angiotensin.
CTGF, a cell fibrosis factor, promotes fibroblast
proliferation and interstitial collagen deposition. In patients
with diabetes, CTGF has an important role in the development of
cardiac fibrosis (9,38). In the present study, TMZ was
demonstrated to reduce the expression of the CTGF protein in
myocardial tissue and myocardial fibroblasts in diabetic rats. TMZ
may be used to reduce the production of myocardial fibroblast
collagen to achieve an anti-fibrotic effect.
In conclusion, the present study demonstrated that
diabetic rats with myocardial fibrosis have elevated LVEDP, LV/TH,
CTGF protein expression and MDA levels at 16 weeks. Diabetic
cardiomyopathy was associated with heart fibrosis and oxidative
stress. TMZ may improve the diastolic function of diabetic rats.
The effect may be associated with the reduction of ROS formation
and CTGF expression in TMZ-treated rats. The present study suggests
that TMZ may protect the heart of diabetic patients. In future
studies, the association between ROS and CTGF, as well as the
mechanism of TMZ to decrease ROS induced by HG, remain to be
elucidated.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The China Health
Promotion Foundation.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and YZ designed the experiment. YZ and YW
performed the animal experiments. SHL and EQ performed the in
vitro cell experiments. HZ and YL performed the
echocardiography. JW, JZ and LP analyzed and interpreted the data.
YZ and SL performed the histological examinations of the heart. JL
and YZ were major contributors in writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the US NIH (publication no. 85-23, revised 1996). All experimental
protocols were approved by the Institutional Animal Care and Use
Committee of Sun Yat-Sen University (IACUC-20140703).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shamhart PE, Luther DJ, Hodson BR, Koshy
JC, Ohanyan V and Meszaros JG: Impact of type 1 diabetes on cardiac
fibroblast activation: Enhanced cell cycle progression and reduced
myofibroblast content in diabetic myocardium. Am J Physiol
Endocrinol Metab. 297:E1147–E1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Heerebeek L, Hamdani N, Handoko ML,
Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ,
Schalkwijk CG, Bronzwaer JG, Diamant M, et al: Diastolic stiffness
of the failing diabetic heart: Importance of fibrosis, advanced
glycation end products, and myocyte resting tension. Circulation.
117:43–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bugyei-Twum A, Advani A, Advani SL, Zhang
Y, Thai K, Kelly DJ and Connelly KA: High glucose induces Smad
activation via the transcriptional coregulator p300 and contributes
to cardiac fibrosis and hypertrophy. Cardiovas Diabetol. 13:892014.
View Article : Google Scholar
|
|
5
|
Dai B, Cui M, Zhu M, Su WL, Qiu MC and
Zhang H: STAT1/3 and ERK1/2 synergistically regulate cardiac
fibrosis induced by high glucose. Cell Physiol Biochem. 32:960–971.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wahab NA, Weston BS and Mason RM:
Connective tissue growth factor CCN2 interacts with and activates
the tyrosine kinase receptor TrkA. J Am Soc Nephrol. 16:340–351.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Li PH, Yang L, Du QS, Guo TT and
Tang X: Connective tissue growth factor mediates high
glucose-induced down-regulation of podocalyxin expression in mouse
podocytes. Nan Fang Yi ke Da Xue Xue Bao (Chinese). 31:839–843.
2011.
|
|
8
|
Kobayashi T, Inoue T, Okada H, Kikuta T,
Kanno Y, Nishida T, Takigawa M, Sugaya T and Suzuki H: Connective
tissue growth factor mediates the profibrotic effects of
transforming growth factor-beta produced by tubular epithelial
cells in response to high glucose. Clin Exp Nephrol. 9:114–121.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLennan SV, Wang XY, Moreno V, Yue DK and
Twigg SM: Connective tissue growth factor mediates high glucose
effects on matrix degradation through tissue inhibitor of matrix
metalloproteinase type 1: Implications for diabetic nephropathy.
Endocrinology. 145:5646–5655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuda S, Gomi F, Katayama T, Koyama Y,
Tohyama M and Tano Y: Induction of connective tissue growth factor
in retinal pigment epithelium cells by oxidative stress. Jpn J
Ophthalmol. 50:229–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda S, Gomi F, Oshima Y, Tohyama M and
Tano Y: Vascular endothelial growth factor reduced and connective
tissue growth factor induced by triamcinolone in ARPE19 cells under
oxidative stress. Invest Ophthalmol Vis Sci. 46:1062–1068. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Gai Y, Liu F, Gao W, Zhang Y, Xu M
and Li Z: Trimetazidine inhibits pressure overload-induced cardiac
fibrosis through NADPH oxidase-ROS-CTGF pathway. Cardiovas Res.
88:150–158. 2010. View Article : Google Scholar
|
|
13
|
Kantor PF, Lucien A, Kozak R and Lopaschuk
GD: The antianginal drug trimetazidine shifts cardiac energy
metabolism from fatty acid oxidation to glucose oxidation by
inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase.
Circ Res. 86:580–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams FM, Tanda K, Kus M and Williams
TJ: Trimetazidine inhibits neutrophil accumulation after myocardial
ischaemia and reperfusion in rabbits. J Cardiovas Pharmacol.
22:828–833. 1993. View Article : Google Scholar
|
|
15
|
Ruixing Y, Wenwu L and Al-Ghazali R:
Trimetazidine inhibits cardiomyocyte apoptosis in a rabbit model of
ischemia-reperfusion. Transl Res. 149:152–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Napoli P, Chierchia S, Taccardi AA,
Grilli A, Felaco M, De Caterina R and Barsotti A: Trimetazidine
improves post-ischemic recovery by preserving endothelial nitric
oxide synthase expression in isolated working rat hearts. Nitric
Oxide. 16:228–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gajdosík A, Gajdosíkova A, Stefek M,
Navarová J and Hozová R: Streptozotocin-induced experimental
diabetes in male wistar rats. Gen Physiol Biophys 18 Spec No.
54–62. 1999.
|
|
18
|
Rennison JH, McElfresh TA, Okere IC,
Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng
KY, et al: High-fat diet postinfarction enhances mitochondrial
function and does not exacerbate left ventricular dysfunction. Am J
Physiol Heart Circ Physiol. 292:H1498–H506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo J, Gao X, Peng L, Sun H and Dai G:
Effects of hydrochlorothiazide on cardiac remodeling in a rat model
of myocardial infarction-induced congestive heart failure. Eur J
Pharmacol. 667:314–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto K, Ishihara K, Tanaka K, Inoue K
and Fushiki T: An adjustable-current swimming pool for the
evaluation of endurance capacity of mice. J Appl Physiol (1985).
81:1843–1849. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan YH, Dong H, Pan Q, Cao YJ, Li H and
Wang HC: Notch signaling may negatively regulate neonatal rat
cardiac fibroblast-myofibroblast transformation. Physiol Res.
60:739–748. 2011.PubMed/NCBI
|
|
22
|
Morici ML, Di Marco A, Sestito D, Candore
R, Cangemi C, Accardo F, Donatelli M, Cataldo MG and Lombardo A:
The impact of coexistent diabetes on the prevalence of coronary
heart disease. J Diabetes Complications. 11:268–273. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isfort M, Stevens SC, Schaffer S, Jong CJ
and Wold LE: Metabolic dysfunction in diabetic cardiomyopathy.
Heart Fail Rev. 19:35–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar D, Lou H and Singal PK: Oxidative
stress and apoptosis in heart dysfunction. Herz. 27:662–668. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeung EH, Pankow JS, Astor BC, Powe NR,
Saudek CD and Kao WH: Increased risk of type 2 diabetes from a
family history of coronary heart disease and type 2 diabetes.
Diabetes Care. 30:154–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fragasso G, Salerno A, Lattuada G, Cuko A,
Calori G, Scollo A, Ragogna F, Arioli F, Bassanelli G, Spoladore R,
et al: Effect of partial inhibition of fatty acid oxidation by
trimetazidine on whole body energy metabolism in patients with
chronic heart failure. Heart. 97:1495–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fragasso G, Perseghin G, De Cobelli F,
Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio
A and Margonato A: Effects of metabolic modulation by trimetazidine
on left ventricular function and phosphocreatine/adenosine
triphosphate ratio in patients with heart failure. Eur Heart J.
27:942–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belardinelli R, Cianci G, Gigli M,
Mazzanti M and Lacalaprice F: Effects of trimetazidine on
myocardial perfusion and left ventricular systolic function in type
2 diabetic patients with ischemic cardiomyopathy. J Cardiovasc
Pharmacol. 51:611–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Belardinelli R, Lacalaprice F, Faccenda E
and Volpe L: Trimetazidine potentiates the effects of exercise
training in patients with ischemic cardiomyopathy referred for
cardiac rehabilitation. Eur J Cardiovas Prev Rehabili. 15:533–540.
2008. View Article : Google Scholar
|
|
30
|
El-Kady T, El-Sabban K, Gabaly M, Sabry A
and Abdel-Hady S: Effects of trimetazidine on myocardial perfusion
and the contractile response of chronically dysfunctional
myocardium in ischemic cardiomyopathy: A 24-month study. Am J
Cardiovasc Drugs. 5:271–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fragasso G, Palloshi A, Puccetti P,
Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O and
Margonato A: A randomized clinical trial of trimetazidine, a
partial free fatty acid oxidation inhibitor, in patients with heart
failure. J Am Coll Cardiol. 48:992–998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fragasso G, Piatti Md PM, Monti L,
Palloshi A, Setola E, Puccetti P, Calori G, Lopaschuk GD and
Margonato A: Short- and long-term beneficial effects of
trimetazidine in patients with diabetes and ischemic
cardiomyopathy. Am Heart J. 146:E182003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao P, Zhang J, Yin XG, Maharaj P,
Narraindoo S, Cui LQ and Tang YS: The effect of trimetazidine on
cardiac function in diabetic patients with idiopathic dilated
cardiomyopathy. Life Sci. 92:633–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Belardinelli R, Solenghi M, Volpe L and
Purcaro A: Trimetazidine improves endothelial dysfunction in
chronic heart failure: An antioxidant effect. Eur Heart J.
28:1102–1108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Ding WY, Wang ZH, Tang MX, Wang
F, Li Y, Zhong M, Zhang Y and Zhang W: Early administration of
trimetazidine attenuates diabetic cardiomyopathy in rats by
alleviating fibrosis, reducing apoptosis and enhancing autophagy. J
Transl Med. 14:1092016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aragno M, Mastrocola R, Alloatti G,
Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O and
Boccuzzi G: Oxidative stress triggers cardiac fibrosis in the heart
of diabetic rats. Endocrinology. 149:380–388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aragno M, Meineri G, Vercellinatto I,
Bardini P, Raimondo S, Peiretti PG, Vercelli A, Alloatti G,
Tomasinelli CE, Danni O and Boccuzzi G: Cardiac impairment in
rabbits fed a high-fat diet is counteracted by
dehydroepiandrosterone supplementation. Life Sci. 85:77–84. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, McLennan SV, Allen TJ, Tsoutsman
T, Semsarian C and Twigg SM: Adverse effects of high glucose and
free fatty acid on cardiomyocytes are mediated by connective tissue
growth factor. Am J Physiol Cell physiol. 297:C1490–500. 2009.
View Article : Google Scholar : PubMed/NCBI
|