Introduction
Thyroid diseases, which include hypothyroxinemia,
hypothyroidism, subclinical hypothyroidism (SCH), hyperthyroidism
and subclinical hyperthyroidism, are the second most common
endocrine diseases during pregnancy (1,2). Thyroid
diseases during pregnancy have been studied extensively in
endocrinology, obstetrics and gynecology (3–5). Vulsma
et al (6) identified that
thyroid hormones exist in umbilical blood from mothers with thyroid
hormone synthesis deficiency, suggesting that free thyroxine (free
T4 or fT4) is delivered through the placenta. Maternal thyroid
hormones serve essential roles in fetal brain development
(gestational week 1–20) (7). Thyroid
diseases during pregnancy severely affect pregnancy outcomes and
neuropsychological development of the offspring (8,9).
Alterations in the secretion of thyroid stimulating hormone (TSH)
or an increase in serum T4 levels result in fetal
neurodevelopmental defects (10,11).
In early pregnancy, thyroid synthesis deficits
(hypothyroidism, SCH and hypothyroxinemia) and positive thyroid
antibodies are associated with impaired intelligence and motor
skills (12,13). By contrast, certain studies have
demonstrated that subclinical hyperthyroidism does not affect
pregnancy outcomes (14–16). According to the Guidelines of the
American Thyroid Association for the Diagnosis and Management of
Thyroid Disease During Pregnancy and Postpartum in 2011 (2011 ATA
Guidelines) (17) and 2012 Chinese
edition of Thyroid Nodules and Differentiated Thyroid Cancer
Management Guidelines (2012 Chinese Guidelines) (18,19),
levothyroxine (L-T4) should be administrated to pregnant women with
SCH who are thyroid peroxidase antibody-positive. Several studies,
however, have claimed that it is not necessary to correct SCH and
isolated hypothyroxinemia (20,21).
Therefore, it is not clear whether L-T4 is beneficial in thyroid
insufficiency.
A comprehensive study to compare different thyroid
levels in pregnant women is lacking. It is not clear whether a
Chinese-specific diagnosis of thyroid dysfunction is necessary.
Since 2012, a new screening standard based on new Chinese
guidelines began to be used and, therefore, two cohorts were
recruited in 2010 and 2014 to evaluate the impact of the change in
screening standards. The ultimate goal of the current study was to
determine thyroid dysfunction in pregnancy in China and evaluate
the effects of an L-T4 supplement on the outcomes and
complications.
Patients and methods
Study subjects
A total of 3,501 pregnant women were enrolled into
the present study from the Department of Obstetrics, Xiangya
Hospital (Changsha, China) between August and December 2010 (named
Cohort 2010; n=825), and between August and December 2014 (named
Cohort 2014; n=2,676). In order to evaluate the impact of the
change in screening standards following the publication of the 2012
Chinese Guidelines, 1,037 patients in the cohort of 2014 (Matched
Cohort 2014) were selected based on age and weight to be compared
with all 825 patients from Cohort 2010 (Table I). In order to evaluate the outcomes
of pregnancy and treatment, other analyses were performed on
subjects selected and enrolled from cohort 2014. The enrollment
criteria for these subjects included: i) Singleton pregnancy, ii)
aged between 20 and 42 years old, iii) thyroid function examined
during the pregnancy. The exclusion criteria included: i) Not
singleton pregnancy (confirmed by ultrasound); ii) aged <20 or
>42 years old; iii) conditions prior to pregnancy, including
diabetes, hypertension, seizure or other convulsive disorders,
pituitary or adrenal diseases, cancer, severe gastrointestinal
diseases, blood system disorders, cerebrovascular diseases,
cardiopulmonary dysfunction and infection; iv) autoimmune diseases,
including systemic lupus erythematosus, antiphospholipid antibody
syndrome and Sjogren's syndrome; v) endocrine diseases prior to
pregnancy, including polycystic ovary syndrome and
hyperprolactinemia; vi) acute diseases during pregnancy, including
appendicitis, pancreatitis and a decrease of whole blood cells; and
vii) history of the use of amiodarone or immunosuppressive agents,
a history of iodine examinations and radiotherapy within 6 months.
The current study was approved by the Institutional Review Board of
Central South University (Changsha, China) and designed according
to the 2011 ATA Guidelines (17) and
2012 Chinese Guidelines (18,19). All
patients provided written informed consent.
 | Table I.Demographic information in cohort
2010 and matched cohort 2014. |
Table I.
Demographic information in cohort
2010 and matched cohort 2014.
| Characteristic | Cohort 2010
(n=825) | Matched cohort 2014
(n=1,037) | P-value (2010 vs.
matched 2014) | Cohort 2014 |
|---|
| Age (years) | 29.1±4.9 | 29.9±4.9 |
0.001 | 29.7±4.2 |
| Weight (kg) | 65.8±10.4 | 67.8±10.4 | <0.001 | 66.3±12.2 |
| Thyroid dysfunction
rate (%) | 29.1 | 82.4 | <0.001 | 78.2 |
Study groups and treatment
For the evaluation of thyroid dysfunction, cohort
2010 and matched cohort 2014 were studied. According to the 2011
ATA Guidelines, patients from all cohorts were divided into the
thyroid disease group (n=132 in cohort 2010; n=539 in matched
cohort 2014; n=1,423 in cohort 2014) and the control/healthy group
(n=693 in cohort 2010, n=498 in matched cohort 2014 and n=1,253 in
cohort 2014). The control groups included healthy pregnant women
with normal thyroid function while the thyroid disease groups
included patients with hypothyroxinemia, hypothyroidism, SCH,
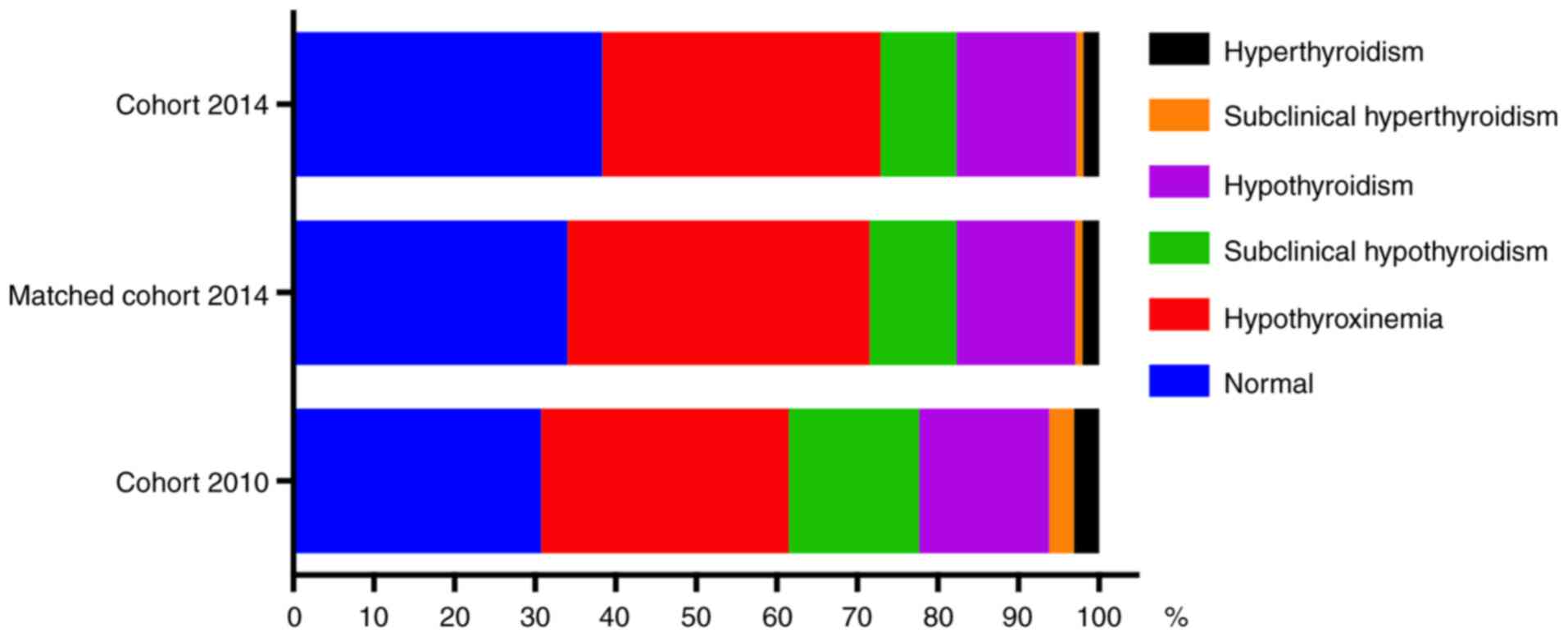
hyperthyroidism and subclinical hyperthyroidism (Fig. 1).
L-T4 (China Associate Pharmaceutical) was
administered orally in selected subjects at an initial dose of
1.5–2.5 µg/kg per day. The dose was then adjusted to <1.5–2.5
µg/kg per day to ensure serum TSH levels were within the normal
range (<3 mIU/l). All L-T4 treatments were administered to
patients within the 2nd and/or 3rd trimester(s). For the evaluation
of L-T4 treatment and comparison of pregnancy outcomes, the total
1,423 patients from cohort 2014 with thyroid disease were divided
into a treatment group (n=231) and a non-treatment group (n=1,192).
Of the 1,192 non-treated patients, 1,111 were divided into 6
different groups [(n=513) normal, (n=379) hypothyroxinemia, (n=108)
hypothyroidism, (n=96) SCH, (n=6) hyperthyroidism and (n=9)
subclinical hyperthyroidism] and the remaining 81 cases did not
deliver at the end of the study due to abortion, induction,
miscarriage or other reasons (Table
II). To evaluate the effects of L-T4 administration, pregnancy
outcomes were compared between three L-T4 treatment subgroup (n=92
in hypothyroidism; n=29 in SCH; n=74 in hypothyroxinemia) and the
corresponding non-treatment subgroup (n=108 in hypothyroidism; n=96
in SCH; n=379 in hypothyroxinemia).
 | Table II.Outcome comparison between subgroups
in non-treated subjects from cohort 2014. |
Table II.
Outcome comparison between subgroups
in non-treated subjects from cohort 2014.
| Outcomes and
complications | Normal, n=513 | Hypothyroxinemia,
n=379 | Hypothyroidism,
n=108 | Subclinical
hypothyroidism, n=96 | Hyperthyroidism,
n=6 | Subclinical
hyperthyroidism, n=9 |
|---|
| Gestational
diabetes mellitus | 38 (7.4) | 39 (10.3) | 12 (11.1) | 5 (5.2) | 0 (0) | 2 (22.2) |
| Intrahepatic
cholestasis of pregnancy | 6 (1.2) | 9 (2.4) | 5
(4.6)a | 4
(4.2)a | 0 (0) | 0 (0) |
| Gestational
hypertension | 6 (1.2) | 17
(4.5)b | 15
(13.9)b | 3 (3.1) | 2
(33.3)b | 0 (0) |
| Premature
birth | 43 (8.4) | 57
(15.0)b | 20
(18.5)b | 16
(16.7)a | 4
(66.7)b | 3
(33.3)a |
| Very LBW | 6 (1.2) | 14
(3.7)a | 4 (3.7) | 2 (2.1) | 0 (0) | 1
(11.1)a |
| LBW | 19 (3.7) | 25 (6.6) | 15
(13.9)b | 10
(10.4)b | 1 (16.7) | 1 (11.1) |
| Large newborns | 43 (8.4) | 34 (9.0) | 4 (3.7) | 1 (1.0) | 0 (0) | 0 (0) |
| Birth asphyxia | 12 (2.3) | 12 (3.2) | 3 (2.8) | 1 (1.0) | 0 (0) | 0 (0) |
| Fetal distress | 14 (2.7) | 19 (5.0) | 7 (6.5) | 2 (2.1) | 0 (0) | 0 (0) |
| Full term PROM | 75 (14.6) | 52 (13.7) | 23 (21.3) | 10 (10.4) | 1 (16.7) | 0 (0) |
| Preterm PROM | 22 (4.3) | 18 (4.7) | 4 (3.7) | 8 (8.3) | 2
(33.3)b | 2
(22.2)a |
| Placental
abruption | 2 (0.4) | 2 (0.5) | 3
(2.8)b | 0 (0) | 0 (0) | 0 (0) |
| Miscarriage | 33 (6.4) | 12
(3.2)a | 0 (0) | 18
(18.8)b | 0 (0) | 3
(33.3)b |
| Fetal
malformation | 21 (4.1) | 13 (3.4) | 1 (0.9) | 3 (3.1) | 0 (0) | 1 (11.1) |
| Mortality | 3 (0.6) | 2 (0.5) | 2 (1.9) | 2 (2.1) | 1
(16.7)b | 0 (0) |
Clinical guidelines for diagnosis
The ATA developed clinical guidelines on the
diagnosis and treatment of thyroid disease during pregnancy and the
postpartum period in order to provide evidence-based
recommendations to inform clinical decision-making. These
guidelines from ATA were first published in 2011 (17) and recently updated in 2017 (22) since significant clinical and
scientific advances have occurred in the field. The task force that
produced these guidelines consisted of international experts in the
field of thyroid disease and pregnancy, as well as international
representatives including the Asia and Oceania Thyroid Association.
Furthermore, the 2012 Chinese Guidelines suggested that high TSH
and fT4 levels were important reassessment markers; thus, these
markers were included in the present study to ensure widespread
acceptance and adoption of the developed guidelines for Chinese
patients.
Thyroid hormone laboratory
measurement
The analytical evaluation of the novel
electro-chemiluminescent immunoassays was performed for the in
vitro quantitative determination of TSH (cat. no. 11731459) and
fT4 (cat. no. 12017709) using the Elecsys 2010 immunoassay system
(Roche Diagnostics, Basel, Switzerland). The results indicated
normal TSH and fT4 values in healthy individuals (97.5% Confidence
Intervals): TSH (0.27–4.2 mIU/l), fT4 (12–22 pmol/l; P5 (5.0th
percentile)=12.87 pmol/l, P10 (10.0th percentile)=13.64 pmol/l).
According to the 2011 ATA Guidelines, the normal ranges of TSH
levels were as follows: Trimester 1, 0.1–2.5 mIU/l; trimester 2,
0.2–3.0 mIU/l; trimester 3, 0.3–3.0 mIU/l; while according to the
2017 ATA Guidelines, the maximum value in all three trimesters
increased to 4.0 mIU/l. The normal range of fT4 in the current
study was based on the 2011 ATA Guidelines for the healthy
population (12.0–22.0 pmol/l for all three trimesters). By
contrast, the normal ranges, according to the 2012 Chinese
Guidelines, were even higher: 0.05–5.17, 0.39–5.22 and 0.60–6.84
mIU/l for TSH in the three trimesters, respectively; and
12.91–22.35, 9.81–17.26 and 9.12–15.71 pmol/l for fT4 in the three
trimesters, respectively.
Diagnostic criteria for thyroid
diseases during pregnancy
Screening for thyroid dysfunction was performed
based on thyroid function tests, including assays for TSH and fT4
as aforementioned. The normal ranges from either the ATA or Chinese
Guidelines were used. Patients were diagnosed as follows:
Hypothyroxinemia: TSH within the normal range, fT4<P5 (5.0th);
hypothyroidism: TSH>upper limit (97.5th) or TSH>10 mIU/l and
fT4<lower limit (2.5th); SCH: upper limit<TSH≤10 mIU/l;
hyperthyroidism: TSH<lower limit, fT4>upper limit and no
syndrome of gestational hyperthyroidism; subclinical
hyperthyroidism: TSH<lower limit and fT4 within the normal
range. According to the previously published analysis method
(23), one value (the most recent or
the worst) was collected for each subject despite the number of
thyroid function exams conducted.
Gestational stages were set from the date of the
last period and verified by ultrasounds; the trimesters were as
follows: Trimester 1, prior to 12 weeks; trimester 2, between 13
and 27 weeks; trimester 3, between 28 weeks and delivery.
Complications [gestational diabetes, gestational hypertension,
intrahepatic cholestasis of pregnancy (ICP)] and adverse outcomes
[premature birth, very low birth weight (VLBW), low birth weight
(LBW), birth asphyxia, fetal distress, premature rupture of
membranes (PROM), placental abruption, miscarriage, fetal
malformation, mortality] were diagnosed according to the 2010 and
2014 Obstetrics and Gynecology and Pediatrics Guidelines, as
described previously (24,25).
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analyses and values were
presented as mean ± standard deviation (SD). When the variable
distribution of the quantitative data was normal, the mean values
of multiple groups were compared using one-way analysis of variance
followed by the Tukey's Honest Significant Difference post hoc
test. For the categorical data, the Chi-square test or Fisher's
exact test (sample size <5) was used. The Odds Ratio and 95%
Confidence Intervals were calculated using the binary variable
regression analysis. Cohen's kappa coefficient (kappa value) was
generated to measure the inter-rater agreement between the
percentages of patients with thyroid disease in accordance with the
two guidelines. P<0.05 was considered to indicate a
statistically significant difference.
Results
Different thyroid levels results in
various outcomes
The age, weight and incidence of thyroid disorder
were compared in the cohort 2010 and matched cohort 2014 groups
(Table I). In past decades, an
increasing proportion of women exhibit delayed childbearing for
educational, social and economic reasons (26,27),
which could explain the advanced maternal age observed in the 2014
matching cohort compared with the 2010 cohort. The mean weight of
the patients was also significantly increased in matched cohort
2014, which is likely due to the association between age and weight
(28,29). Thyroid function was screened by
measuring serum TSH and fT4 levels and the screening rates was
significantly increased in matched 2014 cohort (Table I). The incidence of thyroid disorders
including SCH, hypothyroidism, subclinical hyperthyroidism and
hyperthyroidism were decreased in cohort 2014 or matched cohort
2014 as compared with cohort 2010 (Fig.
1). No significant differences were identified in the number of
pregnancies, number of deliveries, smoking and drinking between the
non-treatment groups (data not shown). However, the proportion of
caesarean sections was significantly higher in the hypothyroxinemia
group compared with the normal group (data not shown).
Patients from cohort 2014 were stratified into
various subgroups with different thyroid abnormalities. The
outcomes of pregnancy and complications were compared between
subgroups in the non-treatment group from cohort 2014. Several
outcomes and complications were significantly different in thyroid
disorder subgroups as compared with the normal subgroup (Table II). The abnormal outcomes of
pregnancy and complications included ICP, gestational hypertension,
premature birth, VLBM, LBM, preterm PROM, miscarriage, placental
abruption and mortality. However, no significant differences were
identified in gestational diabetes mellitus (GDM), large newborns,
birth asphyxia, birth distress, full-term PROM and fetal
malformation between the two groups.
L-T4 administration reduces the odds
of adverse outcomes
To analyze the effects of L-T4 treatment on patients
with thyroid hormone deficiencies, 231 subjects from the
hypothyroidism, SCH and hypothyroxinemia groups in cohort 2014 were
treated with L-T4; the effects were compared with the non-treatment
group. L-T4 significantly reduced the odds of premature birth, and
LBW or VLBW in newborns in the hypothyroxinemia group (Table III). L-T4 also significantly
decreased the odds of gestational hypertension, premature birth and
LBW or VLBW in newborns in the hypothyroidism group.
 | Table III.Outcome comparison between L-T4
treatment group and non-treatment group from cohort 2014. |
Table III.
Outcome comparison between L-T4
treatment group and non-treatment group from cohort 2014.
| Outcomes and
complications | Hypothyroxinemia,
n=74 | Hypothyroidism | Subclinical
hypothyroidism |
|---|
| Subject number | 74 vs. 379 | 92 vs. 108 | 29 vs. 96 |
| (treatment vs.
non-treatment group) |
|
|
|
| Gestational
hypertension | 0.59
(0.13–2.62) | 0.209
(0.059–0.75)b | – |
| [Odd Ratio
(minimum-maximum)] |
|
|
|
| Premature
birth | 0.020
(0.005–0.085)b | 0.253
(0.091–0.70)b |
0.37(0.080–1.72) |
| [Odd Ratio
(minimum-maximum)] |
|
|
|
| LBW or very
LBW | 0.019
(0.003–0.15)a | 0.327
(0.13–0.88)a | – |
| [Odd Ratio
(minimum-maximum)] |
|
|
|
Following this, the treatment group in cohort 2014
were divided into two subgroups based on the thyroid levels
following L-T4 administration: Regular and Irregular. The irregular
subgroup was defined as having thyroid hormone levels not within
the regular range, according to the 2011 ATA Guidelines. In 41
(21.03%) of the total 195 patients from three subgroups
(Hypothyroidism, SCH and Hypothyroxinemia), thyroid hormone levels
returned to regular following treatment with L-T4 (Table IV). No significant differences in
pregnancy outcomes and complications were identified between the
normal and abnormal thyroid groups following treatment. It was
revealed that the odds of GDM occurring in patients from the SCH
subgroup was significantly increased compared with normal subjects
(23.5 vs. 7.4%). No differences in pregnancy outcomes and
complications were observed in patients from the other subgroups,
regardless of their thyroid hormone levels.
 | Table IV.Outcome comparison between
post-treated subjects and normal subjects from cohort 2014. |
Table IV.
Outcome comparison between
post-treated subjects and normal subjects from cohort 2014.
|
|
| Hypothyroidism | Subclinical
hypothyroidism |
Hypothyroxinemia |
|---|
|
|
|
|
|
|
|---|
| Outcomes and
complications | Normal n/a | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal |
|---|
| Number of
cases | 513 | 16 | 76 | 12 | 17 | 13 | 61 |
| Thyroid
diseases |
|
|
|
|
|
|
|
| Gestational
diabetes mellitus | 38 (7.4) | 3 (18.8) | 6 (7.9) | 0 (0) | 4
(23.5)a | 1 (7.7) | 9 (14.8) |
| Gestational
hypertension | 6 (1.2) | 1 (6.2) | 2 (2.6) | 0 (0) | 0 (0) | 0 (0) | 2 (3.3) |
| Premature
birth | 43 (8.4) | 0 (0) | 5 (6.6) | 1 (8.3) | 1 (5.9) | 0 (0) | 2 (3.3) |
| LBW or very
LBW | 25 (4.9) | 0 (0) | 6 (7.9) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) |
| Large
newbornsb | 43 (8.4) | 2 (12.5) | 6 (7.9) | 1 (8.3) | 1 (5.9) | 0 (0) | 4 (6.6) |
| Birth asphyxia | 12 (2.3) | 0 (0) | 2 (2.6) | 0 (0) | 1 (5.9) | 0 (0) | 1 (1.6) |
| Fetal distress | 14 (2.7) | 0 (0) | 1 (1.3) | 0 (0) | 1 (5.9) | 0 (0) | 2 (3.3) |
| Premature rupture
of membranes | 97 (18.9) | 1 (6.2) | 14 (18.4) | 1 (8.3) | 3 (17.6) | 2 (15.4) | 10 (16.4) |
| Miscarriage | 33 (6.4) | 0 (0) | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fetal
malformation | 21 (4.1) | 0 (0) | 2 (2.6) | 0 (0) | 0 (0) | 0 (0) | 2 (3.3) |
Two guidelines provide different
diagnostic criteria for thyroid diseases
Thyroid diseases were diagnosed according to the
2011 ATA and 2012 Chinese Guidelines following the measurement of
serum TSH and fT4 levels. In 0.73% of patients, fT4 levels were
higher than the cutoff values (data not shown). The percentage of
patients diagnosed with hypothyroxinemia, hypothyroidism and SCH
significantly increased when the 2011 ATA Guidelines were used
compared with the 2012 Chinese Guidelines (Table V). The percentage of normal subjects
and patients diagnosed with subclinical hyperthyroidism was
significantly decreased when the 2011 ATA Guidelines were used
compared with the 2012 Chinese Guidelines.
 | Table V.Percentage of patients with thyroid
diseases in cohort 2014 according to the two guidelines. |
Table V.
Percentage of patients with thyroid
diseases in cohort 2014 according to the two guidelines.
|
| Percentage [n
(%)] |
|
|
|---|
|
|
|
|
|
|---|
| Group | 2011 ATA | 2012 Chinese | Chi-square | P-value |
|---|
| Normal | 184 (22.25) | 651 (78.72) | 527.47 | <0.001 |
|
Hypothyroxinemia | 408 (49.33) | 93 (11.25) | 284.11 | <0.001 |
| Hypothyroidism | 111 (13.42) | 3 (0.36) | 109.89 | <0.001 |
| Subclinical
hypothyroidism | 100 (12.09) | 18 (2.18) | 61.36 | <0.001 |
|
Hyperthyroidism | 8 (0.97) | 12 (1.45) | 0.81 | 0.368 |
| Subclinical
hyperthyroidism | 13 (1.57) | 41 (4.96) | 15.00 | <0.001 |
| Syndrome of
gestational hyperthyroidism | 3 (0.36) | 3 (0.36) | 0 | 1.000 |
| Other | 0 (0) | 6 (0.72) | 48.76 | <0.001 |
The number of diagnoses of thyroid dysfunction
during pregnancy was compared when using the 2011 ATA and 2012
Chinese Guidelines. It was demonstrated that the numbers of
diagnoses made in the three trimesters with the two guidelines were
significantly different and that trimester 1 had the highest
accordance rate (Table VI). The
accordance rate was calculated by subtracting number of cases that
received same diagnoses in both guidelines from the total case
number. Similar analysis was conducted in the healthy,
hypothyroxinemia, hypothyroidism, SCH and subclinical
hyperthyroidism groups (data not shown). The two guidelines
resulted in different prevalence rates in all the comparisons.
 | Table VI.Percentage of patients with thyroid
diseases in cohort 2014 according to the two guidelines. |
Table VI.
Percentage of patients with thyroid
diseases in cohort 2014 according to the two guidelines.
|
|
|
| 2012 Chinese |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Group | n | 2011 ATA | + | − | Accordance rate
(%) | Kappa value | P-value |
|---|
| Trimester 1 | 69 | + | 37 | 0.355 | 71.01 | 0.355 | 0.012 |
|
|
| – | 16 | 12 |
|
|
|
| Trimester 2 | 116 | + | 44 | 0.144 | 51.72 | 0.144 | <0.001 |
|
|
| – | 53 | 16 |
|
|
|
| Trimester 3 | 642 | + | 84 | 0.041 | 33.18 | 0.041 | <0.001 |
|
|
| – | 417 | 129 |
|
|
|
| Total | 827 | + | 165 | 0.074 | 38.94 | 0.074 | <0.001 |
|
|
| – | 486 | 157 |
|
|
|
Discussion
Comprehensive experiments were performed to evaluate
the effects of TSH levels on pregnancy outcomes and complications
in China (30–32). The current study indicated that when
pregnancy is compounded by thyroid disorders including
hypothyroidism, SCH and hyperthyroidism, the potential for maternal
and fetal adverse outcomes increases. Hypothyroxinemia and
subclinical hyperthyroidism significantly increases adverse
pregnancy outcomes and complications (7). Furthermore, L-T4 administration in
thyroid deficiency groups may improve pregnancy outcomes and
decrease the odds of complications (33).
Proper diagnosis of thyroid dysfunction during
pregnancy is essential because maternal thyroid diseases complicate
pregnancy (1,2). Therefore, accurate diagnosis guidelines
may provide cutoff values to correctly diagnose thyroid diseases,
allowing appropriate clinical interventions. The metabolism and
hormone levels of pregnant women are different from those in
non-pregnant women (34,35); therefore, it is not appropriate to
use generalized guidelines to detect thyroid diseases during
pregnancy. However, it is not clear which guidelines should be
applied in China for pregnant patients. The current study compared
the 2011 ATA Guidelines and the 2012 Chinese Guidelines. It was
demonstrated that the two guidelines produced different thyroid
function screening rates in all thyroid diseases through the entire
gestational period. As the TSH reference values are relatively high
in the 2012 Chinese Guidelines, more focus should be given to
diagnose SCH in early and late pregnancy.
Thyroid hormones are essential for fetal brain
development, and thyroid abnormalities adversely affect offspring
neuropsychological development (10,14,34).
Thyroid disorders result in premature birth, gestational
hypertension, fetal mortality and other severe adverse outcomes
(36–38). Therefore, it is necessary to screen
thyroid function during early pregnancy. Thyroid diseases,
including hypothyroxinemia, hypothyroidism, SCH, hyperthyroidism
and subclinical hyperthyroidism, are associated with increased
risks of maternal and fetal complications (8,10,14,21).
However, whether all the aforementioned thyroid conditions are
associated with similar pregnancy outcomes or newborn abnormalities
have not been studied previously. The results of the present study
demonstrated that various thyroid levels complicate pregnancy and
fetal development. The comprehensive data from the present study
included various pregnancy complications during different
trimesters. The inconsistencies in the impact of subclinical
hyperthyroidism and hyperthyroidism in cohort 2014 compared with
other studies may be due to the small sample size (9 and 6 cases)
(8,39,40).
The treatment of SCH and hypothyroxinemia is a
debated topic. Administration of L-T4 is beneficial according to
certain studies (41–43), whereas potentially serious and
under-recognized drug interactions can be harmful and dangerous
(44). In addition, studies claimed
that treatment with L-T4 did not cause a significant difference in
offspring development (45,46). L-T4 decreased the number of patients
with thyroid deficiency also having gestational hypertension, LBW
or VLBW babies, premature births and miscarriage, which is
consistent with previously published studies (46,47).
Therefore, the L-T4 administration protocols can be applied in
obstetrics departments in patients with similar thyroid disorders.
Multiple variable analyses confirmed that L-T4 supplementation,
gestational age and clinical classification contribute to TSH and
thyroid hormone levels (48).
During clinical practice conducted by the authors of
the present study, it was suspected that the 2011 ATA Guidelines
may include TSH and fT4 values that are too narrow and low to
assess the outcome of treatment in the Chinese population;
therefore, the current study aimed to test this hypothesis. By
comparing the post-treated subjects with normal subjects from the
non-treatment group, the data demonstrated that no differences in
pregnancy outcomes and complications were identified in spite of
thyroid hormone level improvement, according to the 2011 ATA
Guidelines. These data suggests that the reference TSH level in the
2011 ATA Guidelines (25th and 75th percentiles are 0.1 and 2.5
mIU/l, respectively) may be too narrow and low to assess the
outcome of treatment. Therefore, the authors of the current study
hypothesized that the 2012 Chinese Guidelines could be better due
to the wider normal TSH range (25th and 75th percentiles are 0.05
and 5.17 mIU/l). This conclusion may help to optimize and establish
evidence-based clinical guidelines for the management of thyroid
disorders that would be useful to generalist and subspeciality
physicians, and others providing care for Chinese patients. The
current study may provide evidence for the hypothesis that the
existing guidelines require revision to accommodate the
Asian/Chinese population.
There were limitations in the current study.
Firstly, the current study was conducted in one hospital. Future
studies with larger sample sizes and prospective approaches in
multiple centers are warranted. Secondly, weight was not considered
as a contributor of pregnancy outcomes. It has been reported that
obesity can elevate serum TSH levels (49,50);
therefore, it is possible that SCH may be misdiagnosed. In
addition, it may be informative for future studies to assess
thyroglobulin and thyroid peroxidase antibodies to thoroughly
evaluate the thyroid-autoimmunity-associated thyroidal responses
during pregnancy.
Early screening of thyroid diseases during pregnancy
may allow L-T4 intervention to improve thyroid levels and decrease
several adverse pregnancy outcomes and complications. The current
study suggested that diagnosis of thyroid diseases exhibits
regional specificity and that existing guidelines require
modifications to accommodate the Asian/Chinese population.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Hunan Science
and Technology Department (grant no. 2012FJ4072) and the Hunan
Health and Family Planning Commission (grant no. C2015-003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MZ and MW conceived and designed; MZ, JL, XL and ML
performed the experiments and analyzed the data. ML and XL wrote
the manuscript. All authors critically revised and approved the
final manuscript.
Ethics approval and consent to
participate
The current study was approved by the institutional
review board of Central South University. All patients provided
written informed consent.
Patient consent for publication
All patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allan WC, Haddow JE, Palomaki GE, Williams
JR, Mitchell ML, Hermos RJ, Faix JD and Klein RZ: Maternal thyroid
deficiency and pregnancy complications: Implications for population
screening. J Med Screen. 7:127–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan I, Okosieme OE and Lazarus JH:
Current challenges in the pharmacological management of thyroid
dysfunction in pregnancy. Expert Rev Clin Pharmacol. 10:97–109.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rotondi M, Chiovato L, Pacini F, Bartalena
L and Vitti P: Management of subclinical hypothyroidism in
pregnancy: A comment from the italian society of endocrinology
(SIE) and the italian thyroid association (AIT) to the 2017 ATA
guidelines. ‘The Italian Way’. Thyroid. 28:551–555. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American College of Obstetrics and
Gynecology: ACOG practice bulletin, . Perinatal care at the
threshold of viability. Number 38, September 2002. American College
of Obstetrics and Gynecology. Int J Gynaecol Obstet. 79:181–188.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delitala AP, Capobianco G, Cherchi PL,
Dessole S and Delitala G: Thyroid function and thyroid disorders
during pregnancy: A review and care pathway. Arch Gynecol Obstet.
299:327–338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vulsma T, Gons MH and de Vijlder JJ:
Maternal-fetal transfer of thyroxine in congenital hypothyroidism
due to a total organification defect or thyroid agenesis. N Engl J
Med. 321:13–16. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li
T, Xu YH and Tao FB: Maternal thyroid function in the first twenty
weeks of pregnancy and subsequent fetal and infant development: A
prospective population-based cohort study in China. J Clin
Endocrinol Metab. 96:3234–3241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medenica S, Nedeljkovic O, Radojevic N,
Stojkovic M, Trbojevic B and Pajovic B: Thyroid dysfunction and
thyroid autoimmunity in euthyroid women in achieving fertility. Eur
Rev Med Pharmacol Sci. 19:977–987. 2015.PubMed/NCBI
|
|
9
|
Casey BM and Leveno KJ: Thyroid disease in
pregnancy. Obstet Gynecol. 108:1283–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noten AM, Loomans EM, Vrijkotte TG, van de
Ven PM, van Trotsenburg AS, Rotteveel J, van Eijsden M and Finken
MJ: Maternal hypothyroxinaemia in early pregnancy and school
performance in 5-year-old offspring. Eur J Endocrinol. 173:563–571.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Williams FL, Watson J, Ogston SA, Visser
TJ, Hume R and Willatts P: Maternal and umbilical cord levels of
T4, FT4, TSH, TPOAb, and TgAb in term infants and
neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab.
98:829–838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C,
Teng X, Guo R, Wang H, Li J, et al: Abnormalities of maternal
thyroid function during pregnancy affect neuropsychological
development of their children at 25–30 months. Clin Endocrinol
(Oxf). 72:825–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berbel P, Mestre JL, Santamaria A, Palazón
I, Franco A, Graells M, González-Torga A and de Escobar GM: Delayed
neurobehavioral development in children born to pregnant women with
mild hypothyroxinemia during the first month of gestation: The
importance of early iodine supplementation. Thyroid. 19:511–519.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Behrooz HG, Tohidi M, Mehrabi Y, Behrooz
EG, Tehranidoost M and Azizi F: Subclinical hypothyroidism in
pregnancy: Intellectual development of offspring. Thyroid.
21:1143–1147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casey BM, Dashe JS, Wells CE, McIntire DD,
Leveno KJ and Cunningham FG: Subclinical hyperthyroidism and
pregnancy outcomes. Obstet Gynecol. 107:337–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gilbert EW, Tay CT, Hiam DS, Teede HJ and
Moran LJ: Comorbidities and complications of polycystic ovary
syndrome: An overview of systematic reviews. Clin Endocrinol (Oxf).
89:683–699. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stagnaro-Green A, Abalovich M, Alexander
E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP,
Sullivan S, et al: Guidelines of the american thyroid association
for the diagnosis and management of thyroid disease during
pregnancy and postpartum. Thyroid. 21:1081–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiping T, Tao D, Guang N, Huixia Y and
ZHengpei Z: Guidelines for the diagnosis and treatment of thyroid
disease during pregnancy and postpartum. Chin J Endocrinol Metab.
25:2012.
|
|
19
|
Ya M: Interpretation of the management
guidelines for patients with thyroid nodules and differentiated
thyroid cancer (2012 Chinese edition). Lin Chuang Er Bi Yan Hou Tou
Jing Wai Ke Za Zhi. 27:917–920. 2013.(In Chinese). PubMed/NCBI
|
|
20
|
Habimana L, Twite KE, Daumerie C,
Wallemacq P, Donnen P, Kalenga MK and Robert A: High prevalence of
thyroid dysfunction among pregnant women in lubumbashi, democratic
republic of congo. Thyroid. 24:568–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casey BM, Thom EA, Peaceman AM, Varner MW,
Sorokin Y, Hirtz DG, Reddy UM, Wapner RJ, Thorp JM Jr, Saade G, et
al: Treatment of subclinical hypothyroidism or hypothyroxinemia in
pregnancy. N Engl J Med. 376:815–825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alexander EK, Pearce EN, Brent GA, Brown
RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel
SJ, et al: 2017 Guidelines of the american thyroid association for
the diagnosis and management of thyroid disease during pregnancy
and the postpartum. Thyroid. 27:315–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jianxin L, Shen W, zhongyan S, Weiwei W
and Weiping T: The relationship between thyroid dysfunction and
pregnancy outcomes in pregnant women. Chin J Endocrinol Metab.
30:1058–1062. 2014.
|
|
24
|
Shan Z and Teng W: Reflections on the
iodine nutrition status of pregnancy women in iodine sufficient
areas. Zhonghua Nei Ke Za Zhi. 54:4–5. 2015.(In Chinese).
PubMed/NCBI
|
|
25
|
Zhang Y, Wang H, Pan X, Teng W and Shan Z:
Patients with subclinical hypothyroidism before 20 weeks of
pregnancy have a higher risk of miscarriage: A systematic review
and meta-analysis. PLoS One. 12:e01757082017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gui S: Population growth and sustainable
development in China. China Popul Today. 15:26–14. 1998.PubMed/NCBI
|
|
27
|
Zong Z, Huang J, Sun X, Mao J, Shu X and
Hearst N: Prenatal care among rural to urban migrant women in
China. BMC Pregnancy Childbirth. 18:3012018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Apostolopoulou M, Savopoulos C, Michalakis
K, Coppack S, Dardavessis T and Hatzitolios A: Age, weight and
obesity. Maturitas. 71:115–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heiat A: Impact of age on definition of
standards for ideal weight. Prev Cardiol. 6:104–107. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong X, Liu A, Li Y, Sun H, Li Y, Li C, Yu
X, Fan C, Shan Z and Teng W: The impact of isolated maternal
hypothyroxinemia during the first and second trimester of gestation
on pregnancy outcomes: An intervention and prospective cohort study
in China. J Endocrinol Invest. 2018.
|
|
31
|
Chen S, Zhou X, Zhu H, Yang H, Gong F,
Wang L, Zhang M, Jiang Y, Yan C, Li J, et al: Preconception TSH and
pregnancy outcomes: A population-based cohort study in 184 611
women. Clin Endocrinol (Oxf). 86:816–824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang H, Shao M, Chen L, Chen Q, Yu L, Cai
L, Lin Z, Zhang C and Lu X: Screening strategies for thyroid
disorders in the first and second trimester of pregnancy in China.
PloS one. 9:e996112014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ju R, Lin L, Long Y, Zhang J and Huang J:
Clinical efficacy of therapeutic intervention for subclinical
hypothyroidism during pregnancy. Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
34
|
Stagnaro-Green A and Rovet J: Maternal
thyroid function in pregnancy - a tale of two tails. Nat Rev
Endocrinol. 12:1102016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glinoer D: The regulation of thyroid
function in pregnancy: Pathways of endocrine adaptation from
physiology to pathology. Endocr Rev. 18:404–433. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yazbeck CF and Sullivan SD: Thyroid
disorders during pregnancy. Med Clin North Am. 96:235–256. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Groot L, Abalovich M, Alexander EK,
Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D,
Mandel SJ, et al: Management of thyroid dysfunction during
pregnancy and postpartum: An endocrine society clinical practice
guideline. J Clin Endocrinol Metab. 97:2543–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abalovich M, Gutierrez S, Alcaraz G,
Maccallini G, Garcia A and Levalle O: Overt and subclinical
hypothyroidism complicating pregnancy. Thyroid. 12:63–68. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sarkar S and Bischoff LA: Management of
hyperthyroidism during the preconception phase, pregnancy, and the
postpartum period. Semin Reprod Med. 34:317–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rytter D, Andersen SL, Bech BH,
Halldorsson TI, Henriksen TB, Laurberg P and Olsen SF: Maternal
thyroid function in pregnancy may program offspring blood pressure,
but not adiposity at 20 y of age. Pediatr Res. 80:7–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Negro R, Schwartz A, Gismondi R, Tinelli
A, Mangieri T and Stagnaro-Green A: Universal screening versus case
finding for detection and treatment of thyroid hormonal dysfunction
during pregnancy. J Clin Endocrinol Metab. 95:1699–1707. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kasatkina EP, Samsonova LN, Ivakhnenko VN,
Ibragimova GV, Ryabykh AV, Naumenko LL and Evdokimova YA:
Gestational hypothyroxinemia and cognitive function in offspring.
Neurosci Behav Physiol. 36:619–624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Radetti G, Gentili L, Paganini C,
Oberhofer R, Deluggi I and Delucca A: Psychomotor and audiological
assessment of infants born to mothers with subclinical thyroid
dysfunction in early pregnancy. Minerva Pediatr. 52:691–698.
2000.PubMed/NCBI
|
|
44
|
Sahajpal R, Ahmed RA, Hughes CA and Foisy
MM: Probable interaction between levothyroxine and ritonavir: Case
report and literature review. Am J Health Syst Pharm. 74:587–592.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lazarus JH, Bestwick JP, Channon S,
Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George
LM, et al: Antenatal thyroid screening and childhood cognitive
function. N Engl J Med. 366:493–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vigone MC, Di Frenna M, Guizzardi F,
Gelmini G, de Filippis T, Mora S, Caiulo S, Sonnino M, Bonomi M,
Persani L and Weber G: Mild TSH resistance: Clinical and hormonal
features in childhood and adulthood. Clin Endocrinol (Oxf).
87:587–596. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arslan A, Bas VN, Uytun S and Poyrazoglu
HG: Effects of L-thyroxine treatment on heart functions in infants
with congenital hypothyroidism. J Pediatr Endocrinol Metab.
30:557–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hirsch D, Levy S, Nadler V, Kopel V,
Shainberg B and Toledano Y: Pregnancy outcomes in women with severe
hypothyroidism. Eur J Endocrinol. 169:313–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Santos MI, Limbert C, Marques FC, Rosario
F and Lopes L: Childhood obesity, thyroid function, and insulin
resistance - is there a link? A longitudinal study. J Pediatr
Endocrinol Metab. 28:557–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bilgin H and Pirgon O: Thyroid function in
obese children with non-alcoholic fatty liver disease. J Clin Res
Pediatr Endocrinol. 6:152–157. 2014. View Article : Google Scholar : PubMed/NCBI
|