Introduction
Magnetic resonance imaging (MRI) is a commonly used
diagnostic modality in the pediatric population owing to its high
resolution and absence of radiation exposure (1,2).
High-quality imaging facilitates accurate diagnosis, optimal
treatment and the monitoring of therapeutic responses in pediatric
patients. However, undergoing MRI procedures may be stressful,
particularly for pediatric subjects (3), who tend to experience anxiety and
distress prior to and during scanning. The long duration of
examination, the noise generated during the process and the narrow
confines of MRI devices occasionally lead to failed scans in
pediatric patients (4). To obtain
high-quality images in pediatric patients, sedation is administered
to prevent patient movement and mitigate emotional discomfort.
Dexmedetomidine is an α2-adrenergic
receptor agonist that is widely used for procedures requiring
sedation of pediatric patients due to its sedative and analgesic
characteristics (5,6). Propofol is also an effective and highly
popular sedative agent in pediatric patients (7,8). Several
randomized controlled trials (RCTs) have compared the two drugs in
pediatric patients undergoing MRI. Koroglu et al (9) reported a shorter recovery time in
pediatric patients who received propofol, with a comparable time to
onset of sedation and duration of sedation between the two drugs. A
study by Wu et al (10)
indicated a shorter recovery and onset of sedation for propofol,
which is consistent with the results of a previous meta-analysis by
our group (11), which included RCTs
and non-RCTs. However, in this previous meta-analysis, one RCT
(12) was missed in the pooling of
data, and a novel RCT (13) was
recently published, thus prompting an updated meta-analysis on this
topic. Accordingly, a new meta-analysis with trial sequential
analysis (TSA) performed with.
Materials and methods
Search strategy
The PubMed, Cochrane Library and Web of Knowledge
databases were searched for entries up to August 2018 for potential
trials comparing dexmedetomidine and propofol in pediatric patients
undergoing MRI without restriction by study type or publication
language, according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses statement (14). The following terms were used in the
literature search with various combinations: ‘Dexmedetomidine’ AND
‘propofol’ AND (‘magnetic resonance imaging’ OR ‘MRI’) AND
(‘pediatric’ OR ‘children’ OR ‘child’ OR ‘adolescence’).
Inclusion criteria
Two authors (YT and JM) independently assessed the
trial eligibility. Any disagreement between the two authors was
resolved by the senior author (XZ). Eligible trials were required
to fulfill the following criteria: i) RCT; ii) comparison of
dexmedetomidine and propofol; iii) subjects aged <18 years; and
iv) subjects undergoing MRI.
Data extraction
Baseline variables were extracted from the eligible
studies, including the following: Publication year, first author,
study period, region, number of patinets, patient age, body weight,
sex, MRI machines and details on the intervention. Sedation
parameters were collected, including recovery time, onset time of
sedation, duration of sedation, discharge time and failed sedation.
Timeline parameters are depicted in Fig.
1. The incidence of adverse events with sufficient data for
analysis was also recorded. Desaturation was defined as oxygen
saturation (<93%. The Pediatric Anesthesia Emergence Delirium
scale (PAED) (15) was used to
monitor the behavior of the pediatric patients. Patients with a
PAED score of >10 points had a high risk of emergent delirium.
The number of patients with a PAED score of >10 points at 5 and
10 min after drug discontinuation was recorded as 5-min and 10-min
PAED.
Risk of bias assessment
The major domains of bias in each study were
assessed according to the recommendations from the Cochrane risk of
bias tool (16). In general, the
studies were categorized as having low, high and unknown risk of
bias regarding the following items: Random sequence generation,
allocation concealment, blinding of participants and staff,
blinding of outcome assessment, incomplete outcome data, selective
outcome reporting and other bias.
Trial sequential analysis
Meta-analysis may lead to type I errors due to an
increased risk of random errors when insufficient data are included
and due to multiple testing with new trials. Trial sequential
analysis (TSA) is a program that calculates the required
information size for a meta-analysis with an adjusted threshold for
statistical significance in the cumulative meta-analysis, which may
control the risk of type I and II errors. When the cumulative z
curve crosses the TSA monitoring boundary, a sufficient level of
evidence is achieved for the anticipated intervention effect. If
the z curve dose not cross any boundary and the required
information size has not been reached, evidence for a definite
conclusion is not sufficient. TSA software version 0.9 beta
(Copenhagen Trial Unit, Copenhagen, Denmark) was applied in the
study to estimate the optimal sample size.
Statistical analysis
All the meta-analyses were performed using Revman
5.3 software (Nordic Cochrane Centre, Cochrane Collaboration), with
relative risks (RR) or standard mean differences (SMD) with 95%
confidence intervals (CIs) calculated for dichotomous and
continuous variables, respectively. Heterogeneity among the studies
was explored by a standard Cochrane's Q test and I2. A
fixed- or random-effects model was used to estimate the differences
between groups in the case of absence or presence of heterogeneity
among the studies included. Heterogeneity among the studies was
assessed using I2 statistics with I2>50%
being considered to indicate significant heterogeneity. The
fixed-effects model was selected when I2≤50% and the
random-effects model was used when I2>50%. P<0.05
was considered to indicate statistical significance.
Results
Study search and characteristics
A flow diagram depicting the selection process of
studies is provided in Fig. 2. The
initial search yielded 141 records from the PubMed, Web of
Knowledge and Cochrane Library databases, from which 97 duplicates
were excluded. After review of titles and abstracts, 80 articles
were excluded. A further 11 trials were removed, and the details
are provided in Fig. 2 Finally, 6
studies (9,10,12,13,17,18),
from 5 countries, including Turkey, the USA, Singapore, India and
China, involving 415 pediatric patients (207 receiving
dexmedetomidine and 208 propofol) were included in the final
analysis. The study characteristics are displayed in Table I. Only 2 of the studies indicated the
study period. A total of 3 studies (10,12,17)
reported details of sedation induction. Sevoflurane and nitrous
oxide in oxygen were used for inhalation induction. The
administration methods of dexmedetomidine and propofol are also
provided in Table I. The type of MRI
device was described in two studies (12,13).
Details of sedation effects and adverse events are presented in
Table II. This included data for
recovery time, onset of sedation time, duration of sedation,
discharge time, failed sedation, desatuation, 5 min PAED and 10 min
PAED.
 | Table I.Baseline characteristics of the
studies included in the meta-analysis. Data were shown in the form
of mean ± standard deviation or median (range). |
Table I.
Baseline characteristics of the
studies included in the meta-analysis. Data were shown in the form
of mean ± standard deviation or median (range).
| Item | Koroglu (9), 2005 | Wu (10), 2014 | Bong (17), 2015 | Watt (12), 2016 | Kamal (18), 2017 | Xiao (13), 2017 |
|---|
| Region | Turkey | USA | Singapore | USA | India | China |
| Period | NA |
2009.01–2011.01 | NA | NA | NA |
2016.02–2016.08 |
| Sedation
induction | NA | Inhalation
induction with sevoflurane and nitrous oxide in oxygen | Inhalational
induction with sevoflurane in an oxygen/nitrous oxide mixture | Inhalational
induction with 8% sevoflurane in 4 l/min of nitrous oxide and 2
l/min oxygen | NA | NA |
| Intervention |
|
|
|
|
|
|
|
Dex | IV 1 µg/kg initial
dose for 10 min, followed by continuous infusion of 0.5
µg/kg/h | IV 2 µg/kg initial
dose for 10 min, followed by continuous infusion of 2 µg/kg/h | IV 0.3 µg/kg | IV 1 µg/kg initial
dose over 10 min, with 0.1 mg/kg midazolam, followed by continuous
infusion of 1 µg/kg/h | IV 2 µg/kg initial
dose for 10 min, followed by continuous infusion of 1 µg/kg/h | IV 1 µg/kg initial
dose for 10 min, followed by continuous infusion of 0.3–0.8
µg/kg/h |
|
Pro | IV 3 mg/kg initial
dose for 10 min, followed by continuous infusion of 100
µg/kg/min | IV 2 mg/kg initial
dose, followed by continuous infusion of 200 µg/kg/min | IV 1 mg/kg | IV 300 µg/kg/min
initial dose for 10 mins, then reduction to 250 µg/kg/min | IV 1 mg/kg initial
dose, followed by continuous infusion of 100 µg/kg/min | IV 2.5 mg/kg
initial dose for 10 min, followed by 80–100 µg/kg/min continuous
infusion |
| MRI | NA | NA | NA | 1.5-T GE Exciter
12.0 | NA | 3.0-T GE Propeller
HDMR |
| Number of
subjects | 30/30 | 46/49 | 40/39 | 16/15 | 30/30 | 45/45 |
| (Dex/Pro) |
|
|
|
|
|
|
| Sex (M,
Dex/Pro) | 17/10 | NA | 24/24 | 13/10 | 12/14 | 23/25 |
| Age
(years/months) | 1.00–7.00 | 1.00–7.00 | 2.00–7.00 | 3.00–7.00 | 2.00–10.00 | 1.00–6.00 |
|
Dex | 4.00±1.88 | 51.30±22.10 | 3.00
(3.00–4.00) | 4.60±0.80 | 5.20±2.69 | 3.54±1.93 |
|
Pro | 3.00±2.03 | 47.30±22.40 | 4.00
(3.00–5.00) | 5.10±1.10 | 4.15±2.32 | 3.23±2.12 |
| Weight (kg) |
|
|
|
|
|
|
|
Dex | 14.00±4.14 | 17.90±6.99 | 14.00
(12.70–17.70) | 16.80±5.00 | 16.41±6.21 | 12.42±4.54 |
|
Pro | 14.00±4.57 | 15.40±4.69 | 15.20
(14.00–20.00) | 18.20±4.10 | 14.86±5.49 | 13.61±4.12 |
| MRI duration
(min) |
|
|
|
|
|
|
|
Dex | 22.00±7.14 | 91.50±25.60 | NA | 52.00±11.00 | 23.33±4.64 | 22.20±6.70 |
|
Pro | 25.00±10.14 | 81.70±21.70 | NA | 58.00±12.00 | 25.18±5.01 | 29.50±11.20 |
 | Table II.Details on sedative effects and
adverse events in the studies included in the meta-analysis. Data
were shown in the form of mean ± standard deviation. |
Table II.
Details on sedative effects and
adverse events in the studies included in the meta-analysis. Data
were shown in the form of mean ± standard deviation.
| Item | Koroglu (9), 2005 | Wu (10), 2014 | Bong (17), 2015 | Watt (12), 2016 | Kamal (18), 2017 | Xiao (13), 2017 |
|---|
| Recovery time
(min) |
|
|
|
|
|
|
|
Dex | 27.00±19.05 | 62.50±30.0 | 26.00±18.00 | 39.00±17.00 | 9.02±2.99 | 15.34±5.26 |
|
Pro | 18.00±4.72 | 35.70±10.8 | 22.00±14.00 | 27.00±9.00 | 3.52±1.07 | 8.43±4.51 |
| Onset of sedation
time (min) |
|
|
|
|
|
|
|
Dex | 11.00±4.00 | 24.20±4.84 | NA | 8.00±1.00 | 7.00±1.74 | 16.87±4.72 |
|
Pro | 4.00±1.94 | 16.30±5.54 | NA | 8.00±2.00 | 3.43±1.34 | 11.51±3.92 |
| Duration of
sedation (min) |
|
|
|
|
|
|
| Dex | 47.00±14.93 | NA | 71.00±25.00 | NA | 30.20±5.26 | NA |
|
Pro | 46.00±17.59 | NA | 70.00±28.00 | NA | 28.60±4.61 | NA |
| Discharge time
(min) |
|
|
|
|
|
|
|
Dex | 39.00±24.35 | NA | NA | 97.00±36.00 | NA | NA |
|
Pro | 27.00±6.50 | NA | NA | 91.00±27.00 | NA | NA |
| Failed
sedation |
|
|
|
|
|
|
|
Dex | NA | 15 | NA | NA | 9 | NA |
|
Pro | NA | 1 | NA | NA | 5 | NA |
| Desaturation |
|
|
|
|
|
|
|
Dex | 0 | 0 | NA | NA | 0 | 0 |
|
Pro | 4 | 2 | NA | NA | 0 | 7 |
| 5-min PAED |
|
|
|
|
|
|
|
Dex | NA | 18 | 9 | NA | NA | NA |
|
Pro | NA | 9 | 6 | NA | NA | NA |
| 10-min PAED |
|
|
|
|
|
|
|
Dex | NA | 16 | 5 | NA | NA | NA |
|
Pro | NA | 4 | 1 | NA | NA | NA |
Risk of bias assessment
The risk of bias assessment of the included studies
is provided in Table III. All of
the studies, except 3 studies, maintained a good control of each
domain. Wu et al (10), Watt
et al (12) and Xiao et
al (13) did not include any
details of the random sequence generation. Furthermore, the study
by Xiao et al (13) had an
unclear risk of bias regarding allocation concealment.
 | Table III.Risk of bias assessment for all of
the studies included. |
Table III.
Risk of bias assessment for all of
the studies included.
| First author
(year) | Random sequence
generation | Allocation
concealment | Blinding
(participants and personnel) | Blinding (outcome
assessment) | Incomplete outcome
data | Selective
reporting | Other sources of
bias | (Refs.) |
|---|
| Koroglu (2005) | Low | Low | Low | Low | Low | Low | Low | (9) |
| Wu (2014) |
Unclear | Low | Low | Low | Low | Low | Low | (10) |
| Bong (2015) | Low | Low | Low | Low | Low | Low | Low | (17) |
| Watt (2016) |
Unclear | Low | Low | Low | Low | Low | Low | (12) |
| Kamal (2017) | Low | Low | Low | Low | Low | Low | Low | (18) |
| Xiao (2017) |
Unclear |
Unclear | Low | Low | Low | Low | Low | (13) |
Sedation outcomes
The results of the meta-analysis on sedation
efficacy are provided in Table IV,
which includes recovery time, onset of sedation time, duration of
sedation, discharge time and incidence of failed sedation. The data
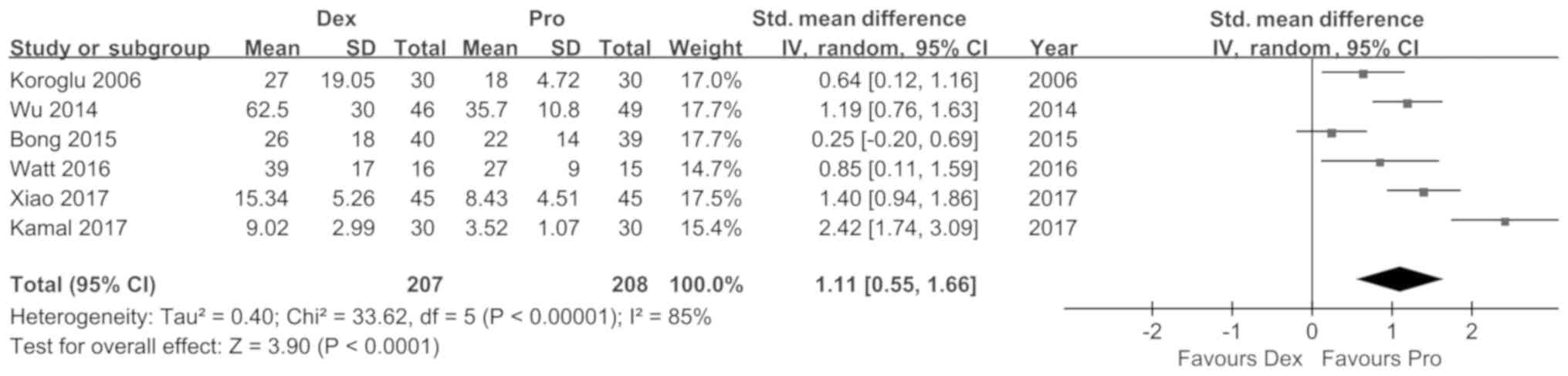
from 6 trials indicated a shorter recovery time [SMD (95%CI): 1.11
(0.55, 1.66), P<0.01, I2=85%; Fig. 3] and onset of sedation time for
propofol compared with dexmedetomidine [SMD (95%CI): 1.44 (0.77,
2.12), P<0.01, I2=86%; Fig. 4]. Pediatric patients who received
propofol were discharged from hospital sooner than those with
dexmedetomidine [SMD (95%CI): 0.49 (0.08, 0.91), P=0.02,
I2=14%; Fig. 5]. The
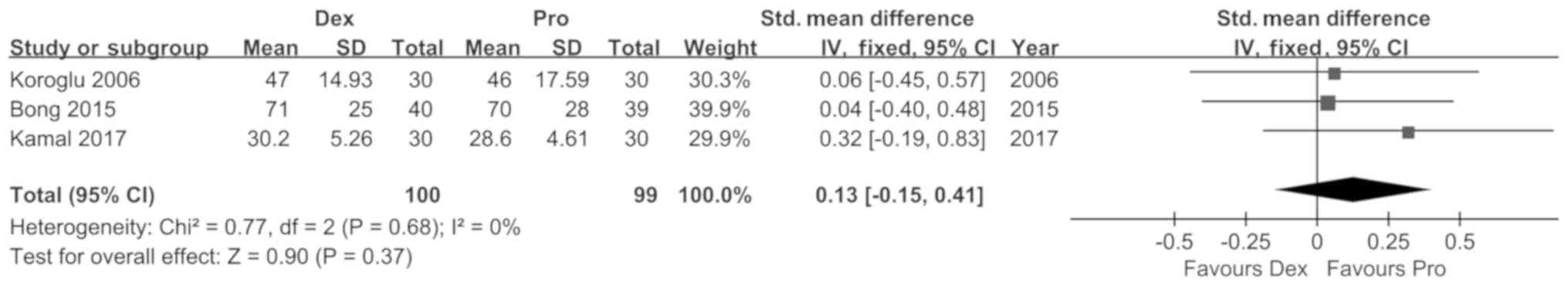
pooling of data from three studies revealed no significant
differences in the duration of sedation between the two
interventions [SMD (95%CI): 0.13 (−0.15, 0.41), P=0.37,
I2=0%; Fig. 6].
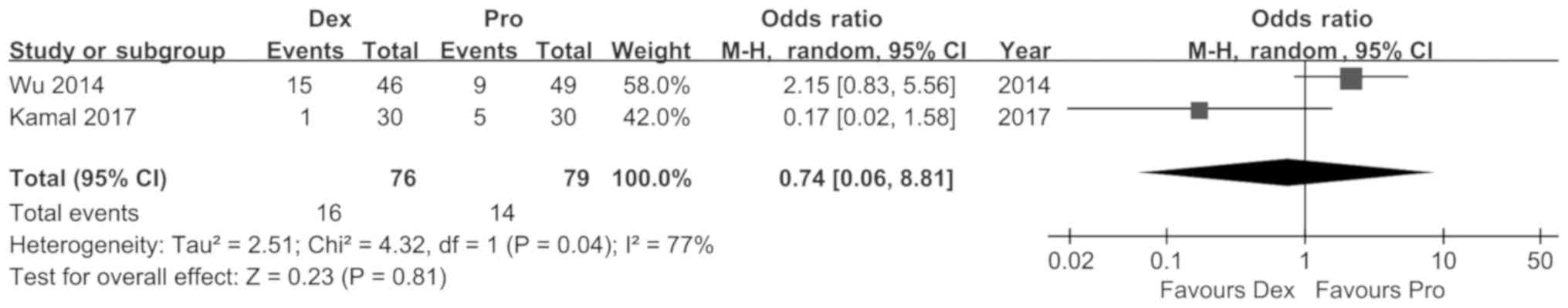
Furthermore, the incidence of failed sedation in the two groups did
not differ significantly [RR (95%CI): 0.74 (0.09, 6.38), P=0.78,
I2=75%; Fig. 7].
 | Table IV.Meta-analysis results of sedative
effects among the studies (Dex vs. Pro). |
Table IV.
Meta-analysis results of sedative
effects among the studies (Dex vs. Pro).
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|---|
| Outcome | df | P-value | RR/SMD [95%
CI] |
Chi2 | I2
(%) | P-Q test |
|---|
| Recovery time | 5 | <0.01 | 1.11 [0.55,
1.66] | 33.62 | 85 | <0.01 |
| Onset of sedation
time | 4 | <0.01 | 1.44 [0.77,
2.12] | 28.33 | 86 | <0.01 |
| Duration of
sedation | 2 | 0.37 | 0.13 [-0.15,
0.41] | 0.77 | 0 | 0.68 |
| Discharge time | 1 | 0.02 | 0.49 [0.08,
0.91] | 1.16 | 14 | 0.28 |
| Failed
sedation | 1 | 0.78 | 0.74 [0.09,
6.38] | 3.96 | 75 | 0.05 |
Adverse events and MRI outcomes
Further outcomes, including adverse events and MRI
duration, are summarized in Table V.
No desaturation was reported in subjects receiving dexmedetomidine
(RR [95%CI]: 0.11 [0.02, 0.55], P<0.01 vs. propofol,
I2=0%; Fig. 8), while
propofol induced a lower incidence of 5-min (RR [95%CI]: 1.86
[1.07, 3.23], P=0.03, I2=0%; Fig. 9) and 10-min (RR [95%CI]: 3.49 [1.50,
8.16], P<0.01, I2=0%; Fig. 10) PAED after sedation was
discontinued. The duration of scanning in the two groups was
relatively similar [SMD (95%CI): −0.35 (−0.82, 0.13), P=0.15,
I2=78%; Fig. 11], and
further information is provided below in regards to sensitivity
analysis. As there were <10 studies in total, no funnel plot
analysis was performed for the detection of publication bias.
 | Table V.Meta-analysis results of adverse
events and MRI parameters among the studies (Dex vs. Pro). |
Table V.
Meta-analysis results of adverse
events and MRI parameters among the studies (Dex vs. Pro).
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|---|
| Outcome | df | P-value | RR/SMD [95%
CI] |
Chi2 | I2
(%) | P-Q test |
|---|
| Desaturation | 4 | <0.01 | 0.11 [0.02,
0.55] |
0.31 | 0 | 0.86 |
| 5-min PAED | 1 | 0.03 | 1.86 [1.07,
3.23] |
0.40 | 0 | 0.53 |
| 10-min PAED | 1 | <0.01 | 3.49 [1.50,
8.16] |
0.01 | 0 | 0.91 |
| MRI duration | 4 | 0.15 | −0.35 [-0.82,
0.13] | 18.10 | 78 | <0.01 |
Heterogeneity and sensitivity
analysis
High heterogeneity in recovery time
(I2=85%, df=5), onset of sedation time
(I2=86%, df=4), failed sedation (I2=77%,
df=1) and MRI duration (I2=78%, df=4) was identified
among the studies. Sensitivity analysis for each comparison
revealed no robust changes in significance, and heterogeneity was
induced when a single study was removed in the analysis of recovery
time (Table VI) and onset of
sedation time (Table VII). For MRI
duration, exclusion of any of the trials did not change the results
and heterogeneity, except for the study by Wu et al
(10) (P<0.01, I2=0%;
Table VIII). Considering the high
risk of bias in the study by Xiao et al (13), all outcomes were analyzed with the
exclusion of this study, which revealed a trend in the incidence of
desaturation toward significance, from 0.008 to 0.07 (Table IX). The trends in any of the other
outcomes were not affected.
 | Table VI.Sensitivity analysis of recovery time
(Dex vs. Pro). |
Table VI.
Sensitivity analysis of recovery time
(Dex vs. Pro).
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|---|
| Study excluded,
first author (year) | P-value | SMD [95% CI] |
Chi2 | I2
(%) | P-Q test | (Refs.) |
|---|
| Koroglu (2005) | <0.01 | 1.20 [0.55,
1.86] | 31.13 | 87 | <0.01 | (9) |
| Wu (2014) | <0.01 | 1.09 [0.39,
1.79] | 32.86 | 88 | <0.01 | (10) |
| Bong (2015) | <0.01 | 1.29 [0.76,
1.81] | 18.34 | 78 | <0.01 | (17) |
| Watt (2016) | <0.01 | 1.15 [0.51,
1.79] | 33.40 | 88 | <0.01 | (12) |
| Kamal (2017) | <0.01 | 0.87 [0.42,
1.31] | 15.50 | 74 | <0.01 | (18) |
| Xiao (2017) | <0.01 | 1.05 [0.38,
1.72] | 30.44 | 87 | <0.01 | (13) |
 | Table VII.Sensitivity analysis of onset of
sedation time (Dex vs. Pro). |
Table VII.
Sensitivity analysis of onset of
sedation time (Dex vs. Pro).
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|---|
| Study excluded,
first author (year) | P-value | SMD [95% CI] |
Chi2 | I2
(%) | P-Q test | (Refs.) |
|---|
| Koroglu (2005) | <0.01 | 1.26 [0.51,
2.01] | 22.27 | 87 | <0.01 | (9) |
| Wu (2014) | <0.01 | 1.43 [0.50,
2.36] | 28.24 | 89 | <0.01 | (10) |
| Watt (2016) | <0.01 | 1.75 [1.26,
2.24] | 9.93 | 70 | 0.02 | (12) |
| Kamal (2017) | <0.01 | 1.25 [0.51,
1.98] | 21.29 | 86 | <0.01 | (18) |
| Xiao (2017) | <0.01 | 1.50 [0.59,
2.41] | 27.05 | 89 | <0.01 | (13) |
 | Table VIII.Sensitivity analysis of magnetic
resonance imaging time (Dex vs. Pro). |
Table VIII.
Sensitivity analysis of magnetic
resonance imaging time (Dex vs. Pro).
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|---|
| Study excluded,
first author (year) | P-value | SMD [95% CI] |
Chi2 | I2
(%) | P-Q test | (Refs.) |
|---|
| Koroglu (2005) | 0.26 | −0.35 [0.97,
0.26] | 18.07 | 83 | <0.01 | (9) |
| Wu (2014) | <0.01 | −0.58 [-0.84,
−0.32] | 1.77 | 0 | 0.62 | (10) |
| Watt (2016) | 0.27 | −0.32 [-0.88,
0.25] | 17.73 | 83 | <0.01 | (12) |
| Kamal (2017) | 0.33 | −0.29 [-0.88,
0.30] | 16.68 | 82 | <0.01 | (18) |
| Xiao (2017) | 0.39 | −0.22 [-0.73,
0.29] | 11.43 | 74 | 0.01 | (13) |
 | Table IX.Sensitivity analysis of incidence of
desaturation (Dex vs. Pro). |
Table IX.
Sensitivity analysis of incidence of
desaturation (Dex vs. Pro).
|
|
|
| Heterogeneity |
|
|---|
|
|
|
|
|
|
|---|
| Study excluded,
first author (year) | P-value | RR [95% CI] |
Chi2 | I2
(%) | P-Q test | (Refs.) |
|---|
| Koroglu (2006) | 0.03 | 0.10 [0.01,
0.78] | 0.32 | 0 | 0.57 | (9) |
| Wu (2014) | 0.02 | 0.08 [0.01,
0.63] | 0.06 | 0 | 0.08 | (10) |
| Kamal (2017) | <0.01 | 0.11 [0.02,
0.55] | 0.31 | 0 | 0.86 | (18) |
| Xiao (2017) | 0.07 | 0.15 [0.02,
1.16] | 0.09 | 0 | 0.76 | (13) |
TSA
TSA was performed for the outcomes that included
>5 trials. It was performed to analyze the results of recovery
time (Fig. 12). The TSA monitoring
boundary was crossed by the cumulative z curve, indicating the firm
evidence provided by the current results of recovery time. No
boundaries were crossed in the analysis of onset of sedation
time.
Discussion
The present meta-analysis summarizes the data from
current RCTs comparing dexmedetomidine and propofol in pediatric
patients undergoing MRI. It was indicated that propofol was
associated with a shorter recovery time and onset of sedation time
than dexmedetomidine. Dexmedetomidine and propofol were comparable
in terms of sedation duration, the incidence of failed sedation and
MRI duration. Propofol induced a lower incidence of 5-min and
10-min PAED, as well as a higher incidence of desaturation.
To date, two previous meta-analysis have been
published on this topic; however, the meta-analysis by Fang et
al (19) was deemed to be not as
accurate. A new meta-analysis was published by our group in 2017
(11), which revealed that propofol
had a shorter onset of sedation time and recovery time than
dexmedetomidine. The new meta-analysis of the present study
revealed similar results for these parameters, indicating better
sedative effects achieved with propofol. Compared with the previous
meta-analysis, the present study includes a recently published RCT
(13). Furthermore, the study by
Watt et al (12) was missed
in the previous analysis, thus encouraging more careful attention
in the selection process.
More variables were analyzed in the present analysis
compared with previous meta-analysis (11). This result may be attributed to the
quicker recovery from propofol than dexmedetomidine. Safety is as
important as the efficacy of the interventions in clinical trials;
consequently, the occurrence of desaturation, a severe condition
requiring immediate treatment, was analyzed. It is thought that
dexmedetomidine causes less airway collapse than propofol (12). This may explain why a higher
incidence of desaturation was observed in pediatric patients who
received propofol. For pediatric patients with respiratory
disorders, caution should be exercised when considering the use of
dexmedetomidine. To assess emergence delirium, a PAED scale was
used to evaluate patients in terms of restlessness, eye contact,
inconsolability, purposeful actions and consciousness of the
environment (20,21). Scores of >10 points indicated a
high risk for the occurrence of emergence delirium (10). Two studies indicated a lower
incidence of a PAED score of >10 points for propofol at 5 and 10
min after sedation, compared with dexmedetomidine. TSA is
increasingly used to detect the risk for type I errors and to test
the level of evidence of a meta-analysis (22). In the present study, the Z curve of
recovery time crossed the monitoring boundary, indicating firm
evidence for propofol as a preference over dexmedetomidine in terms
of sedative effects.
In addition, a quality assessment of the studies
included in the present meta-analysis was performed. Most of the
trials were of high quality, indicating a reliable evidence level
of the results. Heterogeneity was identified in the outcomes of
recovery time (I2=85%), onset time of sedation
(I2=86%), failed sedation (I2=77%) and MRI
duration (I2=78%). Only the recovery time, onset time of
sedation and MRI duration were deduced from >2 trials. No
significant change of heterogeneity emerged when sensitivity
analysis was performed on recovery time and onset time of sedation.
It was assumed that the high heterogeneity originated from the
inconsistency in sedation details and different sample sources.
Removing the study by Wu et al (10) changed the results and heterogeneity
of MRI duration (P<0.00001, I2=0%). MRI duration
varied depending on body parts and sequences for scanning; however,
no details of these indexes were available. It may be speculated
that the high heterogeneity was caused by the diversity of body
parts and scanning sequences.
The present meta-analysis had several limitations,
the first of which was the relatively small number of studies for
comparison, although two new studies were included compared with
the previous meta-analysis by our group. Data regarding certain
items in the trials were not available to us, despite numerous
efforts in contacting the authors of those trials. Consequently, a
thorough analysis of each variable was not possible. Furthermore,
the sample size in each of the studies was relatively small. In
addition, the high heterogeneity among the studies limited the
credibility of the study.
In conclusion, the present meta-analysis indicated
that dexmedetomidine and propofol had comparable sedation effects.
TSA provided supportive evidence favoring propofol over
dexmedetomidine in terms of shorter recovery time and onset of
sedation time, as well as a faster discharge from hospital, and a
lower incidence of PAED score >10. Propofol is recommended for
sedation of pediatric patients during MRI; however, considering the
possibility of desaturation, it should be used with caution.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used in the meta-analysis are available
from the corresponding author on reasonable request.
Authors' contributions
YT and XZ designed the study. JM and QZ performed
the literature search and data extraction. JL and QZ analyzed the
data. YT and JM drafted the manuscript. XZ revised the draft.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
TSA
|
trial sequential analysis
|
|
RCT
|
randomized controlled trial
|
|
PAED
|
Pediatric Anesthesia Emergence
Delirium
|
References
|
1
|
Callen DJ, Shroff MM, Branson HM, Lotze T,
Li DK, Stephens D and Banwell BL: MRI in the diagnosis of pediatric
multiple sclerosis. Neurology. 72:961–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moore MM, Gustas CN, Choudhary AK,
Methratta ST, Hulse MA, Geeting G, Eggli KD and Boal DK: MRI for
clinically suspected pediatric appendicitis: An implemented
program. Pediatr Radiol. 42:1056–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartman JH, Bena J, McIntyre S and Albert
NM: Does a photo diarydecrease stress and anxiety in children
undergoing magnetic resonance imaging? A randomized, controlled
study. J Radiol Nurs. 28:122–128. 2009. View Article : Google Scholar
|
|
4
|
McJury M and Shellock FG: Auditory Noise
associated with MR procedures: A review. J Magn Reson Imaging.
12:37–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li BL, Ni J, Huang JX, Zhang N, Song XR
and Yuen VM: Intranasal dexmedetomidine for sedation in children
undergoing transthoracic echocardiography study-a prospective
observational study. Paediatr Anaesth. 25:891–896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuen VM, Li BL, Cheuk DK, Leung MKM, Hui
TWC, Wong IC, Lam WW, Choi SW and Irwin MG: A randomised controlled
trial of oral chloral hydrate vs. intranasal dexmedetomidine before
computerised tomography in children. Anaesthesia. 72:1191–1195.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Millar K, Bowman AW, Burns D, McLaughlin
P, Moores T, Morton NS, Musiello T, Wallace E, Wray A and Welbury
RR: Children's cognitive recovery after day-case general
anesthesia: A randomized trial of propofol or isoflurane for dental
procedures. Paediatr Anaesth. 24:201–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiaretti A, Benini F, Pierri F, Vecchiato
K, Ronfani L, Agosto C, Ventura A, Genovese O and Barbi E: Safety
and efficacy of propofol administered by paediatricians during
procedural sedation in children. Acta Paediatr. 103:182–187. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koroglu A, Demirbilek S, Teksan H, Sagir
O, But AK and Ersoy MO: Sedative, haemodynamic and respiratory
effects of dexmedetomidine in children undergoing magnetic
resonance imaging examination: Preliminary results. Br J Anaesth.
94:821–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Mahmoud M, Schmitt M, Hossain M and
Kurth D: Comparison of propofol and dexmedetomedine techniques in
children undergoing magnetic resonance imaging. Paediatr Anaesth.
24:813–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Q, Shen L, Zhang X, Li J and Tang Y:
Dexmedetomidine versus propofol on the sedation of pediatric
patients during magnetic resonance imaging (MRI) scanning: A
meta-analysis of current studies. Oncotarget. 8:102468–102473.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watt S, Sabouri S, Hegazy R, Gupta P and
Heard C: Does dexmedetomidine cause less airway collapse than
propofol when used for deep sedation? J Clin Anesth. 35:259–267.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao Y, He P, Jing G, Wang Q and Wen J:
Comparison of sedative effect of dexmedetomide injection and
propofol injection in pediatric patients undergoing magnetic
resonance imaging. Zhongguo Lin Chuang Yao Li Xue Za Zhi.
33:1764–1767. 2017.(In Chinese).
|
|
14
|
Knobloch K, Yoon U and Vogt PM: Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement and publication bias. J Craniomaxillofac Surg. 39:91–92.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringblom J, Wahlin I and Proczkowska M: A
psychometric evaluation of the Pediatric Anesthesia Emergence
Delirium scale. Paediatr Anaesth. 28:332–337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bong CL, Lim E, Allen JC, Choo WL, Siow
YN, Teo PB and Tan JS: A comparison of single-dose dexmedetomidine
or propofol on the incidence of emergence delirium in children
undergoing general anaesthesia for magnetic resonance imaging.
Anaesthesia. 70:393–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamal K, Asthana U, Bansal T, Dureja J,
Ahlawat G and Kapoor S: Evaluation of efficacy of dexmedetomidine
versus propofol for sedation in children undergoing magnetic
resonance imaging. Saudi J Anaesth. 11:163–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang H, Yang L, Wang X and Zhu H: Clinical
efficacy of dexmedetomidine versus propofol in children undergoing
magnetic resonance imaging: A meta-analysis. Int J Clin Exp Med.
8:11881–11889. 2015.PubMed/NCBI
|
|
20
|
Chandler JR, Myers D, Mehta D, Whyte E,
Groberman MK, Montgomery CJ and Ansermino JM: Emergence delirium in
children: A randomized trial to compare total intravenous
anesthesia with propofol and remifentanil to inhalational
sevoflurane anesthesia. Paediatr Anaesth. 23:309–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Isik B, Arslan M, Tunga AD and Kurtipek O:
Dexmedetomidine decreases emergence agitation in pediatric patients
after sevoflurane anesthesia without surgery. Paediatr Anaesth.
16:748–753. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wetterslev J, Jakobsen JC and Gluud C:
Trial sequential analysis in systematic reviews with meta-analysis.
BMC Med Res Methodol. 17:392017. View Article : Google Scholar : PubMed/NCBI
|