Introduction
With the increasing demand in diagnostic and
therapeutic cardiovascular interventions, the major concern
regarding the use of contrast medium (CM) is the deterioration of
renal function referred to as contrast-induced acute kidney injury
(CI-AKI). Nash et al (1)
reported CI-AKI as the third leading cause of hospital-acquired
acute renal failure, accounting for 11% of all cases. The incidence
of CI-AKI varies considerably, depending on the patient population
studied (2,3). As the baseline renal function worsens,
there is a sharp increase in the rate of CI-AKI, and up to 26.6% of
patients with severe pre-existing chronic kidney disease (CKD) are
at risk of developing CI-AKI (4).
CI-AKI resolves spontaneously in most cases, although transient
dialysis may occasionally be required. Approximately 18.6% of
patients with moderate-to-severe CKD who develop CI-AKI progress to
irreversible renal dysfunction, leading to prolonged hospital stay,
elevated medical costs, poor long-term clinical outcome and
increased risk of death, persistent dialysis or major adverse
cardiovascular events (5,6). However, at present, no definitive
treatment is available for this complication (7).
Statins are known to possess pleiotropic effects
(anti-oxidant, anti-inflammatory and anti-thrombotic),
independently of their intended effects on blood cholesterol levels
(8,9). Statins also improve endothelial
function (10), increase nitric
oxide bioavailability (11), prevent
CM-induced renal tubular epithelial cell apoptosis, restore
survival signaling pathways (12)
and reduce the uptake of iodinated CM from the urinary space
(13), which may counteract the
specific pathophysiological mechanisms underlying CI-AKI and exert
renoprotective effects. Although several clinical trials have
indicated that short-term high-loading-dose statin administration
correlates with a significant reduction in the incidence of CI-AKI,
and a recent Bayesian network meta-analysis comparing the relative
efficacy of multiple pharmacological interventions concluded that
high-dose statins plus hydration may be the most effective strategy
for the prevention of CI-AKI (12,14–20).
These previous studies mainly focused on statin-naive patients,
while frequently excluding patients with severe renal
impairment.
In the real-world setting, patients diagnosed with
coronary artery disease always receive long-term statin therapy and
occasionally develop advanced CKD. Thus, the aim of the present
study was to examine the effect of high-dose statin reload on renal
function among patients with moderate-to-severe CKD and long-term
statin use undergoing percutaneous coronary intervention (PCI) or
coronary artery angiography (CAG).
Materials and methods
Study population
The present study was a single-center retrospective
clinical trial performed at Peking University First Hospital
(Beijing, China). Consecutive patients with stage-3 or −4 CKD on
long-term statin treatment who were identified through a medical
history review and underwent scheduled PCI or CAG between January
2012 and December 2015 were enrolled. The exclusion criteria were
stage-1 or −2 CKD, end-stage renal disease (ESRD) requiring
hemodialysis or peritoneal dialysis, other causes of AKI prior to
catheterization, unavailable serum creatinine (SCr) value 48–72 h
after the procedure or missing data on CM dosage, acute ST-segment
elevation myocardial infarction (STEMI), cardiogenic shock or
hemodynamic instability, and administration of iodinated CM during
the week preceding the procedure. Eligible patients were then
assigned to the statin-loading group and the no statin-loading
group, according to whether they were administered high-dose
statins (atorvastatin ≥40 mg or rosuvastatin ≥10 mg) within 24 h
prior to the procedure.
Study protocol
The angiographic reports saved in the Innova IGS 520
(GE Medical Systems SCS) and the corresponding medical records of
the patients were retrieved and reviewed. The demographic data and
medical history of the patients were recorded. The following
clinical information was also documented: Final diagnosis, left
ventricular ejection fraction (LVEF), category and dosage of
chronically or pre-procedurally administered statins, baseline SCr
and estimated glomerular filtration rate (eGFR), SCr peak level and
eGFR value at 48–72 h after PCI or CAG, and occurrence of
in-hospital adverse events, including dialysis, all-cause death,
stent thrombosis, as well as cerebral infarction. The procedural
characteristics, particularly preparatory hydration and type or
volume of CM used, were recorded.
The eGFR values were calculated from baseline and
peak post-procedural SCr concentrations using the CKD Epidemiology
Collaboration equation for ‘white and other’, which illustrates the
specific algorithm of eGFR (21).
Renal function was classified according to the stages set by the
National Kidney Foundation (USA) Kidney Disease Outcomes Quality
Initiative as follows: Stage 1, CKD with eGFR ≥90 ml/min/1.73
m2, considered normal; stage 2, CKD with eGFR 60–89
ml/min/1.73 m2, considered mildly impaired; stage 3, CKD
with eGFR 30–59 ml/min/1.73 m2, considered moderately
impaired; stage 4, CKD with eGFR 15–29 ml/min/1.73 m2,
considered severely impaired; and stage 5, CKD with eGFR <15
ml/min/1.73 m2, considered ESRD (22).
Endpoints and definitions
Given the difference in baseline SCr levels between
the two groups, the percent change of peri-procedural SCr levels
was selected as the primary endpoint. Additional endpoints included
the absolute value of SCr change, eGFR value at 48–72 h after PCI
or CAG according to the CI-AKI definition, incidence rate of CI-AKI
(defined as a SCr concentration increase by ≥0.5 mg/dl or ≥25%
above baseline within 48–72 h after contrast exposure) (23) and composite in-hospital adverse
events. The absolute difference in SCr levels was calculated as the
baseline minus post-operative peak SCr concentration, while the
percent change was the ratio of absolute change and baseline SCr
concentration.
Non-ST-segment elevation acute coronary syndrome
(NSTE-ACS) included unstable angina and non-STEMI. The contrast
volume exceeding 140 ml was considered as a high-dose CM load
(24).
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 (IBM Corp.). The Shapiro-Wilk test was used to examine
the normality of distribution. Normally distributed continuous
variables were expressed as the mean ± standard deviation and
analyzed using independent Student's t-tests. Non-normally
distributed variables were presented as the median (interquartile
range) and comparisons were performed using Mann-Whitney U-tests.
All categorical data, expressed as absolute numbers (percentages)
were compared between the two groups using Chi-squared or Fisher's
exact tests. Stratified analyses were also performed in each
pre-specified subgroup, and multiple linear regression was applied
to adjust for age, sex, medical history and baseline differences in
clinical and procedural factors. All statistical analyses were
two-tailed. P<0.05 was considered to indicate statistical
significance.
Results
Patient population and baseline
characteristics
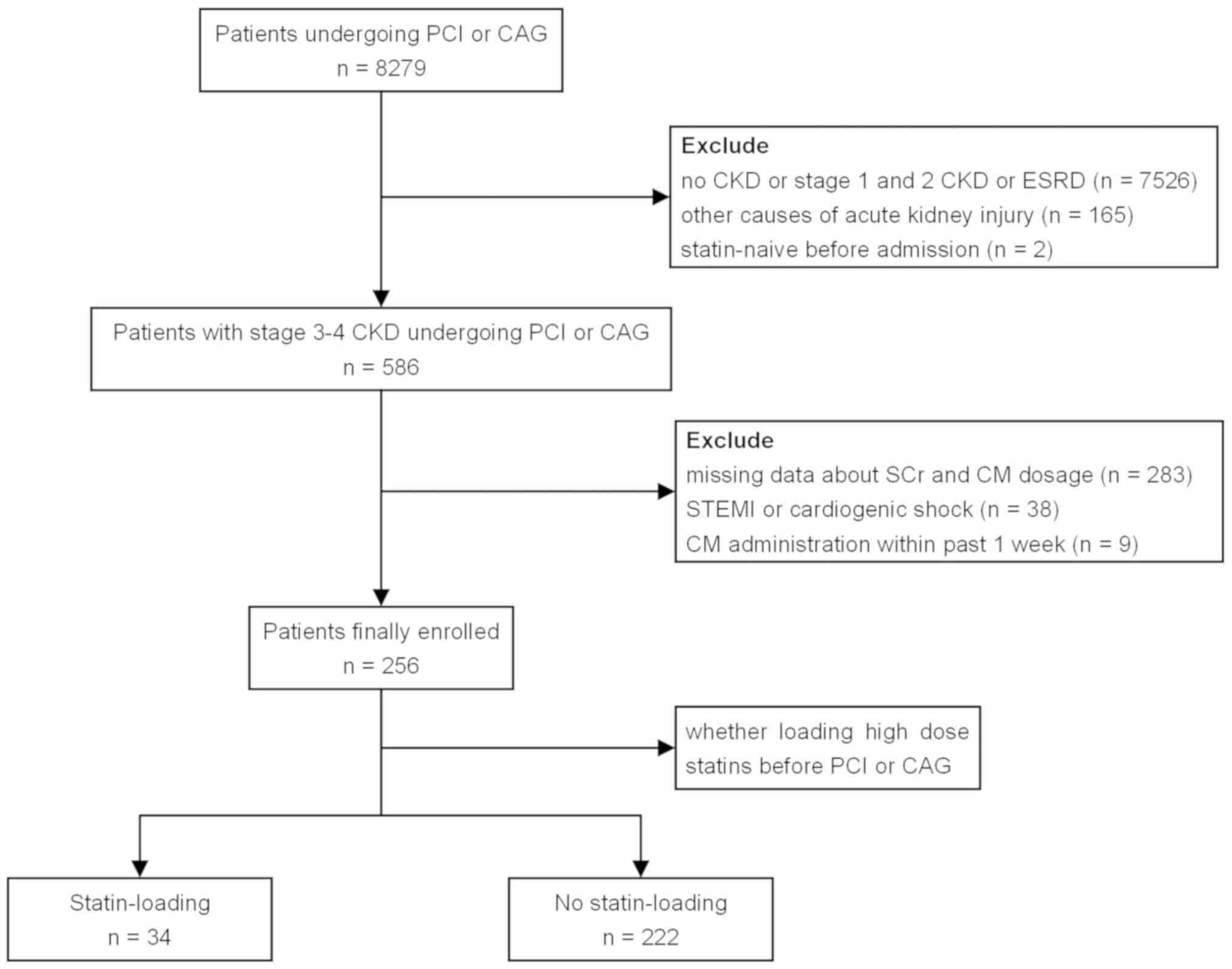
A total of 256 patients were considered eligible for
final analysis and were assigned to the statin-loading group (n=34)
or the no statin-loading group (n=222; Fig. 1).
The mean age of the participants was 71.38±10.27
years and 160 (62.5%) were male. There were no significant
differences with respect to age, sex, height, body mass index,
medical history or LVEF between the statin-loading and no
statin-loading groups (P>0.05). The baseline SCr level and the
percentage of severe renal insufficiency were similar between the
two groups (P>0.05). The baseline eGFR value was numerically
lower in the statin-loading group, but the difference was not
statistically significant (P=0.054). Compared with that in the no
statin-loading group, the number of patients diagnosed with
NSTE-ACS was significantly higher in the statin-loading group (97.1
vs. 83.3%; P=0.036). No significant difference was observed
regarding the categories of statins chronically administered
(P=0.255 for atorvastatin and P=0.262 for rosuvastatin; Table I).
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
|
| Statin-loading
group | No-statin loading
group |
|
|---|
|
|
|
|
|
|---|
| Characteristic | (n=34) | (n=222) | P-value |
|---|
| Age (years) | 70.41±11.89 | 71.52±10.02 | 0.558 |
| Male | 23 (67.6) | 137 (61.7) | 0.506 |
| Height (cm) | 165
(158.75–171.25) | 165
(160.00–171.00) | 0.677 |
| BMI
(kg/m2) | 27.01±5.13 | 26.00±3.51 | 0.297 |
| Diabetes
mellitus | 15 (44.1) | 100 (45.0) | 0.919 |
| Hypertension | 28 (82.4) | 195 (87.8) | 0.408 |
| Hyperlipidemia | 25 (73.5) | 144 (64.9) | 0.321 |
| Current
smoking | 10 (29.4) | 60 (27.0) | 0.771 |
| Previous PCI | 14 (41.2) | 88 (39.6) | 0.865 |
| Previous CABG | 1 (2.9) | 9 (4.1) | >0.999 |
| Previous MI | 9 (26.5) | 60 (27.0) | 0.946 |
| LVEF (%) | 60.48±16.61 | 65.39±12.75 | 0.118 |
| Cardiac
presentation |
|
|
|
|
SCAD | 1 (2.9) | 37 (16.7) | 0.036 |
|
NSTE-ACS | 33 (97.1) | 185 (83.3) | 0.036 |
| Baseline SCr
(µmol/l) | 139.41±33.80 | 129.82±42.27 | 0.208 |
| Baseline eGFR
(ml/min/1.73 m2) | 42.27±9.45 | 46.08±10.87 | 0.054 |
| Severe CKD | 4 (11.8) | 21 (9.5) | 0.755 |
| Chronic statins
administered |
|
|
|
|
Atorvastatin | 29 (85.3) | 170 (76.6) | 0.255 |
|
Rosuvastatin | 4 (11.8) | 44 (19.8) | 0.262 |
| Number of ≥50%
stenotic vessels | 2.24±0.96 | 2.18±0.93 | 0.750 |
| Culprit vessel |
|
|
|
|
LAD | 29 (85.3) | 184 (82.9) | 0.726 |
|
LCX | 20 (58.8) | 135 (60.8) | 0.825 |
|
RCA | 23 (67.6) | 145 (65.3) | 0.790 |
| LM | 4 (11.8) | 16 (7.2) | 0.317 |
| Graft
vessel | 0 (0.0) | 4 (1.8) | >0.999 |
| Number of
stents | 1.50±0.83 | 1.72±1.04 | 0.238 |
| PCI | 31 (91.2) | 206 (92.8) | 0.725 |
| GPI
administration | 7 (20.6) | 80 (36.0) | 0.077 |
| Hydration | 23 (67.6) | 139 (62.6) | 0.571 |
| CM type |
|
|
|
|
Iohexol | 4 (11.8) | 57 (25.7) | 0.076 |
|
Iodixanol | 18 (52.9) | 53 (23.9) | <0.001 |
|
Iopamidol | 7 (20.6) | 65 (29.3) | 0.294 |
|
Iopromide | 5 (14.7) | 47 (21.2) | 0.383 |
| CM dose (ml) | 129.85±37.39 | 151.23±53.08 | 0.025 |
| High-dose CM
load | 13 (38.2) | 133 (59.9) | 0.017 |
The number of vessels with stenosis ≥50% and stents
deployed, culprit vessels, as well as usage of glycoprotein
IIb/IIIa inhibitors, were well-balanced (P>0.05). Of the 256
patients, 63.3% received peri-procedural hydration (n=162), without
any significant difference between the two groups (67.6 vs. 62.6%;
P=0.571). However, the volume of CM and the proportion of patients
receiving high-dose CM were significantly decreased in the
statin-loading group (P=0.025 and P=0.017, respectively). In the
statin-loading group, a markedly higher proportion of patients was
exposed to iso-osmolar iodixanol than that in the no statin-loading
group (52.9 vs. 23.9%; P<0.001; Table
I).
Changes in renal function
parameters
The normal range of SCr is 59–104 µmol/l for males
and 45–84 µmol/l for females (25).
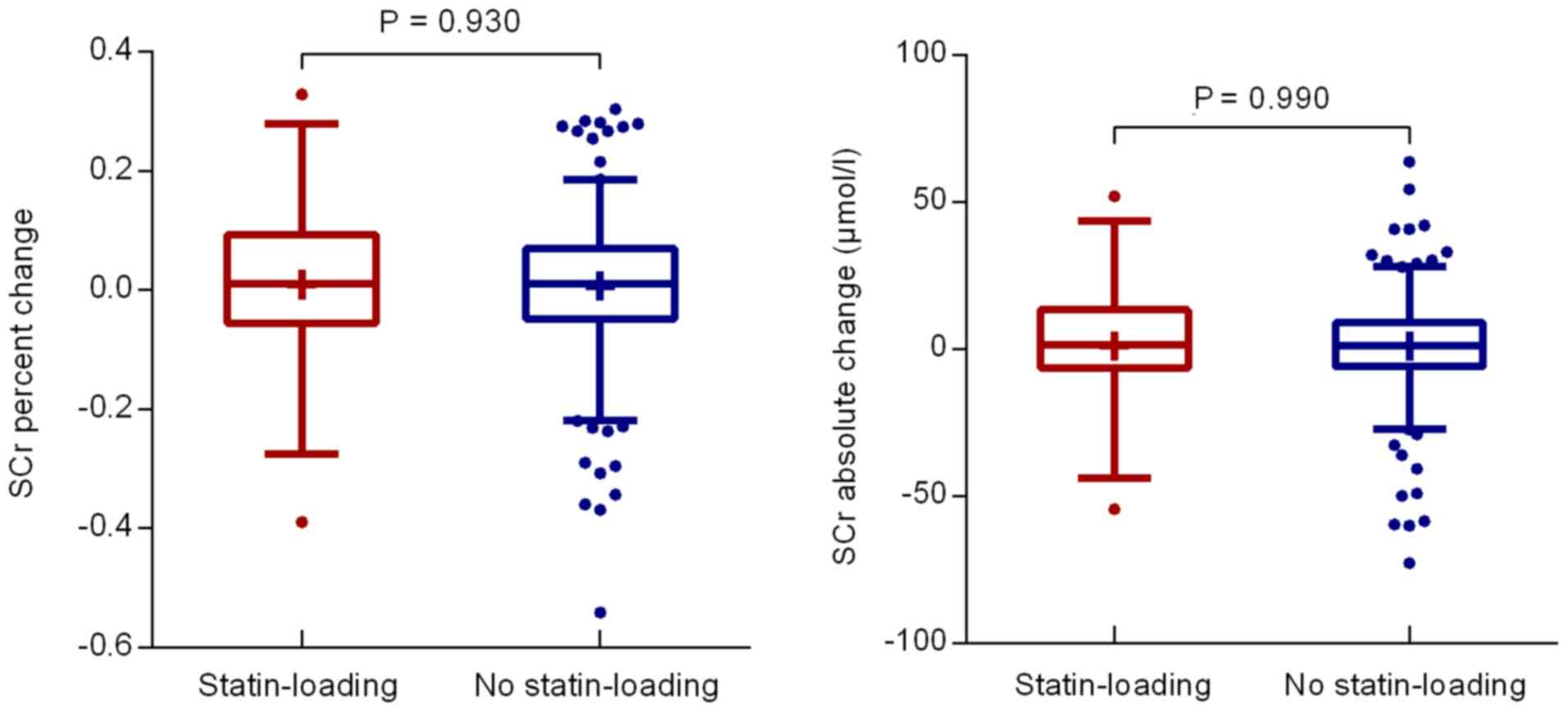
A decrease in post-procedural peak SCr levels in either of the two
groups was observed, while the percent change in the SCr
concentration was not significantly different between the two
groups (0.91±13.81 vs. 0.71±12.11%; P=0.930; Fig. 2). There was also no significant
difference in the absolute SCr change (1.10±20.33 vs. 1.06±17.27
µmol/l; P=0.990; Fig. 2). The eGFR
value at 48–72 h after PCI or CAG was above baseline and comparable
between the two groups (P=0.119; Table
II). The baseline and post-procedural SCr levels were slightly
higher in the statin-loading group compared with those in the no
statin-loading group (P=0.208 and P=0.252; Table II).
 | Table II.Changes in renal function after
administration of CM. |
Table II.
Changes in renal function after
administration of CM.
|
| Statin-loading
group | No statin-loading
group |
|
|---|
|
|
|
|
|
|---|
| Renal function
parameters | (n=34) | (n=222) | P-value |
|---|
| eGFRa (ml/min/1.73 m2) |
|
|
|
|
Baseline | 42.27±9.45 | 46.08±10.87 | 0.054 |
| Post
procedure (48–72 h) | 43.69±11.35 | 47.26±12.54 | 0.119 |
| SCrb (µmol/l) |
|
|
|
|
Baseline | 139.41±33.80 | 129.82±42.27 | 0.208 |
| Post
procedure (48–72 h) | 138.32±40.48 | 128.77±45.78 | 0.252 |
Incidence of CI-AKI
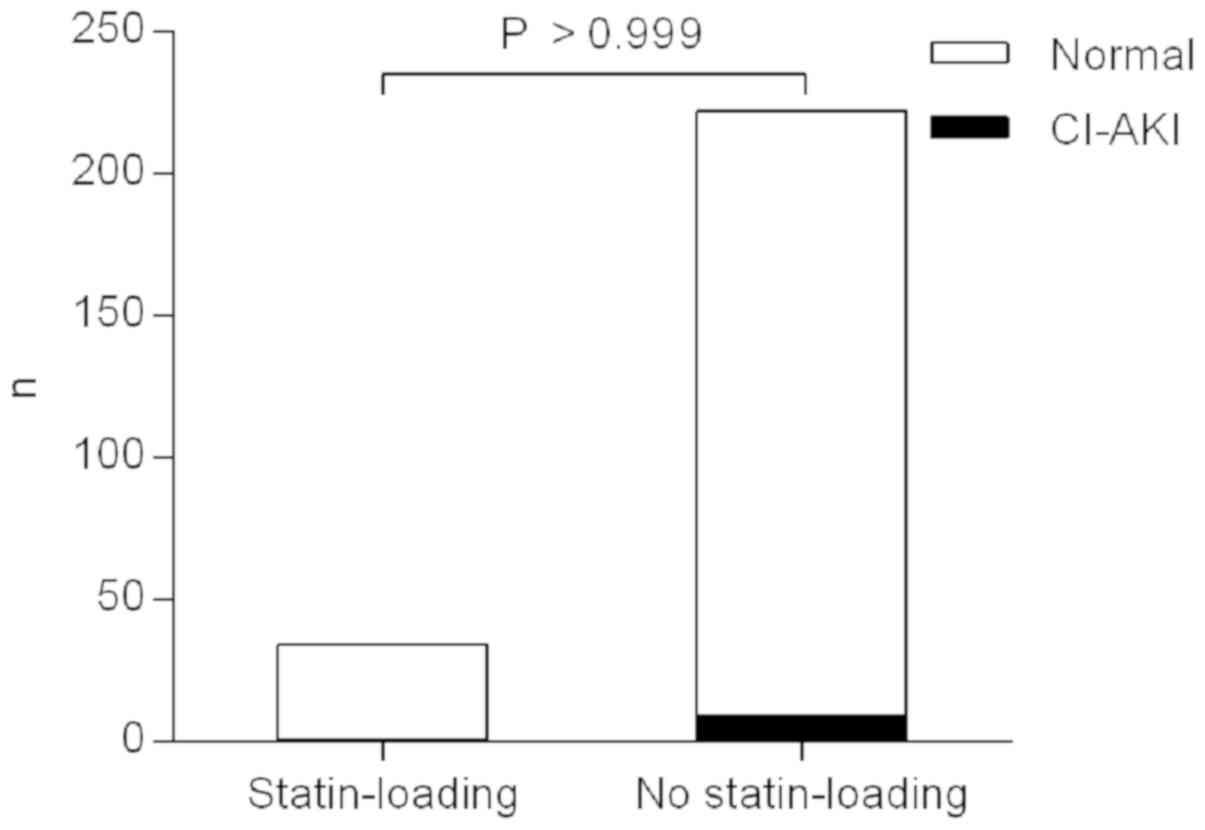
One patient (2.9%) in the statin-loading group and 9
patients (4.1%) in the no statin-loading group developed CI-AKI
within 48–72 h of CM administration. The CI-AKI rate was similar
between the two groups (P>0.999, Fisher's exact test; Fig. 3 and Table III).
 | Table III.CI-AKI occurrence and in-hospital
adverse events. |
Table III.
CI-AKI occurrence and in-hospital
adverse events.
|
| Statin-loading
group | No statin-loading
group |
|
|---|
|
|
|
|
|
|---|
| In-hospital adverse
event | (n=34) | (n=222) | P-value |
|---|
| CI-AKI | 1 (2.9) | 9 (4.1) | >0.999 |
| Dialysis | 0 (0.0) | 5 (2.3) | – |
| Death | 0 (0.0) | 0 (0.0) | – |
| Stent
thrombosis | 0 (0.0) | 2 (0.9) | – |
| Cerebral
infarction | 0 (0.0) | 1 (0.5) | – |
| Composite
endpoint | 0 (0.0) | 8 (3.6) | 0.602 |
In-hospital clinical outcome
No deaths were reported in either group. No
dialysis, stent thrombosis or cerebral infarction occurred in the
statin-loading group, whereas the corresponding incidence of
dialysis, stent thrombosis and cerebral infarction in the no
statin-loading group was 2.3, 0.9 and 0.5%, respectively. There was
no significant difference in the in-hospital composite adverse
events of all-cause death, dialysis, stent thrombosis and cerebral
infarction after CM exposure (0.0 vs. 3.6%; P=0.602, Fisher's exact
test; Table III).
Subgroup analyses
Stratified analyses according to the presence of
severe renal functional impairment, concomitant diabetes mellitus,
adequate hydration, administration of high-dose CM, selection of an
iso-osmolar CM and advanced age (≥75 years) demonstrated consistent
results when the statin-loading and no statin-loading groups were
compared. Of note, in the high-dose CM and elderly patient
subgroups, the eGFR value post-procedure was significantly lower in
the statin-loading group compared with that in the no
statin-loading group (P=0.034 and P=0.043, respectively; Table IV).
 | Table IV.Subgroup analyses of differences in
study endpoints. |
Table IV.
Subgroup analyses of differences in
study endpoints.
| Subgroup | Statin-loading | No
statin-loading | P-value |
|---|
| eGFR (ml/min/1.73
m2) |
|
|
|
|
<30 |
|
|
|
|
Number of
patients | 4 | 21 |
|
|
Change in SCr
(%) | −7.08±8.31 | 3.84±12.44 | 0.108 |
|
Post-procedure
eGFR | 25.19±1.27 | 25.42±6.62 | 0.947 |
|
CI-AKI | 0 (0) | 2 (9.5) | >0.999 |
|
≥30 |
|
|
|
|
Number of
patients | 30 | 201 |
|
|
Change in SCr
(%) | 1.97±14.14 | 0.38±12.06 | 0.510 |
|
Post-procedure
eGFR | 46.15±9.64 | 49.54±10.68 | 0.103 |
|
CI-AKI | 1 (3.3) | 7 (3.5) | >0.999 |
| Diabetes |
|
|
|
|
Yes |
|
|
|
|
Number of
patients | 15 | 100 |
|
|
Change in SCr
(%) | −3.44±10.38 | 0.15±12.38 | 0.289 |
|
Post-procedure
eGFR | 39.93±9.92 | 45.47±13.69 | 0.069 |
|
CI-AKI | 0 (0) | 5 (5) | >0.999 |
| No |
|
|
|
|
Number of
patients | 19 | 122 |
|
|
Change in SCr
(%) | 4.34±15.42 | 1.17±11.91 | 0.302 |
|
Post-procedure
eGFR | 46.66±11.77 | 48.73±11.37 | 0.463 |
|
CI-AKI | 1 (5.3) | 4 (3.3) | 0.520 |
| Hydration |
|
|
|
|
Yes |
|
|
|
|
Number of
patients | 23 | 139 |
|
|
Change in SCr
(%) | −1.61±14.71 | 0.16±12.53 | 0.543 |
|
Post-procedure
eGFR | 40.32±9.66 | 43.98±12.61 | 0.117 |
|
CI-AKI | 1 (4.3) | 7 (5.0) | >0.999 |
| No |
|
|
|
|
Number of
patients | 11 | 83 |
|
|
Change in SCr
(%) | 6.17±10.42 | 1.63±11.38 | 0.213 |
|
Post-procedure
eGFR | 50.74±11.80 | 52.75±10.38 | 0.554 |
|
CI-AKI | 0 (0) | 2 (2.4) | >0.999 |
| CM dose (ml) |
|
|
|
|
≥140 |
|
|
|
|
Number of
patients | 13 | 133 |
|
|
Change in SCr
(%) | −2.84±13.78 | 0.15±11.84 | 0.393 |
|
Post-procedure
eGFR | 39.96±9.85 | 47.58±12.46 | 0.034 |
|
CI-AKI | 1 (7.7) | 6 (4.5) | 0.487 |
|
<140 |
|
|
|
|
Number of
patients | 21 | 89 |
|
|
Change in SCr
(%) | 3.23±13.64 | 1.54±12.51 | 0.585 |
|
Post-procedure
eGFR | 45.99±11.82 | 46.78±12.71 | 0.796 |
|
CI-AKI | 0 (0) | 3 (3.4) | >0.999 |
| CM |
|
|
|
|
Iodixanol |
|
|
|
|
Number of
patients | 18 | 53 |
|
|
Change in SCr
(%) | −1.29±16.55 | −0.18±14.23 | 0.784 |
|
Post-procedure
eGFR | 40.90±12.05 | 40.83±10.84 | 0.981 |
|
CI-AKI | 1 (5.6) | 2 (3.8) | >0.999 |
|
Other |
|
|
|
|
Number of
patients | 16 | 169 |
|
|
Change in SCr
(%) | 3.39±9.85 | 0.99±11.39 | 0.417 |
|
Post-procedure
eGFR | 46.83±9.95 | 49.28±12.38 | 0.444 |
|
CI-AKI | 0 (0) | 7 (4.1) | >0.999 |
| Age (years) |
|
|
|
|
≥75 |
|
|
|
|
Number of
patients | 16 | 97 |
|
|
Change in SCr
(%) | 2.16±15.83 | −0.66±12.90 | 0.436 |
|
Post-procedure
eGFR | 40.29±11.35 | 46.61±11.48 | 0.043 |
|
CI-AKI | 1 (6.3) | 5 (5.2) | >0.999 |
|
<75 |
|
|
|
|
Number of
patients | 18 | 125 |
|
|
Change in SCr
(%) | −0.20±12.09 | 1.77±11.40 | 0.498 |
|
Post-procedure
eGFR | 46.71±10.76 | 47.76±13.33 | 0.749 |
|
CI-AKI | 0 (0) | 4 (3.2) | >0.999 |
Multiple linear regression
The analysis revealed that a low baseline eGFR value
(β=0.911, P<0.001) and current smoking status (smokers vs.
non-smokers: β=−2.469, P=0.019) were significantly associated with
a reduction in the eGFR value at 48–72 h after PCI or CAG, after
adjusting for age, sex, medical history, and baseline
heterogeneities in clinical and procedural factors. By contrast,
high-dose statin reload exerted no significant effect on the
post-procedural eGFR value (P=0.618; Table V). The ‘constant’ β0 in
the equation represents the potentially significant influencing
factors of the post-procedural eGFR value not included as an
independent variable (P<0.001; Table
V).
 | Table V.Multivariate analysis for
post-procedural eGFR value. |
Table V.
Multivariate analysis for
post-procedural eGFR value.
| Variable in
model | Partial regression
coefficient | t statistic | P-value |
|---|
| Constant
(β0) | 11.335 | 3.574 | <0.001 |
| Statin loading | 0.650 | 0.499 | 0.618 |
| Age ≥75 years | −1.632 | −1.794 | 0.074 |
| Male sex | 1.430 | 1.454 | 0.147 |
| Diabetes
mellitus | −0.969 | −1.086 | 0.279 |
| Hypertension | −0.629 | −0.469 | 0.640 |
| Hyperlipidemia | −0.965 | −1.031 | 0.304 |
| Current
smoking | −2.469 | −2.370 | 0.019 |
| NSTE-ACS | −1.784 | −1.454 | 0.147 |
| Baseline
eGFRa | 0.911 | 20.136 | <0.001 |
| ≥2 vessels
diseasedb | −0.478 | −0.495 | 0.621 |
| Iodixanol
administration | −1.912 | −1.852 | 0.065 |
| High-dose CM
load | −1.028 | −1.137 | 0.257 |
| Adequate
hydration | −1.682 | −1.698 | 0.091 |
Discussion
Several clinical trials have been designed to
evaluate the efficacy of statins in the prevention of CI-AKI, with
controversial results. Furthermore, only few studies have
investigated the renoprotective role of statin reload in patients
with moderate-to-severe CKD receiving long-term statin therapy who
undergo cardiac catheterization (12,14–19,26–29). The
major results of the present study indicate that, compared with no
statin loading, high-dose statin pre-treatment does not further
protect renal function, reduce the occurrence of CI-AKI or improve
in-hospital clinical outcome for such patients.
An eGFR of <60 ml/min/1.73 m2 is
currently generally accepted as the threshold for risk of CI-AKI
(30). Certain retrospective and
observational trials indicated that prophylactic administration of
statins prior to catheterization may be associated with lower risk
of CI-AKI among CKD patients, and this early benefit translated
into shorter hospital stay, along with improved long-term clinical
outcome (31–34). Certain prospective, randomized and
controlled trials also investigated whether peri-procedural
high-dose statins efficiently protect the renal function of CKD
patients. Quintavalle et al (12) reported that 80 mg atorvastatin load
within 24 h prior to CM exposure significantly reduced the
incidence rate of CI-AKI in patients with moderate CKD undergoing
PCI or CAG. Shehata and Hamza (14)
investigated diabetic patients with mild-to-moderate renal
impairment, and observed that the incidence of CI-AKI was lower
among patients receiving atorvastatin 80 mg daily for 48 h prior to
elective PCI. The TRACK-D trial, including 2,998 Chinese patients
with type 2 diabetes mellitus coincident with mild-to-moderate CKD
who underwent coronary or peripheral arterial angiography,
demonstrated that short-term high-dose rosuvastatin lowered the
rate of CI-AKI and worsening heart failure during a 30-day
follow-up (15). Recently, a network
meta-analysis, including 150 trials with 31,631 participants that
synchronously assessed different treatments, reported that
high-dose statins plus hydration may be regarded as the best
strategy to prevent CI-AKI (20).
Accordingly, in the present study, high-loading-dose statins were
generally prescribed for higher-risk patients with lower baseline
eGFR values.
In line with the results of the present study,
peri-procedural high-dose simvastatin administration in patients
with renal insufficiency undergoing CAG was not associated with any
differences in the mean peak increase of SCr, incidence of CI-AKI,
length of hospital stay or short-term clinical outcome in the
PROMISS trial (35). Similarly, Toso
et al (36) performed a
prospective, single-center study on CKD patients, revealing that
the mean increase in SCr, CI-AKI rate, in-hospital mortality and
requirement for dialysis did not significantly differ between the
high-dose atorvastatin and placebo groups. Consistent results were
reported in all of the prospectively defined higher-risk subgroups
(36). However, unlike those in the
present study, one of the enrollment criteria in the PROMISS trial
included normal or only mildly impaired renal function (baseline
SCr ≥1.1 mg/dl). More importantly, recent statin users were
excluded from all of the above-mentioned trials. By contrast, the
present study included patients on chronic statin administration
with coexisting moderate-to-severe CKD.
Acikel et al (19) compared the efficacy of short-term and
long-term statin therapy for CI-AKI prevention. They demonstrated
that the SCr and eGFR values at 48 h after elective CAG were
significantly better in the high-dose atorvastatin and chronic
statin therapy groups compared with those in control subjects,
whereas no differences were observed in renal function parameters
between the high-dose and chronic statin therapy groups. While
certain results of the above study were similar to those of the
present study, in terms of the comparable benefits of the two
statin regimens, the discrepancy in the inclusion criteria of the
two studies is noteworthy. Acikel et al (19) excluded patients with a
moderate-to-severe decrease in eGFR, while the present study
focused on a patient population with moderate-to-severe CKD.
In the NAPLES II trial, 80 mg atorvastatin load
prior to CM exposure failed to lower the CI-AKI rate in the
subgroup with severe CKD (12).
Patti et al (33) also
observed that patients treated with a variety of statins undergoing
PCI exhibited a 90% risk reduction of CI-AKI, apart from those with
a creatinine clearance <40 ml/min, possibly due to the multiple
irreversible pathogenetic mechanisms underlying the development of
advanced renal failure (33,37). One meta-analysis of 31 prospective
randomized trials reported that the effect of statin therapy on
renal outcome was markedly affected by kidney function, and the
relative effect was significantly reduced in patients with more
advanced kidney dysfunction (38).
Since the majority of the patients analyzed in the present study
had moderate-to-severe CKD, with severe CKD accounting for ~10% of
the cases, it is reasonable to hypothesize that the beneficial
effects of high-loading-dose statins on renal function are offset.
This may also partly explain the significantly lower
post-procedural eGFR values in the higher-risk statin-loading group
observed in the high-dose CM and elderly patient subgroup analyses,
despite the application of various precautionary strategies.
Lower creatinine clearance is associated with a
higher frequency of death or myocardial infarction during the
initial hospital stay and at 1 year among patients with CKD
undergoing PCI. Furthermore, during the initial hospital stay, a
stepwise increase in hemorrhagic complications with declining
creatinine clearance is observed (39). The results of two previous large
registry cohorts demonstrated that prescription of statins
correlated with a significant improvement in subsequent outcomes,
including death and composite endpoints of death, myocardial
infarction and target vessel revascularization in the mild CKD
stratum (40,41). According to the dyslipidemia
management guidelines, patients diagnosed with ACS should receive
moderate-to-high-intensity statin therapy for atherosclerotic
cardiovascular disease as a secondary prevention (42,43). The
patient-centered approach proposed by the National Lipid
Association Expert Panel recommends that patients with ACS or
stage-3B-4 CKD are classified as very high- or high-risk and,
therefore, high-intensity statin therapy should be considered
(44). The updated European Clinical
Practice Guidelines also recommend that high-intensity statin
therapy should be initiated as early as possible in recently
diagnosed ACS or CKD patients (45).
On the basis of this evidence, long-term statin administration must
be advocated in CKD patients and those diagnosed with NSTE-ACS, as
in the present study.
Distinct from previous studies, the peak SCr values
within 48–72 h post-PCI or -CAG declined in the two groups of the
present study, and the CI-AKI rate was merely 3.9%, which is
notably lower compared with that reported in the literature; these
results may be attributed to the sufficient preventive measures.
The principles of CI-AKI prevention and management include using as
low as reasonably achievable volumes of CM, selecting the least
toxic iodinated CM, and hydration with isotonic crystalloid
solution 12 h prior to and at least 24 h after the procedure
(46–48). Thus, in the present study, in
addition to statin administration, 63.3% of the patients received
adequate hydration. Furthermore, the mean CM dosage (129.85±37.39
vs. 151.23±53.08 ml; P=0.025) was markedly lower and the proportion
of iso-osmolar iodixanol use was higher in patients with lower
baseline eGFR values.
There are several limitations to the present study.
First, due to the retrospective design and lack of randomization,
there was significant heterogeneity with respect to baseline
clinical or procedural variables between the two groups, and a
causal association cannot be verified from the present analyses.
Second, the eligible patients in the statin-loading group were
relatively few, and only a small proportion of patients developed
in-hospital adverse events, making this trial underpowered and
possibly inconclusive. Third, the enrolled patients were followed
up for only 48–72 h, and the peak in SCr levels may have been
missed. However, the majority of the patients developing CI-AKI
experience an increase in SCr >0.5 mg/dl within 24 h after CM
exposure (49); hence, the SCr peak
may have been missed in only a small number of cases. Although all
the patients included in the present study were administered
statins chronically, the definitive duration of statin treatment
remains undetermined. Next, the endpoints of the present study were
limited to in-hospital events; consequently, the impact of
high-loading-dose statins on the medium- or long-term clinical
outcome and permanent functional state of the kidney was not
evaluated. In addition, the present study only used SCr or eGFR
values to reflect renal function, whereas the levels of serum
cystatin C, a more sensitive and reliable renal injury biomarker
allowing an early diagnosis of CI-AKI (50), were not determined. Finally, the
present study provided no information on the lipid profile of the
patients, changes in the highly sensitive C-reactive protein levels
or monitoring of statin-associated adverse effects.
In conclusion, routine short treatment with
high-dose atorvastatin or rosuvastatin on the background of chronic
therapy prior to cardiac catheterization confers no added benefit
to the renal function of patients with moderate-to-severe CKD or a
reduction of the risk of in-hospital adverse events. These results
require confirmation in further prospective and multi-center
clinical trials.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH collected, analyzed and interpreted the patient
data, and was a major contributor in writing the manuscript. BoZ
revised the manuscript and helped to interpret the data. BiZ
analyzed the data for the work. XGW and QPS made contributions to
the acquisition of data. MC designed the present study. All authors
have read and approved the final version of this manuscript.
Ethics approval and consent to
participate
The current study was a retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nash K, Hafeez A and Hou S:
Hospital-acquired renal insufficiency. Am J Kidney Dis. 39:930–936.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCullough PA, Adam A, Becker CR, Davidson
C, Lameire N, Stacul F and Tumlin J; CIN Consensus Working Panel, :
Epidemiology and prognostic implications of contrast-induced
nephropathy. Am J Cardiol. 98:5K–13K. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owen RJ, Hiremath S, Myers A, Fraser-Hill
M and Barrett BJ: Canadian Association of Radiologists consensus
guidelines for the prevention of contrast-induced nephropathy:
Update 2012. Can Assoc Radiol J. 65:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai TT, Patel UD, Chang TI, Kennedy KF,
Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC,
Rumsfeld JS and Spertus JA: Contemporary incidence, predictors, and
outcomes of acute kidney injury in patients undergoing percutaneous
coronary interventions: Insights from the NCDR Cath-PCI registry.
JACC Cardiovasc Interv. 7:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gruberg L, Mehran R, Dangas G, Mintz GS,
Waksman R, Kent KM, Pichard AD, Satler LF, Wu H and Leon MB: Acute
renal failure requiring dialysis after percutaneous coronary
interventions. Catheter Cardiovasc Interv. 52:409–416. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maioli M, Toso A, Leoncini M, Gallopin M,
Musilli N and Bellandi F: Persistent renal damage after
contrast-induced acute kidney injury: Incidence, evolution, risk
factors and prognosis. Circulation. 125:3099–3107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azzalini L, Spagnoli V and Ly HQ:
Contrast-induced nephropathy: From pathophysiology to preventive
strategies. Can J Cardiol. 32:247–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rikitake Y, Kawashima S, Takeshita S,
Yamashita T, Azumi H, Yasuhara M, Nishi H, Inoue N and Yokoyama M:
Anti-oxidative properties of fluvastatin, an HMG-CoA reductase
inhibitor, contribute to prevention of atherosclerosis in
cholesterol-fed rabbits. Atherosclerosis. 154:87–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonetti PO, Lerman LO, Napoli C and Lerman
A: Statin effects beyond lipid lowering-are they clinically
relevant? Eur Heart J. 24:225–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chello M, Goffredo C, Patti G, Candura D,
Melfi R, Mastrobuoni S, Di Sciascio G and Covino E: Effects of
atorvastatin on arterial endothelial function in coronary bypass
surgery. Eur J Cardiothorac Surg. 28:805–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Almuti K, Rimawi R, Spevack D and Ostfeld
RJ: Effects of statins beyond lipid lowering: Potential for
clinical benefits. Int J Cardiol. 109:7–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quintavalle C, Fiore D, De Micco F,
Visconti G, Focaccio A, Golia B, Ricciardelli B, Donnarumma E,
Bianco A, Zabatta MA, et al: Impact of a high loading dose of
atorvastatin on contrast-induced acute kidney injury. Circulation.
126:3008–3016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCullough PA, Khambatta S and Jazrawi A:
Minimizing the renal toxicity of iodinated contrast. Circulation.
124:1210–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shehata M and Hamza M: Impact of high
loading dose of atorvastatin in diabetic patients with renal
dysfunction undergoing elective percutaneous coronary intervention:
A randomized controlled trial. Cardiovasc Ther. 33:35–41. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han Y, Zhu G, Han L, Hou F, Huang W, Liu
H, Gan J, Jiang T, Li X, Wang W, et al: Short-term rosuvastatin
therapy for prevention of contrast-induced acute kidney injury in
patients with diabetes and chronic kidney disease. J Am Coll
Cardiol. 63:62–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leoncini M, Toso A, Maioli M, Tropeano F,
Villani S and Bellandi F: Early high-dose rosuvastatin for
contrast-induced nephropathy prevention in acute coronary syndrome:
Results from the PRATO-ACS Study (Protective Effect of Rosuvastatin
and Antiplatelet Therapy On contrast-induced acute kidney injury
and myocardial damage in patients with Acute Coronary Syndrome). J
Am Coll Cardiol. 63:71–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patti G, Ricottini E, Nusca A, Colonna G,
Pasceri V, D'Ambrosio A, Montinaro A and Di Sciascio G: Short-term,
high-dose Atorvastatin pretreatment to prevent contrast-induced
nephropathy in patients with acute coronary syndromes undergoing
percutaneous coronary intervention (from the ARMYDA-CIN
[atorvastatin for reduction of myocardial damage during
angioplasty-contrast-induced nephropathy] trial. Am J Cardiol.
108:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozhan H, Erden I, Ordu S, Aydin M, Caglar
O, Basar C, Yalcin S and Alemdar R: Efficacy of short-term
high-dose atorvastatin for prevention of contrast-induced
nephropathy in patients undergoing coronary angiography. Angiology.
61:711–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acikel S, Muderrisoglu H, Yildirir A,
Aydinalp A, Sade E, Bayraktar N, Bal U and Ozin B: Prevention of
contrast-induced impairment of renal function by short-term or
long-term statin therapy in patients undergoing elective coronary
angiography. Blood Coagul Fibrinolysis. 21:750–757. 2010.PubMed/NCBI
|
|
20
|
Su X, Xie X, Liu L, Lv J, Song F, Perkovic
V and Zhang H: Comparative effectiveness of 12 treatment strategies
for preventing contrast-induced acute kidney injury: A systematic
review and bayesian network meta-analysis. Am J Kidney Dis.
69:69–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Kidney Foundation: K/DOQI
clinical practice guidelines for chronic kidney disease:
Evaluation, classification, and stratification. Am J Kidney Dis. 39
(Suppl 1):S1–S266. 2002.PubMed/NCBI
|
|
23
|
Stacul F, van der Molen AJ, Reimer P, Webb
JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement
O, et al: Contrast induced nephropathy: Updated ESUR Contrast Media
Safety Committee guidelines. Eur Radiol. 21:2527–2541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Briguori C, Manganelli F, Scarpato P, Elia
PP, Golia B, Riviezzo G, Lepore S, Librera M, Villari B, Colombo A
and Ricciardelli B: Acetylcysteine and contrast agent-associated
nephrotoxicity. J Am Coll Cardiol. 40:298–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horn PS, Feng L, Li Y and Pesce AJ: Effect
of outliers and nonhealthy individuals on reference interval
estimation. Clin Chem. 47:2137–2145. 2001.PubMed/NCBI
|
|
26
|
Jo SH, Hahn JY, Lee SY, Kim HJ, Song YB,
Choi JH, Choi SH, Lee SH and Gwon HC: High-dose atorvastatin for
preventing contrast-induced nephropathy in primary percutaneous
coronary intervention. J Cardiovasc Med (Hagerstown). 16:213–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abaci O, Arat Ozkan A, Kocas C, Cetinkal
G, Sukru Karaca O, Baydar O, Kaya A and Gurmen T: Impact of
rosuvastatin on contrast-induced acute kidney injury in patients at
high risk for nephropathy undergoing elective angiography. Am J
Cardiol. 115:867–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galal H, Nammas W and Samir A: Impact of
high dose versus low dose atorvastatin on contrast induced
nephropathy in diabetic patients with acute coronary syndrome
undergoing early percutaneous coronary intervention. Egypt Heart J.
67:329–336. 2015. View Article : Google Scholar
|
|
29
|
Bidram P, Roghani F, Sanei H, Hedayati Z,
Golabchi A, Mousavi M, Hajiannejad A, Pourheidar B, Badalabadi MM,
Gharaati M, et al: Atorvastatin and prevention of contrast induced
nephropathy following coronary angiography. J Res Med Sci. 20:1–6.
2015.PubMed/NCBI
|
|
30
|
Rear R, Bell RM and Hausenloy DJ:
Contrast-induced nephropathy following angiography and cardiac
interventions. Heart. 102:638–648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Attallah N, Yassine L, Musial J, Yee J and
Fisher K: The potential role of statins in contrast nephropathy.
Clin Nephrol. 62:273–278. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khanal S, Attallah N, Smith DE,
Kline-Rogers E, Share D, O'Donnell MJ and Moscucci M: Statin
therapy reduces contrast-induced nephropathy: An analysis of
contemporary percutaneous interventions. Am J Med. 118:843–849.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patti G, Nusca A, Chello M, Pasceri V,
D'Ambrosio A, Vetrovec GW and Di Sciascio G: Usefulness of statin
pretreatment to prevent contrast-induced nephropathy and to improve
long-term outcome in patients undergoing percutaneous coronary
intervention. Am J Cardiol. 101:279–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida S, Kamihata H, Nakamura S, Senoo
T, Manabe K, Motohiro M, Sugiura T and Iwasaka T: Prevention of
contrast-induced nephropathy by chronic pravastatin treatment in
patients with cardiovascular disease and renal insufficiency. J
Cardiol. 54:192–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jo SH, Koo BK, Park JS, Kang HJ, Cho YS,
Kim YJ, Youn TJ, Chung WY, Chae IH, Choi DJ, et al: Prevention of
radiocontrast medium-induced nephropathy using short-term high-dose
simvastatin in patients with renal insufficiency undergoing
coronary angiography (PROMISS) trial-a randomized controlled study.
Am Heart J. 155:499 e1–e8. 2008. View Article : Google Scholar
|
|
36
|
Toso A, Maioli M, Leoncini M, Gallopin M,
Tedeschi D, Micheletti C, Manzone C, Amato M and Bellandi F:
Usefulness of atorvastatin (80 mg) in prevention of
contrast-induced nephropathy in patients with chronic renal
disease. Am J Cardiol. 105:288–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall WD: Abnormalities of kidney function
as a cause and a consequence of cardiovascular disease. Am J Med
Sci. 317:176–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou W, Lv J, Perkovic V, Yang L, Zhao N,
Jardine MJ, Cass A, Zhang H and Wang H: Effect of statin therapy on
cardiovascular and renal outcomes in patients with chronic kidney
disease: A systematic review and meta-analysis. Eur Heart J.
34:1807–1817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Latif F, Kleiman NS, Cohen DJ, Pencina MJ,
Yen CH, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ and Saucedo JF;
EVENT Investigators, : In-hospital and 1-year outcomes among
percutaneous coronary intervention patients with chronic kidney
disease in the era of drug-eluting stents: A report from the EVENT
(Evaluation of Drug Eluting Stents and Ischemic Events) registry.
JACC Cardiovasc Interv. 2:37–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Natsuaki M, Furukawa Y, Morimoto T, Sakata
R and Kimura T; CREDO-Kyoto PCI/CABG Registry Cohort-2
Investigators, : Renal function and effect of statin therapy on
cardiovascular outcomes in patients undergoing coronary
revascularization (from the CREDO-Kyoto PCI/CABG Registry
Cohort-2). Am J Cardiol. 110:1568–1577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dasari TW, Cohen DJ, Kleiman NS, Keyes MJ,
Yen CH, Hanna EB and Saucedo JF: Statin therapy in patients with
chronic kidney disease undergoing percutaneous coronary
intervention (from the Evaluation of Drug Eluting Stents and
Ischemic Events Registry). Am J Cardiol. 113:621–625. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stone NJ, Robinson JG, Lichtenstein AH,
Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D,
Lloyd-Jones DM, et al: 2013 ACC/AHA guideline on the treatment of
blood cholesterol to reduce atherosclerotic cardiovascular risk in
adults: A report of the American College of Cardiology/American
Heart Association Task Force on Practice Guidelines. J Am Coll
Cardiol. 63:2889–2934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Downs JR and O'Malley PG: Management of
dyslipidemia for cardiovascular disease risk reduction: Synopsis of
the 2014 U.S. Department of Veterans Affairs and U.S. Department of
Defense clinical practice guideline. Ann Intern Med. 163:291–297.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jacobson TA, Ito MK, Maki KC, Orringer CE,
Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al:
National Lipid Association recommendations for patient-centered
management of dyslipidemia: Part 1-executive summary. J Clin
Lipidol. 8:473–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Catapano AL, Graham I, De Backer G,
Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser
U, Pedersen TR, et al: 2016 ESC/EAS guidelines for the management
of dyslipidaemias. Eur Heart J. 37:2999–3058. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCullough PA, Choi JP, Feghali GA,
Schussler JM, Stoler RM, Vallabahn RC and Mehta A: Contrast-induced
acute kidney injury. J Am Coll Cardiol. 68:1465–1473. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for Percutaneous
Coronary Intervention: Executive summary: A report of the American
College of Cardiology Foundation/American Heart Association Task
Force on Practice Guidelines and the Society for Cardiovascular
Angiography and Interventions. Circulation. 124:2574–2609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kolh P, Windecker S, Alfonso F, Collet JP,
Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, et al:
2014 ESC/EACTS Guidelines on myocardial revascularization: The Task
Force on Myocardial Revascularization of the European Society of
Cardiology (ESC) and the European Association for Cardio-Thoracic
Surgery (EACTS). Developed with the special contribution of the
European Association of Percutaneous Cardiovascular Interventions
(EAPCI). Eur J Cardiothorac Surg. 46:517–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guitterez NV, Diaz A, Timmis GC, O'Neill
WW, Stevens MA, Sandberg KR and McCullough PA: Determinants of
serum creatinine trajectory in acute contrast nephropathy. J Interv
Cardiol. 15:349–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Briguori C, Visconti G, Rivera NV,
Focaccio A, Golia B, Giannone R, Castaldo D, De Micco F,
Ricciardelli B and Colombo A: Cystatin C and contrast-induced acute
kidney injury. Circulation. 121:2117–2122. 2010. View Article : Google Scholar : PubMed/NCBI
|