Introduction
According to previous studies, severe and chronic
hypoxia leads to neuronal death in the Cornu Ammonis 3 (CA3) and
CA4 regions in hippocampus dentate gyrus, indicating that neuronal
apoptosis in this brain region is one of the main causes of chronic
hypobaric hypoxia-induced cognitive impairment (1).
Cold inducible RNA-binding protein (CIRP) was
screened as the DNA damage-induced gene transcript initially, which
plays a key role in controlling the cell response under various
environmental stresses, such as low temperature and ultraviolet
light (2,3). Previous studies have revealed that CIRP
migrates from the nucleus to the cytoplasm under environmental
stress, which regulates its target messenger RNA (mRNA) at the
post-transcriptional level and exerts a neuroprotective effect
(4,5). For example, CIRP can inhibit the
neuronal apoptosis through inhibiting the mitochondrial apoptosis
pathway during mild hypothermia (6).
Besides, CIRP protein in cortical neuron of rats inhibits
H2O2-induced neuronal apoptosis under low
temperature, thereby protecting the brain. There have been reports
that CIRP is up-regulated in acute mild (8% O2) or
severe (1% O2) hypoxia response (7). However, the expression features of CIRP
in brain tissues under chronic hypobaric hypoxia remain unclear,
and whether CIRP can serve as a neuroprotective factor under
chronic hypobaric hypoxia has not been confirmed (8).
As the most important transcription factor in cell
hypoxia response, hypoxia-inducible factor-1α (HIF-1α) is closely
related to the hypoxia-induced neuronal apoptosis (9). Under hypoxic stress, HIF-1α can inhibit
its anti-apoptosis effect through increasing the anti-apoptotic
protein, B-cell lymphoma 2 (Bcl-2) (10–12).
Besides, HIF-1α is related to neuronal apoptosis after brain injury
through regulating p53 and Bcl-2 nineteen-kilodalton interacting
protein 3 (BNIP3) in apoptotic neurons (13). To weaken the hypoxia-induced neuronal
apoptosis in the brain region of cognitive function, clarifying the
detailed regulatory mechanism of HIF-1α under hypoxic stress and
searching for protective factors are of medical significance.
Chang et al (14), found that CIRP can bind to mRNA of
HIF-1α and several protein translation factors on polysomes, and
increase the protein translation under cell stress. Considering
that CIRP plays an important role in the stress-induced neuronal
apoptosis, it is assumed as a neuroprotective factor. CIRP can be
involved in HIF-1α-mediated neuronal apoptosis under chronic
hypobaric hypoxia and exert a neuroprotective effect. A microRNA
(miRNA) is a small and non-coding RNA, which plays a vital role in
the regulation of such biological processes as cell
differentiation, proliferation and apoptosis. Under hypoxic
conditions, a hypoxia-sensitive miRNA family named hypoxamiRs will
be induced, and these miRNAs are specifically involved in
controlling various processes, such as tumorigenesis, angiogenesis
and apoptosis.
To confirm the above hypothesis, dynamic changes in
CIRP/HIF-1α expression and neuronal apoptosis were detected in rats
exposed to chronic hypobaric hypoxia and SH-SY5Y cells exposed to
hypoxia (1% O2). To investigate the potential
association between CIRP change and hypoxia-induced neuronal
apoptosis, the effects of CIRP overexpression on HIF-1α expression
and neuronal apoptosis were detected.
Materials and methods
Materials
Main reagents
Rabbit anti-human CIRP, HIF-1α, Bax, Bcl-2,
caspase-3 and β-actin polyclonal antibodies were purchased from
ProteinTech Group, Inc. (Chicago, IL, USA) (1:300; cat. nos.
10209-2-AP, 20960-1-AP, 50599-2-Ig, 12789-1-AP, 19677-1-AP,
20536-1-AP, respectively), rabbit anti-human cleaved caspase-3
(1:200; cat. no. 9661, Cell Signaling Technology, Danvers, USA) and
Opti-MEM Medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) were also used.
The present study was approved by the Ethics
Committee of Liaocheng Third People's Hospital (Liaocheng,
China).
Model establishment
In vivo hypobaric hypoxia animal models
Newborn male Sprague-Dawley rats (n=40) were kept in
an animal room of the Research Institute in cages with 12/12 h
dark-light cycle before exposure to hypobaric hypoxia and provided
with sufficient pellet feed and water at 23°C. The humidity was
60%. All rats were randomly divided into normal control group (n=6)
and hypoxia group (n=6).
In vitro chronic 1% hypoxic cell models
Human neuron-like SH-SY5Y neuroblastoma cells
(ATCC® CRL-2266™) were placed in the RPMI-1640 medium
containing 2 mM L-glutamine supplemented with 10% heat-inactivated
fetal bovine serum and 100 U/ml penicillin/streptomycin. The
culture was kept in a standard wet incubator with 5% CO2
at 37°C, and the original medium was replaced with fresh medium
once every 2 days. When 90% cells were fused, the medium was
divided as 1:4. Cells were placed in the calibration gas containing
1% O2 or 3% O2 (the concentration of
CO2 was adjusted to 5% under these two conditions) and
the cells were placed in a humidified microaerophilic culture
system (DWS HypOxystation) to prepare the anaerobic environment.
The cells were kept in an incubator at 37°C at different times.
Control culture was kept for the same time under normal oxygen
content.
Methods
Terminal-deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) analysis
TUNEL was carried out to evaluate cell apoptosis
according to the manufacturer protocol. The procedure was as
follows: Induced apoptotic cells were fixed in 4% paraformaldehyde
phosphate-buffered saline (PBS) at room temperature for 30 min,
washed with PBS 3 times and then incubated on ice using 0.1% Triton
X-100. After that, the treated cells were mixed with TUNEL reaction
mixture, followed by reaction in the dark for 1 h at 37°C. The
nuclei were labelled with Hoechst-33342. Subsequently, the cells
were observed under a fluorescence microscope to count the
proportion of cell apoptosis.
Western blot analysis
After exposure for the specified time, 3 rats in
each group were decollated and western blot analysis was performed
to determine HIF-1α, CIRP, cleaved caspase-3/caspase-3 and
Bax/Bcl-2 levels in the hippocampus. The hippocampus was removed
from brain tissue of rats after cervical dislocation and rapidly
placed in prepared pre-cooled 0.9% NaCl solution. Resected tissue
was preserved in liquid nitrogen. Samples of tissue and cells were
lysed and homogenized, after which the concentration of protein
obtained was determined. Western blot analysis was carried out and
FluorChem FC2 imaging system (ProteinSimple, San Jose, CA, USA)
based on ECLO was used to detect the immune response signal. Gray
values of bands in each group were analyzed using ImageJ software.
Each protein band was normalized into β-actin value and presented
as the intensity ratio. Western blot analyses were performed in
triplicate.
Flow cytometry for analysis of cell
apoptosis
Flow cytometry was performed for further analysis of
cell apoptosis. After hypoxic exposure, SH-SY5Y cells were treated
with trypsin, centrifuged at 3,000 × g for 8 min at 4°C and washed
twice. Then, the cells were re-suspended using binding buffer, and
added with 5 ml FITC-labeled Annexin-V and 5 ml PI, followed by
incubation in the dark at room temperature for 15 min. Samples were
analyzed within 1 h after staining.
Plasmid construction and
transfection
CIRP complementary deoxyribonucleic acid (cDNA) was
cloned in pEGFP-N2 vector and control transfection was performed
via pEGFP-N2 without CIRP. Overexpression of CIRP in cells was
confirmed via western blot analysis using anti-CIRP antibody in
accordance with the protocol. According to procedures provided by
the manufacturer, SH-SY5Y cells were transfected with CIRP cDNA
using Lipofectamine 2000 transfection reagents. In brief, the cells
were inoculated into a 6-well plate with 3×105 cells in
each well and grew overnight until 80% of cells were fused.
Transfection complex composed of 2.5 µg pEGFP-N2 vector plasmid DNA
or pEGFP-N2-CIRP plasmid DNA and 6 µl Lipofectamine reagent was
added into the well with Opti-MEM medium. Transfection efficiency
and viability of cells were analyzed at 48 h after lipid
transfection.
Statistical analysis
Continuous variables were presented as mean ±
standard error of mean (SEM) and Student's t test was applied for
analysis. Statistical analyses were completed using GraphPad Prism
v.5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
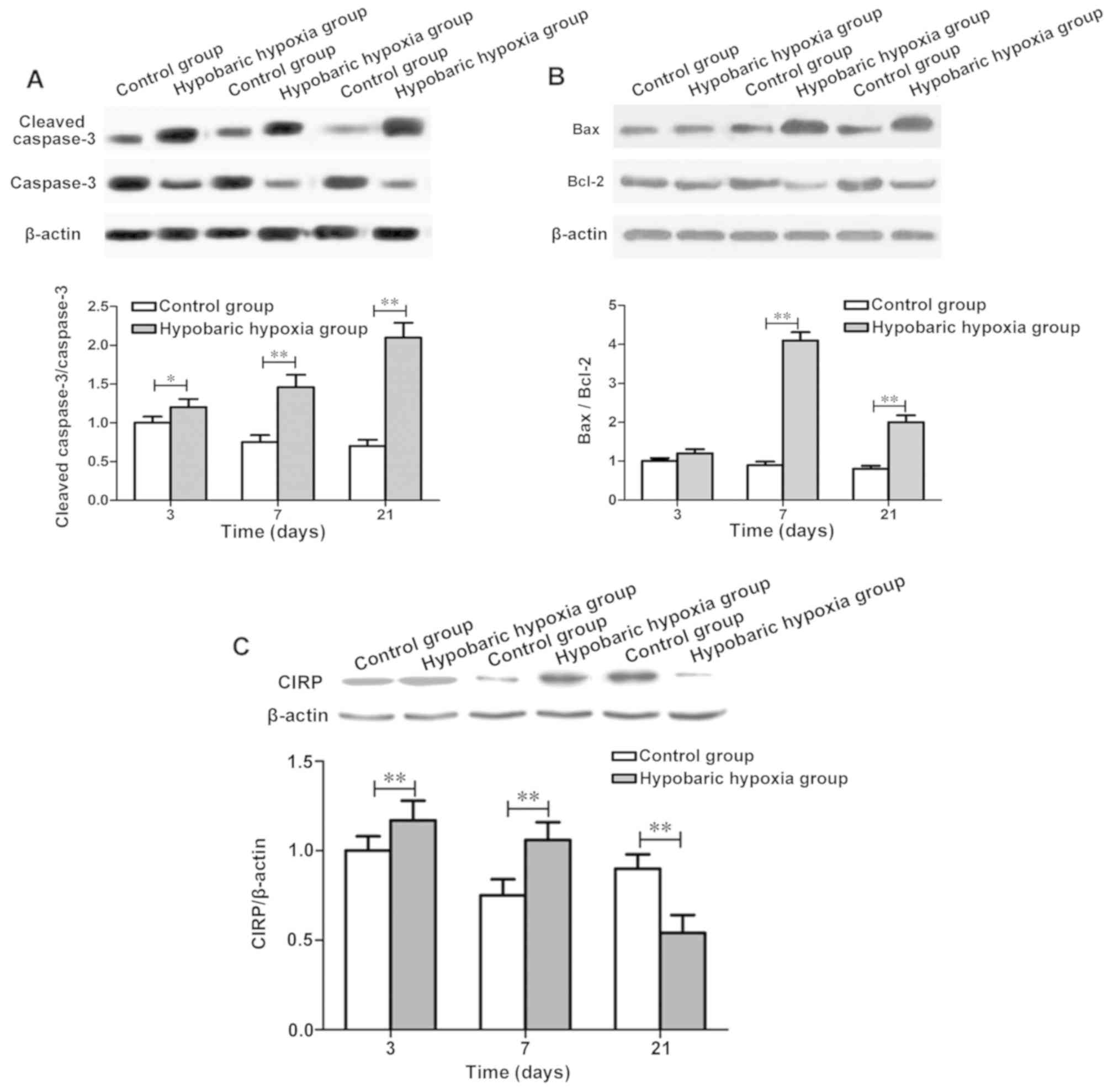
Exposure to chronic hypobaric hypoxia
led to the increased apoptotic rate of hippocampal neurons in rats
and significant changes in CIRP expression
To investigate the effect of exposure to chronic
hypoxia on hypoxia-sensitive hippocampal neurons, adult rats were
placed in an animal decompression chamber under 349 mmHg. The
expression of apoptosis-related proteins, caspase-3, Bcl-2
associated X protein (Bax) and Bcl-2, in hippocampal neurons were
detected, and the CIRP expression during hypobaric hypoxia was also
detected at day (d) 3, 7 and 21. Compared with those in control
group, the cleaved caspase-3/caspase-3 and Bax/Bcl-2 ratios in
hypoxia group at 7 d and 21 d were significantly increased
(Fig. 1A and B). Results of western
blot analysis revealed that the CIRP levels at 3 and 7 d in hypoxia
group were obviously higher than those in control group
(P<0.01), while the CIRP level at 21 d in hypoxia group was
obviously lower than that in control group (P<0.01). CIRP was
induced at the early stage of hypoxia exposure, and inhibited
continuously with the prolongation of exposure time (Fig. 1C).
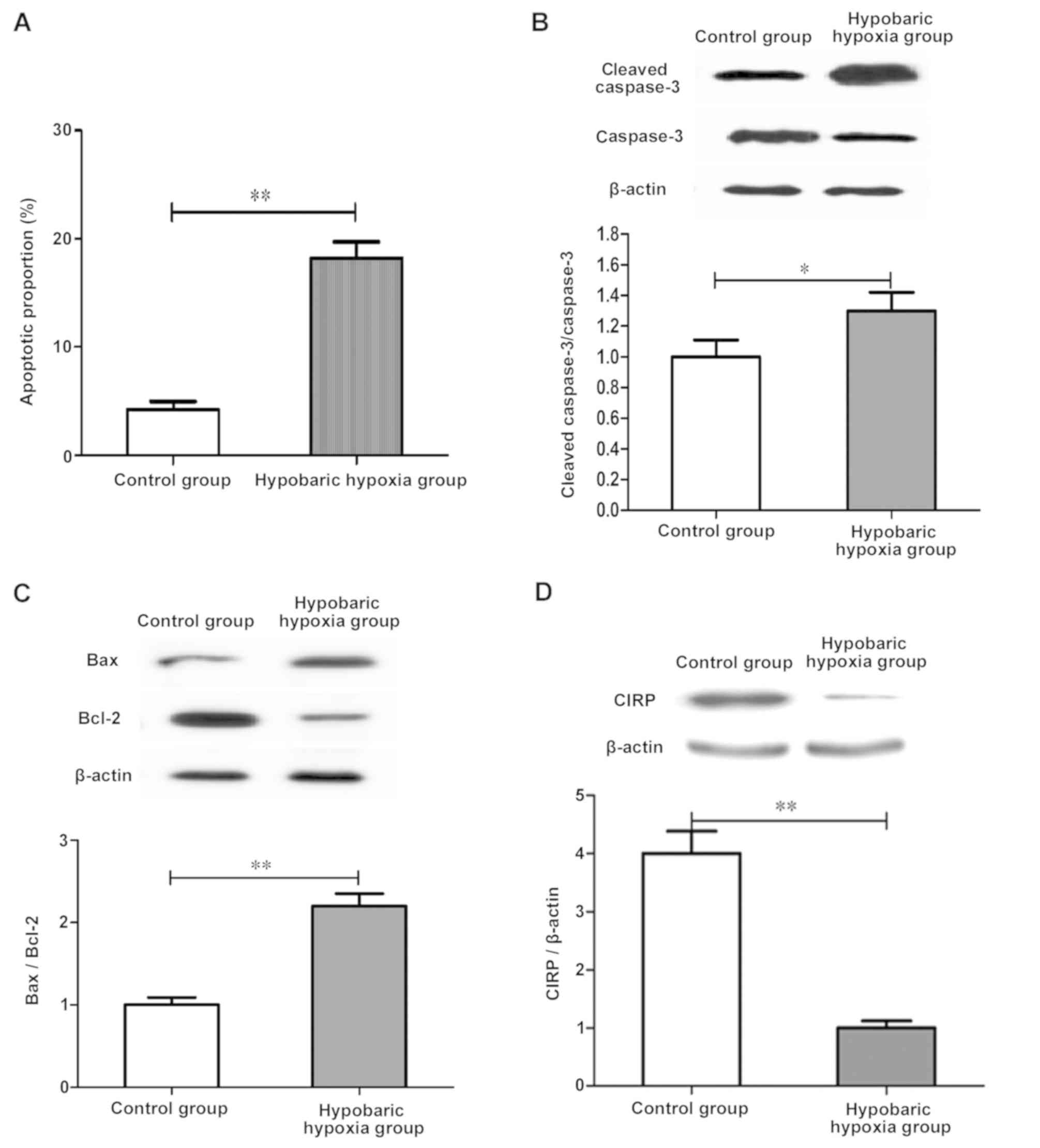
Exposure to chronic hypobaric hypoxia
induces apoptosis of SH-SY5Y cells and significantly reduces the
CIRP expression
To detect the role of CIRP in hypoxic-related
neuronal apoptosis, the in vitro chronic hypoxia model was
constructed. SH-SY5Y cells were cultured in an anoxic chamber with
1% O2 for 48 h to simulate the chronic hypoxic condition
in tissues. Results of western blot analysis showed that compared
with those in control group, the CIRP expression was significantly
decreased, and cleaved caspase-3/caspase-3 and Bax/Bcl-2 ratios
were significantly increased in hypoxia group (P<0.01; Fig. 2B and C). Therefore, it is speculated
that exposure to 1% hypoxia for 48 h leads to the increased
apoptotic rate of SH-SY5Y cells.
The CIRP expression was detected after exposure to
1% hypoxia, and results of western blot analysis manifested that
the CIRP expression in hypoxia group was remarkably decreased
compared with that in control group, which was consistent with
in vivo results (Fig.
2D).
Overexpression of CIRP inhibits the
upregulation of HIF-1α in hypoxia and inhibits hypoxia-induced
neuronal apoptosis
To study the potential association between CIRP
decrease and hypoxia-induced brain injury, the effects of CIRP
overexpression on HIF-1α expression and hypoxia-induced apoptosis
were detected. SH-SY5Y cells were transfected with p-EGFP-N2-CIRP
plasmid, and apoptosis was detected after exposure to hypoxia (1%
O2) for 48 h. The overexpression of CIRP in transfected
cells was confirmed via western blot analysis (Fig. 3C), which obviously decreased the
HIF-1α expression under 1% hypoxic conditions (P<0.01; Fig. 3B) and significantly reduced neuronal
apoptosis induced by hypoxia (Fig.
3A). The above results suggest that the overexpression of CIRP
can alleviate hypoxia and induce apoptosis of SH-SY5Y cells by
regulating HIF-1α expression.
Discussion
The aim of the present study was to investigate the
molecular mechanism of CIRP participating in apoptosis during
chronic hypobaric hypoxia stress. It was found that the CIRP
expression was downregulated in hippocampal neurons and SH-SY5Y
cells of rats exposed to hypoxia. Moreover, the overexpression of
CIRP could effectively inhibit the upregulation of HIF-1α, thus
inhibiting the hypoxia-induced neuronal apoptosis.
CIRP is involved in the neuronal apoptosis induced
by a variety of environmental stresses, such as low temperature,
oxidative stress, inflammation and DNA damage (15,16). In
cortical neurons of rats, CIRP inhibits the etoposide-induced
apoptosis through regulating levels of p53 and its downstream
targets (8). To the best of our
knowledge, no studies are available on the role of CIRP under
chronic hypobaric hypoxia. It was found in the present study that
in the hippocampus of rats exposed to chronic hypobaric hypoxia,
CIRP expression was increased at early exposure, decreased after 7
d and continuously inhibited. Accordingly, the neuronal apoptosis
and proportion of apoptosis-related proteins in the hippocampal CA3
region were increased from 7 d after exposure. In SH-SY5Y cells
exposed to 1% O2, CIRP expression was increased in the
first 12 h of exposure, then decreased at 24 h after hypoxia
exposure and continuously inhibited. Therefore, it can be
speculated that the downregulation of CIRP may be involved in the
chronic hypobaric hypoxia-induced neuronal apoptosis.
The role of HIF-1α, the most important transcription
factor in cell hypoxia response, in hypoxia-induced apoptosis has
been discussed widely (17). HIF-1α
can initiate the hypoxia-mediated apoptosis by increasing the
expression of Bcl-2 binding protein, thus inhibiting the
anti-apoptotic effect of Bcl-2 (11). Chang et al found that CIRP can
bind to mRNA of HIF-1α and several protein translation factors on
polysomes, and increase the protein translation under cell stress
(14). In the present study, the
overexpression of CIRP obviously decreased the HIF-1α level and the
apoptotic rate of SH-SY5Y cells exposed to 1% O2,
suggesting that the overexpression of CIRP can inhibit the HIF-1α
expression and alleviate the hypoxia-induced apoptosis. Recently,
Luo et al (18), reported
several HIF inhibitors under chronic hypoxia, and found that
several kinds of genes, such as peroxiredoxin 2 (PRDX2) and PRDX4,
inhibit the HIF-1α mRNA level and transcriptional activity.
Therefore, it is speculated that CIRP may repress HIF-1α during
chronic hypobaric hypoxia-induced neuronal apoptosis.
CIRP significantly increased HIF-1α expression under
normoxia compared with that under hypoxia. Several previous studies
have proved that HIF-1α accumulates under hypoxia (19–21).
Wang et al (21), confirmed
that the accumulation of HIF-1α under normoxia is possibly related
to the increased glycolysis or glutamine dissolution. It has been
proved that CIRP is widely involved in cellular metabolism, so it
can be inferred that the transfection of CIRP under normoxia can
change the cellular metabolism, resulting in the accumulation of
HIF-1α. Moreover, HIF-1α is mainly regulated by the protein
stability in an oxygen-dependent way. Under normoxia, HIF-1α can be
rapidly degraded by the proteasome, failing to exert its functions.
In the present study, the apoptotic rate and levels of
apoptosis-related proteins in cells transfected with CIRP under
normoxia had no difference from those in control group. It can be
observed that although CIRP transfection significantly increases
the HIF-1α accumulation under normoxia, it seemingly has no effect
on apoptosis of SH-SY5Y cells. The detailed mechanism of CIRP in
promoting HIF-1α under normoxia remains to be clarified.
In conclusion, the present study indicates that
exposure to hypobaric hypoxia leads to hypoxia injury in the
hippocampus of rats and neuronal apoptosis. At the same time,
hypoxia exposure to 1% O2 increases levels of HIF-1α and
apoptosis-related proteins, and apoptotic rate of SH-SY5Y cells.
CIRP is considered to exert a neuroprotective effect under chronic
hypobaric hypoxia stress. The overexpression of CIRP can
effectively inhibit the HIF-1α expression in cells, thus
alleviating the hypoxia-induced apoptosis. However, the CIRP
expression decreases gradually with the prolongation of exposure
time.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC assisted with TUNEL analysis and wrote the
manuscript. LC and QT were responsible for model establishment. WW
performed western blot analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Liaocheng Third People's Hospital (Liaocheng,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maiti P, Singh SB, Muthuraju S, Veleri S
and Ilavazhagan G: Hypobaric hypoxia damages the hippocampal
pyramidal neurons in the rat brain. Brain Res. 1175:1–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan F, Zarate J, Choudhury A, Rupprecht R
and Bradley TM: Osmotic stress of salmon stimulates upregulation of
a cold inducible RNA binding protein (CIRP) similar to that of
mammals and amphibians. Biochimie. 86:451–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishiyama H, Higashitsuji H, Yokoi H, Itoh
K, Danno S, Matsuda T and Fujita J: Cloning and characterization of
human CIRP (cold-inducible RNA-binding protein) cDNA and
chromosomal assignment of the gene. Gene. 204:115–120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang JJ, Tang C and Nie PT: The
cytoprotective mechanisms of CIRP upon stresses. Sheng Li Ke Xue
Jin Zhan. 44:67–71. 2013.(In Chinese). PubMed/NCBI
|
|
5
|
Al-Fageeh MB and Smales CM: Cold-inducible
RNA binding protein (CIRP) expression is modulated by alternative
mRNAs. RNA. 15:1164–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang HT, Xue JH, Zhang ZW, Kong HB, Liu
AJ, Li SC and Xu DG: Cold-inducible RNA-binding protein inhibits
neuron apoptosis through the suppression of mitochondrial
apoptosis. Brain Res. 1622:474–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li S, Zhang Z, Xue J, Liu A and Zhang H:
Cold-inducible RNA binding protein inhibits
H2O2-induced apoptosis in rat cortical
neurons. Brain Res. 1441:47–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HN, Ahn SM and Jang HH: Cold-inducible
RNA-binding protein, CIRP, inhibits DNA damage-induced apoptosis by
regulating p53. Biochem Biophys Res Commun. 464:916–921. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J
and Le AD: Resveratrol inhibits hypoxia-induced accumulation of
hypoxia-inducible factor-1alpha and VEGF expression in human tongue
squamous cell carcinoma and hepatoma cells. Mol Cancer Ther.
4:1465–1474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1alpha in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takubo K, Goda N, Yamada W, Iriuchishima
H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et
al: Regulation of the HIF-1alpha level is essential for
hematopoietic stem cells. Cell Stem Cell. 7:391–402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zaidi AU, McDonough JS, Klocke BJ, Latham
CB, Korsmeyer SJ, Flavell RA, Schmidt RE and Roth KA:
Chloroquine-induced neuronal cell death is p53 and Bcl-2
family-dependent but caspase-independent. J Neuropathol Exp Neurol.
60:937–945. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang ET, Parekh PR, Yang Q, Nguyen DM and
Carrier F: Heterogenous ribonucleoprotein A18 (hnRNP A18) promotes
tumor growth by increasing protein translation of selected
transcripts in cancer cells. Oncotarget. 7:10578–10593.
2016.PubMed/NCBI
|
|
15
|
Khan MM, Yang WL, Brenner M, Bolognese AC
and Wang P: Cold-inducible RNA-binding protein (CIRP) causes
sepsis-associated acute lung injury via induction of endoplasmic
reticulum stress. Sci Rep. 7:413632017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiang X, Yang WL, Wu R, Zhou M, Jacob A,
Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al: Cold-inducible
RNA-binding protein (CIRP) triggers inflammatory responses in
hemorrhagic shock and sepsis. Nat Med. 19:1489–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai S, Huang ML, Hsu CY and Chao KS:
Inhibition of hypoxia inducible factor 1alpha causes
oxygen-independent cytotoxicity and induces p53 independent
apoptosis in glioblastoma cells. Int J Radiat Oncol Biol Phys.
55:1027–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo W and Wang Y: HIF repressors under
chronic hypoxia. Aging (Albany NY). 8:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doe MR, Ascano JM, Kaur M and Cole MD: Myc
posttranscriptionally induces HIF1 protein and target gene
expression in normal and cancer cells. Cancer Res. 72:949–957.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iida Y, Aoki K, Asakura T, Ueda K,
Yanaihara N, Takakura S, Yamada K, Okamoto A, Tanaka T and Ohkawa
K: Hypoxia promotes glycogen synthesis and accumulation in human
ovarian clear cell carcinoma. Int J Oncol. 40:2122–2130.
2012.PubMed/NCBI
|
|
21
|
Wang H, Zhao L, Zhu LT, Wang Y, Pan D, Yao
J, You QD and Guo QL: Wogonin reverses hypoxia resistance of human
colon cancer HCT116 cells via downregulation of HIF-1α and
glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol Carcinog.
53 (Suppl 1):E107–E118. 2014. View
Article : Google Scholar : PubMed/NCBI
|