Introduction
Bone remodeling has been described as a cycle that
consists of three major consecutive overlapping phases: Resorption,
reversal and formation (1). The bone
systems homeostatic balance requires communication between
osteoblasts and osteoclasts, which occurs at various stages of bone
remodeling, and includes three modes: Direct, paracrine and
cell-bone matrix (1). Through direct
communication between osteoclasts and osteoblasts, membrane-bound
ligands and receptors interact and initiate intracellular
signaling. Gap junctions can also form between contact cells,
allowing the passage of small water-soluble molecules (2). Communication between cells can also
occur through diffusible paracrine factors, including growth
factors, cytokines, chemokines and other small molecules, which are
secreted by either cell type or acting on the other via diffusion
(3). Growth factors and a variety of
other molecules previously buried in the bone matrix have been
demonstrated to be released by osteoclasts during bone resorption
(2,3). However, it is undetermined as to which
of these cell communicators serves a key role in osteoblast
activity.
1α, 25-dihydroxyvitamin D3 [1α,
25(OH)2D3], the active form of vitamin
D3, is a potent inducer of receptor activator of NF-κB
ligand (RANKL), a key molecule that is secreted by osteoblasts in
osteoclastogenesis (4). The
expression of vitamin D receptors on osteoblast cells enables
direct responses to vitamin D3. The magnitude of effects
in response to Vitamin D3 is dependent on the presence
of a number of factors in the cell microenvironment, including
parathyroid hormone (PTH), calcium/phosphors level, transforming
growth factor-β1 (TGF-β1) and insulin-like growth factor 1 (IGF-1)
(5).
Despite vitamin D3 being successfully
used in the management of conditions including psoriasis (6) and various cancer types (7), the use of vitamin D3 in the
treatment of osteoporosis has been prevented due to its calcemic
activity and the consensus that it is associated with osteoclastic
bone resorption (8–10). Eldecalcitol (ELD), formerly known as
ED-71, is an analog of 1α,25-(OH)2D3 that
includes a hydroxypropyloxy residue at the 2β position (11). ELD was previously developed to
increase the inhibitory effect on bone resorption and was approved
in Japan as a therapeutic drug for the treatment of osteoporosis in
2011 (12–14). It has been previously reported that
ELD lowered the biochemical and histological parameters of bone
resorption in a ovariectomized rat model of osteoporosis (15). These aforementioned effects were
observed without sustained hypercalcemia or hypercalciuria
(12).
Previous studies have demonstrated that TGF-β and
IGF-1, which are released from the bone matrix during osteoclastic
bone resorption, serve an important role in osteoblast activities
including receptor activator of NF-κB ligand (RANKL) expression and
cell migration (16–18). The present study aimed to determine
the role of proteins, which are released by bone slices during
osteoclastic bone resorption, in the regulation of osteoblast
activity. The current study also provides additional data to
understand how ELD affects osteoblasts in a distinct cell
microenvironment, for example, in the presence or absence of
osteoclastic bone resorption. Osteoblast cell culture models were
established in vitro, with differentiated osteoclast cell
culture supernatants (OCS) or bone resorption culture supernatant
(BRS). Osteoblastic induction was performed using MC3T3-E1
pre-osteoblast cells and the viability, differentiation and
RANKL/osteoprotegerin (OPG) expression of osteoblast cells was
determined.
Materials and methods
Pre-osteoclast culture and
osteoclastic induction
Murine RAW264.7 monocytic cells were purchased from
the Type Culture Collection of the Chinese Academy of Sciences and
cultured in α-minimum essential medium (α-MEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 10 U/l penicillin and 100 mg/l streptomycin at
37°C in a humidified atmosphere containing 5% CO2.
Recombinant soluble mouse RANKL and macrophage colony-stimulating
factor (M-CSF) were purchased from R&D Systems, Inc. for use in
osteoclast differentiation. The cells were seeded in six-well plate
(5×105 cells/well) or 24-well plate (3×104
cells/well) and cultured at 37°C for 6 days in α-MEM supplemented
with 10% FBS, 30 ng/ml M-CSF and 50 ng/ml RANKL. The culture medium
was collected on day 6.
Establishment of bone resorption
model
Cortical bone slices (5×5 mm) of fresh bovine femur
slices (0.1 mm) were purchased from the Immunodiagnostic Systems,
Ltd. (cat. no. DT-1BON1000-96) and used to create the bone
resorption model according to the protocol described previously
(19,20).
RAW 264.7 cells were seeded into 24-well plates
(3×104 cells/well) and cultured in α-MEM supplemented
with 10% FBS, 10 U/l penicillin and 100 mg/l streptomycin with the
prepared cortical bone slices. After 24 h, cells were treated with
30 ng/ml M-CSF and 50 ng/ml RANKL at 37°C for 6 days (21). The culture medium was replaced every
2 days and was collected on day 6. Supernatants were centrifuged
(400 × g 4°C for 10 min), filtered through a 0.22 mm
polyethersulfone membrane filter (EMD Millipore) and stored at
−20°C.
Osteoblast cell culture
Murine MC3T3-E1 pre-osteoblast cells were purchased
from the Type Culture Collection of the Chinese Academy of
Sciences. MC3T3-E1 cells were cultured in α-MEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS, 10 U/l
penicillin and 100 mg/l streptomycin. Six groups were formed: i)
MC3T3-E1 that were cultured with OCS; ii) MC3T3-E1 that were
cultured with BRS; iii) MC3T3-E1 that were cultured with OCS + ELD
and iv) MC3T3-E1 that were cultures with BRS + ELD; v) MC3T3-E1
that were cultured with ELD; and vi) CON group. Exponentially
growing cells were plated into six-well plates (6×103
cells/well) for reverse transcription-quantitative PCR (RT-qPCR)
and western blot analysis. After incubation at 37°C for 24 h, cells
were transferred to a medium containing 75% (v/v) α-MEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, 10 U/l
penicillin, 100 mg/l streptomycin and 25% (v/v) OCS or BRS. ELD
(10−7 M) was added to the ELD group (Chugai
Pharmaceutical Co., Ltd.).
Cell viability assay
The MC3T3-E1 pre-osteoblast cells were harvested and
seeded into 96 well plates (1×104 cells/well) with a
total volume of 200 µl culture medium. Cells were incubated for 24,
48 and 72 h at 37°C. After the cells were treated for the indicated
times, Cell Counting Kit-8 (MedChem Express) was used (20 µl/well)
and cells were incubated for 3 h at 37°C. Subsequently, absorbance
at 450 nm was read for all plates using an automated microplate
spectrophotometer (Bio Rad Laboratories, Inc.). Each experiment was
repeated at least three times.
Tartrate-resistant acid phosphatase
(TRAP) staining
RAW 264.7 cells were seeded into 24-well plates and
cultured in α-MEM supplemented with aforementioned stimuli for 6
days. Cells were fixed with 4% paraformaldehyde for at least 15 min
at room temperature and stained for TRAP using a TRAP-staining
solution containing 0.1 M sodium acetate (pH 5.0) and 0.01%
naphthol AS-MX phosphate (Sigma-Aldrich; Merck KGaA) as a substrate
and 0.03% red violet LB salt (Sigma-Aldrich; Merck KGaA) as a stain
for the reaction product in the presence of 50 mM sodium tartrate
for 15 min at 37°C. Staining was observed by light microscopy
(magnification, ×100 and ×400; Olympus Corporation). Cell nuclei
were counterstained with hematoxylin for 2 min at room temperature.
Multinucleated TRAP-positive cells with at least three nuclei were
scored as osteoclasts (22).
RT-qPCR
Total RNA was extracted from RAW 264.7 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
was synthesized using PrimeScript™ RT reagent kit (Takara Bio,
Inc.) according to the manufacturer's protocol. Alkaline
phosphatase (ALP), RUNX2, RANKL and OPG mRNA expression were
assessed on days 1, 3 and 7 using qPCR that was performed using 1
µl cDNA template in a 10 µl total volume with the TB
Green® Premix Ex Taq™ (Takara Bio, Inc.) using MyiQ™
Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.). The thermocycling conditions used were: Initial denaturation
for 10 sec at 95°C, followed by 40 cycles of 5 sec at 95°C, 31 sec
at 58°C and 30 sec at 72°C. The data were collected at 72°C in each
cycle. The mRNA value was normalized to that of the housekeeping
gene GAPDH. The results are presented as the relative gene
expression. The fold-change in gene expression relative to the
control was calculated using the 2−∆∆Cq method (23) with GraphPad Prism software (version
6.0; GraphPad Software, Inc.). The primer sequences are presented
in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| ALP |
|
| Forward |
CGCCATGACATCCCAGAAAG |
| Reverse |
GCCTGGTAGTTGTTGTGAGC |
| RUNX2 |
|
| Forward |
GCCCAGGCGTATTTCAGATG |
| Reverse |
GGTAAAGGTGGCTGGGTAGT |
| RANKL |
|
| Forward |
GTACTTTCGAGCGCAGATGG |
| Reverse |
TCCAACCATGAGCCTTCCAT |
| OPG |
|
| Forward |
ATGAACAAGTGGCTGTGCTG |
| Reverse |
TAAGAGTGGTCAGGGCAAGG |
| GAPDH |
|
| Forward |
TGGCCTTCCGTGTTCCTAC |
| Reverse |
GAGTTGCTGTTGAAGTCGCA |
Western blot analysis
MC3T3-E1 cells were harvested on days 1, 3 and 7 and
lysed using RIPA lysis buffer (Beijing ComWin Biotech Co., Ltd.).
Following measurement of protein concentration using a
Bicinchoninic Acid assay kit (Beyotime Institute of Biotechnology),
the protein samples (50 µg) were mixed with 1/4 volume of 5X SDS
loading buffer and heated at 95°C for 5 min. Following separation
by 10–15% SDS-PAGE, proteins were transferred to PVDF membranes.
The membranes were blocked with 5% BSA diluted in TBS supplemented
with 0.1% Tween-20 at room temperature for 1 h. Western blot
analysis was performed using: Rabbit anti-ALP antibody (1:1,000;
cat. no. ab83259; Abcam), mouse anti-RUNX2 antibody (1:1,000; cat.
no. ab76956; Abcam), rabbit anti-RANKL antibody (1:1,000; cat. no.
sc-9073; Santa Cruz Biotechnology, Inc.), rabbit anti-OPG antibody
(1:1,000; cat. no. ab73400; Abcam) and mouse anti-GAPDH (1:2,000;
cat. no. ab8245; Abcam), overnight at 4°C. The secondary antibodies
used were horseradish peroxidase (HRP)-conjugated goat anti-rabbit
IgG (1:2,000; cat. no. #14708; Cell Signaling Technology, Inc.) for
ALP, RANKL and OPG and HRP-conjugated rabbit anti-mouse IgG
(1:1,000; cat. no. #58802; Cell Signaling Technology, Inc.) for
GAPDH and RUNX2, at room temperature for 1 h. Protein bands were
visualized using an enhanced chemiluminescence reagent (Millipore;
Merck KGaA) and western blot images were captured using a FluorChem
E System (ProteinSimple) and quantified using ImageJ software
(version 1.41; National Institute of Health).
Statistical analysis
The data are expressed as the mean ± standard
deviation. All experiments were performed in triplicate. GraphPad
Prism 6.0 software was used to analyze the obtained data (GraphPad
Software, Inc.). A one-way ANOVA was used for multiple group
comparisons and the mean value of each group was compared using the
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant result.
Results
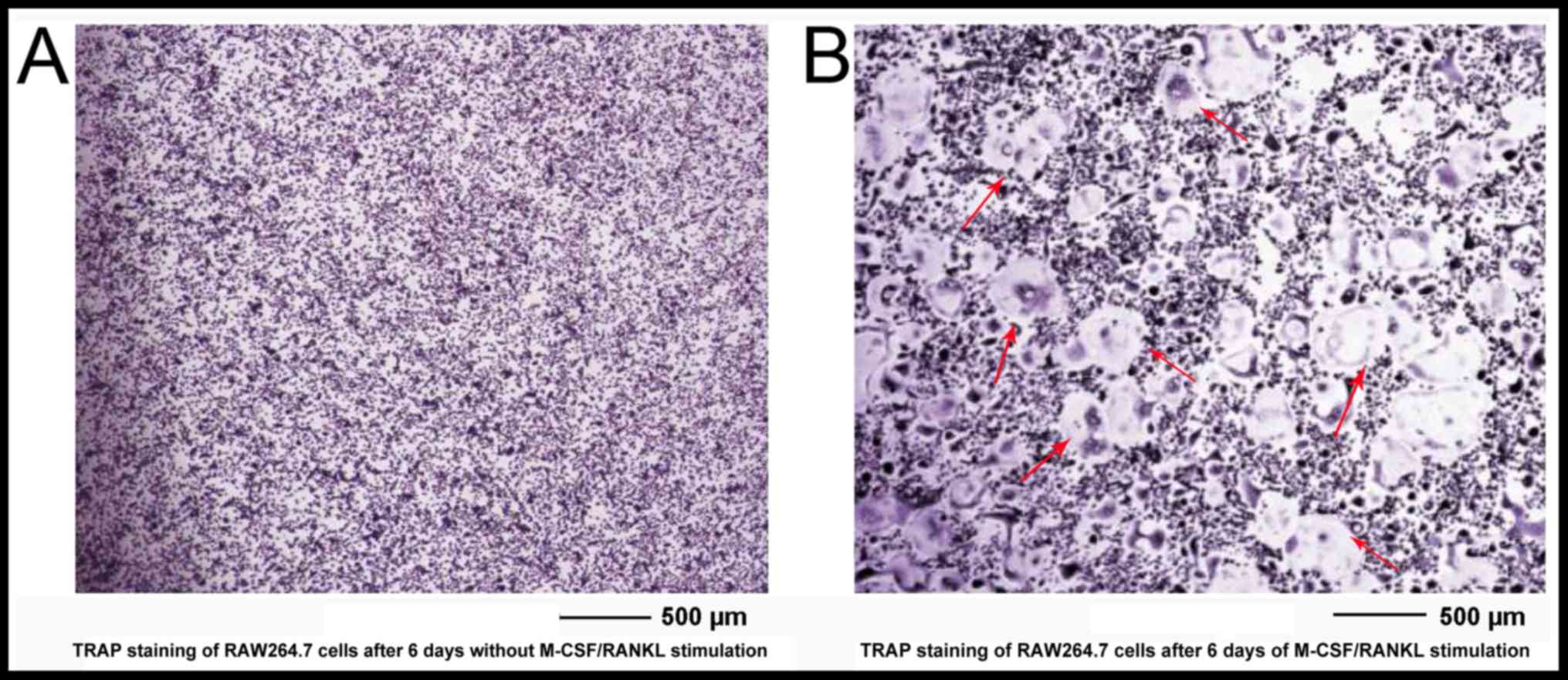
TRAP staining indicates that RAW 264.7
cells could be differentiated into functioning osteoclasts
Since the identification of the gene encoding RANKL,
a cocktail of soluble forms of RANKL and M-CSF (also known as
CSF-1) has been used to generate osteoclast-like cells in
vitro in the absence of osteoblasts, simplifying the analysis
of osteoclast differentiation (24).
The results of the current study observed that 50 ng/ml RANKL could
stimulate RAW264.7 cells to develop into TRAP-positive cells after
1 day and multinucleated cells after 3–4 days. During RANKL-induced
differentiation, RAW264.7 cells started to undergo the
characteristic morphological changes after 3 days with increasing
cell-cell fusion into large and multinucleated cells. TRAP staining
of RAW264.7 cultured without stimuli (Fig. 1A) or with 30 ng/ml M-CSF and 50 ng/ml
RANKL (Fig. 1B) were observed on day
6. An increase in TRAP staining and cell fusion were observed in
the RANKL/M-CSF-induced differentiation group compared with the
control group on day 6. TRAP-positive multinucleated cells (MNCs)
containing three or more nuclei were counted as osteoclasts.
Osteoclast culture supernatant,
osteoclast bone resorption supernatant and ELD regulate MC3T3-E1
pre-osteoblast viability and ALP activity
The OCS and OCS + ELD treatment significantly
increased MC3T3-E1 cell viability after 48 and 72 h compared with
the control group (CON), whilst ELD exerted no significant effects
on MC3T3-E1 cell viability compared with CON (Fig. 2A). However, BRS alone, ELD + BRS and
ELD alone significantly reduced MC3T3-E1 cell viability at 24 h,
when compared with CON (Fig. 2B).
ALP activity is a marker of early stage osteoblast differentiation
(25). OCS and ELD significantly
reduced ALP activity, and combined OCS + ELD treatment exerted an
additional inhibitory effect; however, no significant difference
was observed between the OCS and ELD alone groups (Fig. 2C). BRS enhanced MC3T3-E1 cell ALP
activity, and this increased effect was also present in the BRS-ELD
treatment. However, ELD treatment decreased ALP activity when
compared with the CON group (Fig.
2D).
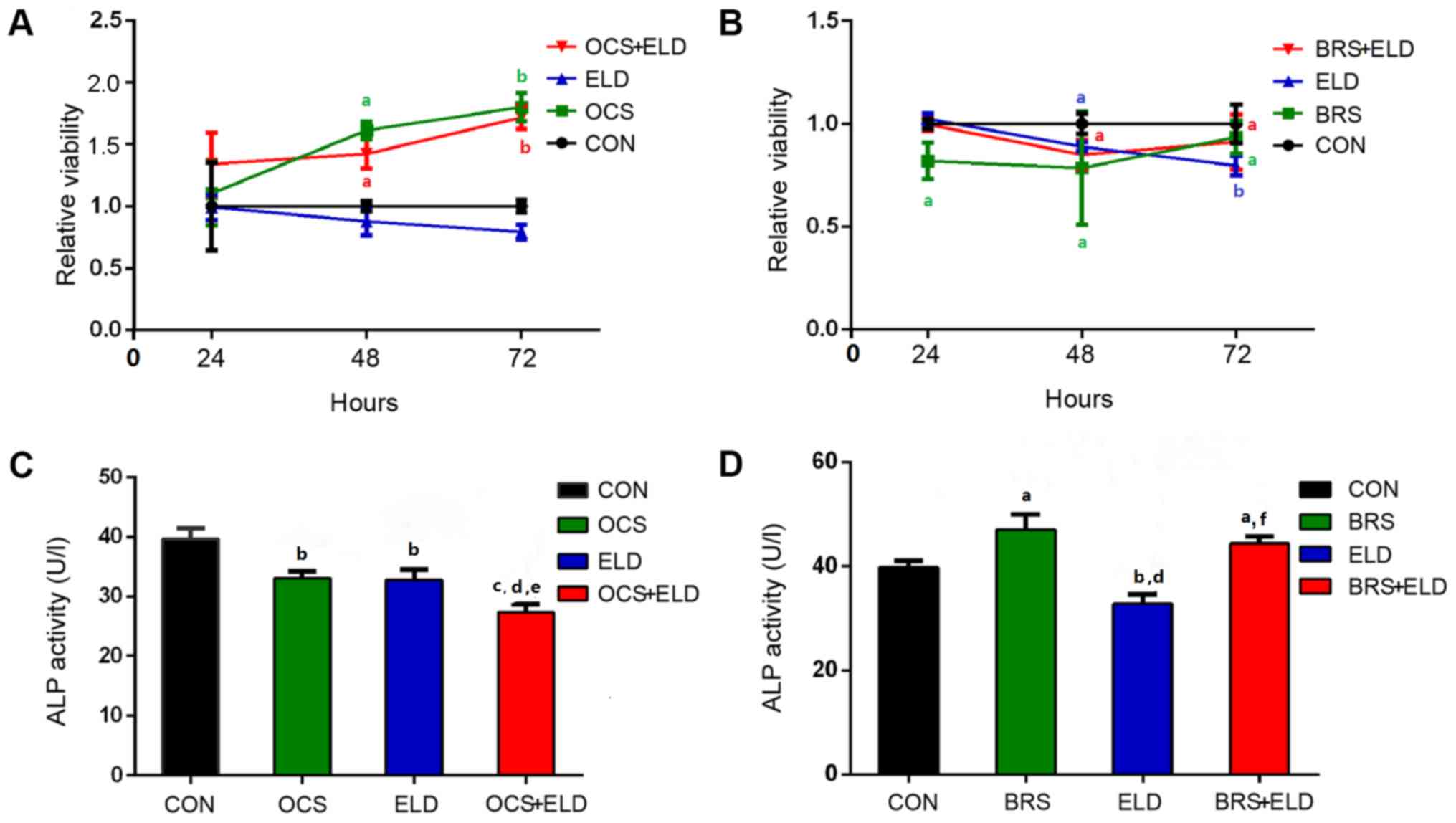 | Figure 2.Osteoclasts culture supernatant,
osteoclasts bone resorption supernatant and ELD distinctly
regulated MC3T3-E1 pre-osteoblast viability and ALP activity.
Relative cell viability of MC3T3-E1 cells treated with (A) OCS +
ELD, ELD and OCS and (B) BRS + ELD, ELD and BRS with the associated
controls. ALP activity in MC3T3-E1 cells treated with (C) OCS +
ELD, ELD and OCS and (D) BRS + ELD, ELD and BRS with the associated
controls. aP<0.05, bP<0.01 and
cP<0.001 vs. CON; dP<0.01 vs. OCS or
BRS; eP<0.05 and fP<0.001 vs. ELD. ELD,
eldecalcitol; ALP, alkaline phosphatase; OCS, differentiated
osteoclast cell culture supernatants; BRS, bone resorption culture
supernatant; CON, control. |
Osteoclast culture supernatant and ELD
reduces osteogenic marker expression and increases RANKL/OPG
ratio
ALP and RUNX2 mRNA expression was detected in
MC3T3-E1 cells cultured with OCS, ELD or OCS + ELD. The results of
RT-qPCR demonstrated that ELD and OCS treatment reduced the
expression of osteogenic markers (ALP and RUNX2) in MC3T3-E1 cells
when compared with the control group on days 1, 3 and 7; and their
combination exerted an additional inhibitory effect on day 3
(Fig. 3A and B). ELD, OCS and ELD +
OCS all enhanced RANKL expression and reduced OPG on days 1, 3 and
7, resulting in an increased RANKL/OPG ratio in MC3T3-E1 cells
(Fig. 3C-E). However, no difference
was observed between the ELD and CON groups on days 1, 3 or 7
(Fig. 3C-E). This observation
suggests that ELD may affect osteoblasts through the medium
secreted by osteoclasts, rather than directly acting on
osteoblasts.
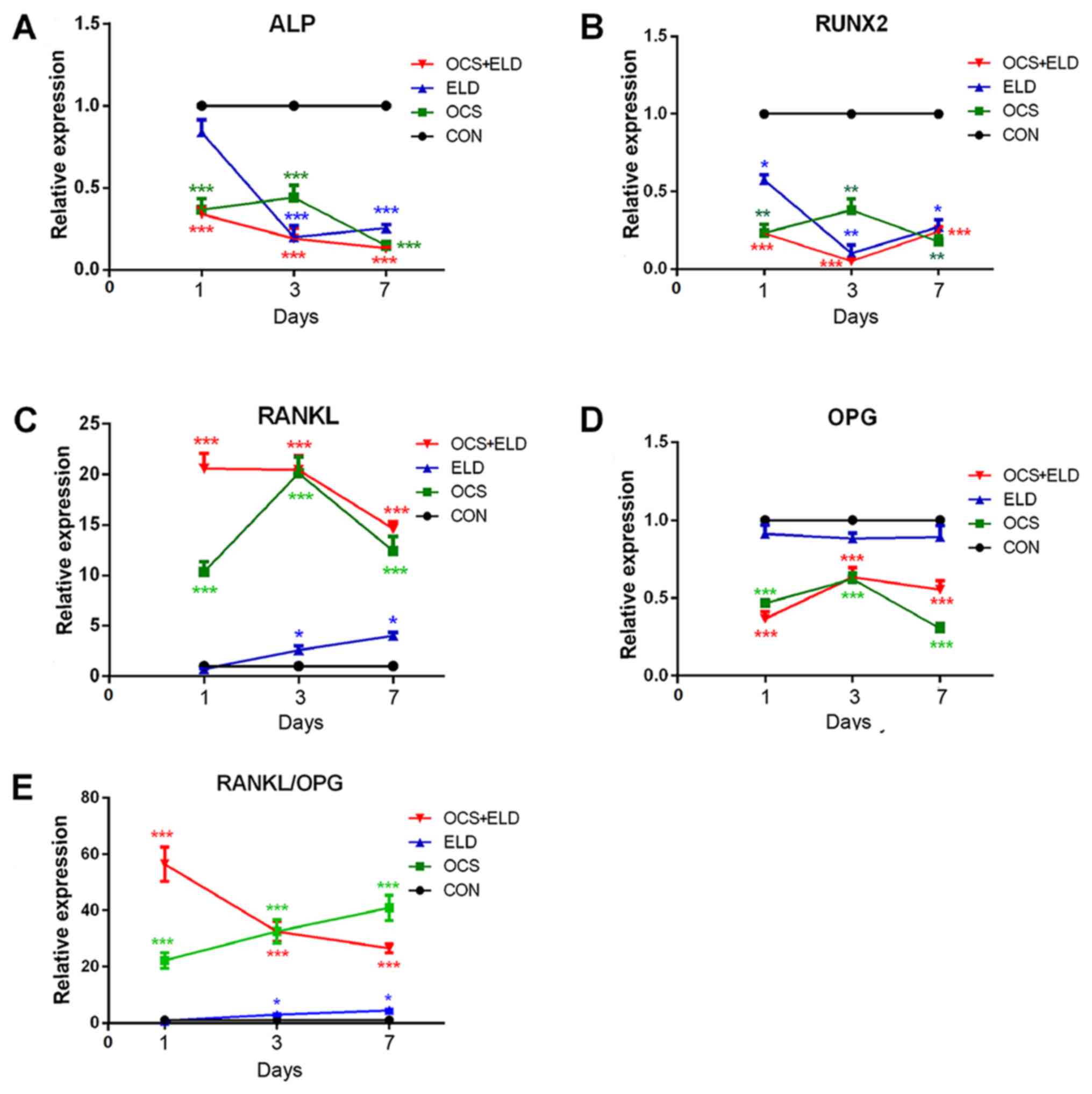 | Figure 3.mRNA expression of pre-osteoblasts
osteogenic markers and RANKL/OPG expression in MC3T3-E1 cells
treated with osteoclasts culture supernatant and ELD. Reverse
transcription-quantitative PCR analysis of (A) ALP, (B) RUNX2, (C)
RANKL, (D) OPG and (E) RANKL/OPG expression in MC3T3-E1 cells
cultured in the presence of OCS or ELD and OCS + ELD. *P<0.05,
**P<0.01 and ***P<0.001 vs. CON. RANKL, receptor activator of
NF-κB ligand; OPG, osteoprotegerin; ELD, eldecalcitol; ALP,
alkaline phosphatase; RUNX2, runt-related transcription factor 2;
OCS, differentiated osteoclast cell culture supernatants; CON,
control. |
Western blot analysis was performed to measure
RUNX2, RANKL and OPG expression on days 1, 3 and 7. The results
exhibited similar patterns with that of mRNA expression, in that
RUNX2 expression was significantly decreased in ELD and OCS
treatment groups when compared with CON on days 1, 3 and 7
(Fig. 4A and B). In addition,
enhanced RANKL expression (Fig. 4A and
C), decreased OPG expression on days 1 and 3 (Fig. 4A and D) and increased the RANKL/OPG
ratio (Fig. 4E) were also observed
in the ELD and OCS treatment groups compared with CON. These
results indicated that cells cultured with OCS + ELD reduced RUNX2
expression and increased the RANKL/OPG ratio.
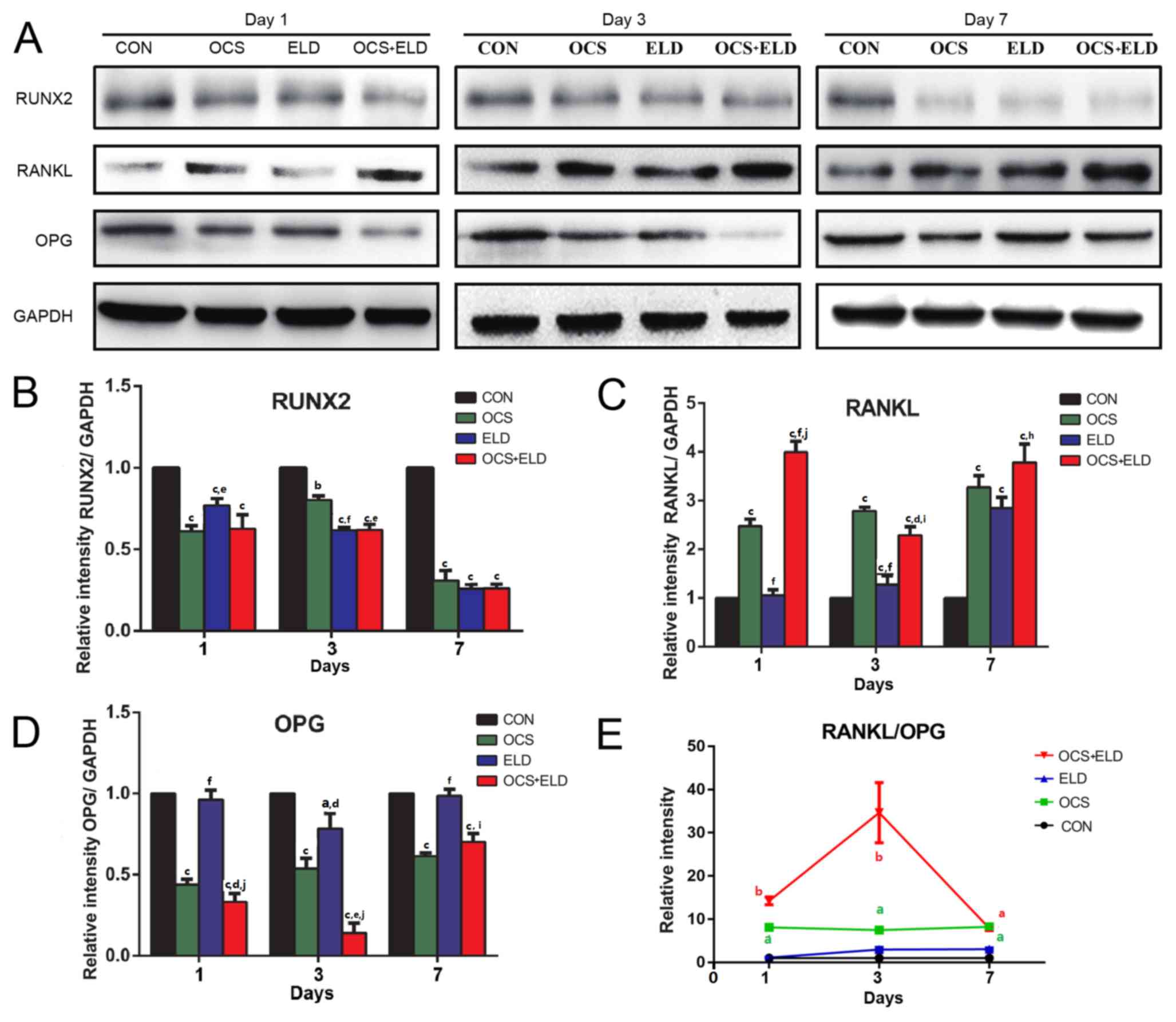 | Figure 4.Western blot analysis of osteoclasts
culture supernatant and ELD on the MC3T3-E1 pre-osteoblasts
osteogenic markers and RANKL/OPG expressions. (A) western blot
analysis of (A) RUNX2, RANKL, OPG and GADPH expression with
subsequent quantification of (B) RUNX2, (C) RANKL, (D) OPG and (E)
RANKL/OPG in MC3T3-E1 cells treated with OCS, ELD or OCS + ELD.
aP<0.05, bP<0.01 and
cP<0.001 vs. CON; dP<0.05,
eP<0.01, fP<0.001 vs. OCS;
hP<0.05, iP<0.01 and
jP<0.001 vs. ELD. ELD, eldecalcitol; RANKL, receptor
activator of NF-κB ligand; OPG, osteoprotegerin; RUNX2,
runt-related transcription factor 2; OCS, differentiated osteoclast
cell culture supernatants; CON, control. |
ELD induces the inhibition of
osteogenic marker expression and increases the RANKL/OPG ratio and
these actions are reversed by co-culture with bone resorption
supernatant
ELD significantly inhibited ALP and RUNX2 mRNA
expression in MC3T3-E1 cells (Fig. 5A
and B). However, BRS greatly increased the mRNA expression of
osteogenic markers ALP and RANKL when compared with CON (Fig. 5A and C). This promotive effect was
also exhibited by the BRS + ELD group (Fig. 5A-C), while ELD showed a significant
inhibitory effect on ALP activity on days 3 and 7 and on RUNX2
expression on days 1, 3 and 7 when compared with CON (Fig. 5A and B). BRS promoted RANKL
expression when compared with CON on days 1, 3 and 7. However, for
the ELD group this was evident only on day 7. Compared with the ELD
group, BRS treatment potentiated RANKL expression over 15-fold on
day 3. An additional promotive effect on RANKL expression was
exhibited by the ELD + BRS group on days 1 and 7 (Fig. 5C). Furthermore, no significant
differences were observed in the mRNA expression levels of OPG in
the BRS or BRS + ELD groups when compared with CON group, whilst
the RANKL/OPG ratio was increased in the BRS and BRS + ELD groups
due to the increased RANKL expression on days 1, 3 and 7 compared
with the CON group (Fig. 5D and E).
The results of the western blot analysis were almost in concordance
with the mRNA expression data (Fig.
6A). The BRS group exhibited upregulated RUNX2 expression and
ELD exhibited downregulated RUNX2 expression when compared with the
CON group on days 1, 3 and 7. The ELD + BRS group upregulated the
RUNX2 expression (Fig. 6B), and
treatments with ELD alone, BRS alone and ELD + BRS all enhanced
RANKL expression (Fig. 6C) and
RANKL/OPG ratio in MC3T3-E1 cells on days 1, 3 and 7 (Fig. 6E). Significantly different values
were exhibited by the BRS, BRS + ELD were observed on day 7 for OPG
expression compared with CON (Fig.
6D). The aforementioned results demonstrated that ELD induced
the inhibition of osteogenic marker expression and increased the
RANKL/OPG ratio, and these effects could be reversed by co-culture
with osteoclast BRS.
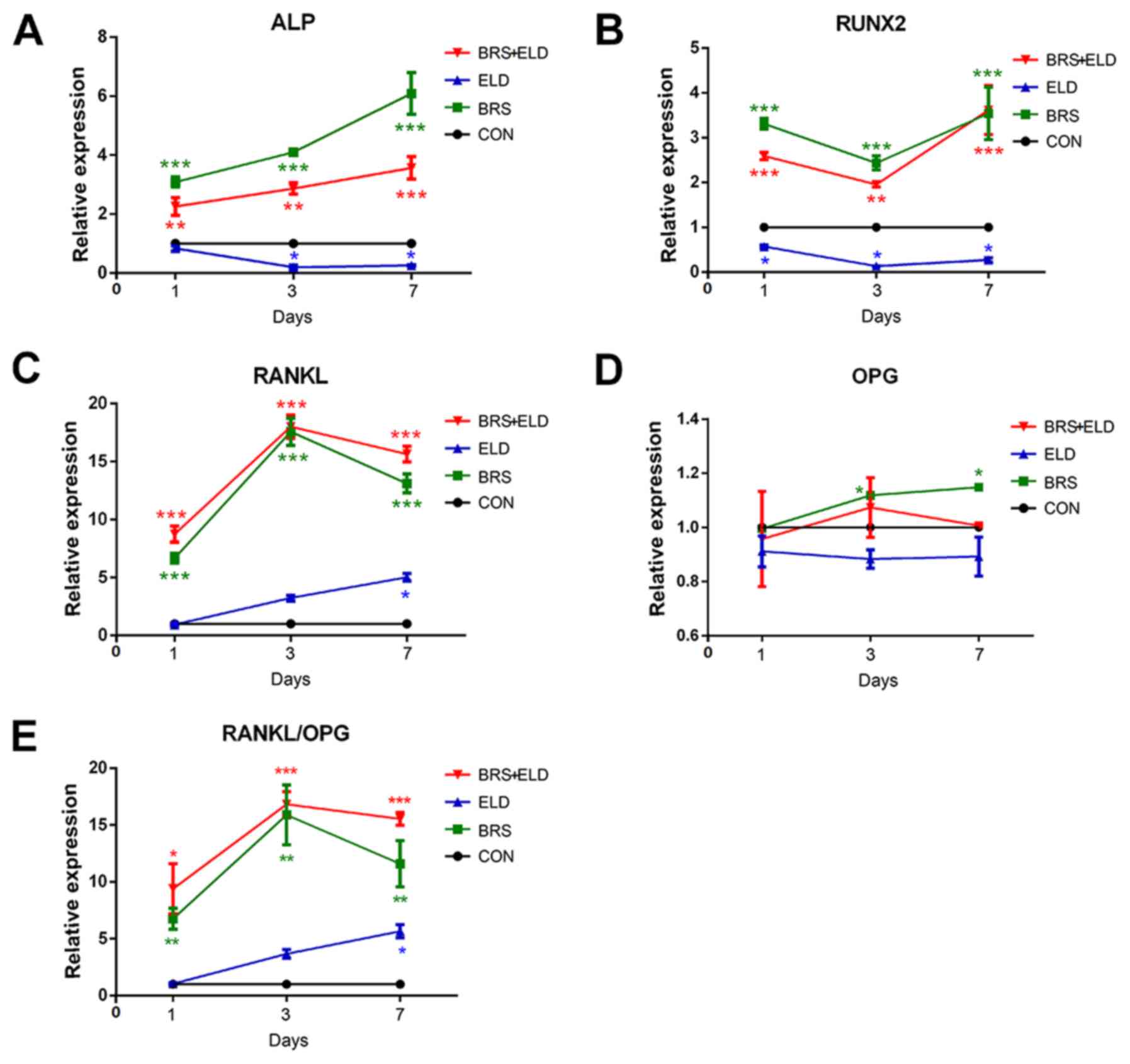 | Figure 5.Effect of bone resorption supernatant
and ELD on the expression of mRNA of MC3T3-E1 pre-osteoblasts
osteogenic markers and RANKL/OPG expressions. Reverse
transcription-quantitative PCR analysis of (A) ALP, (B) RUNX2, (C)
RANKL, (D) OPG and (E) RANKL/OPG expression in MC3T3-E1 cells
cultured in the presence of BRS or ELD and BRS + ELD. *P<0.05,
**P<0.01 and ***P<0.001 vs. CON. RANKL, receptor activator of
NF-κB ligand; OPG, osteoprotegerin; ELD, eldecalcitol; ALP,
alkaline phosphatase; RUNX2, runt-related transcription factor 2;
BRS, bone resorption culture supernatant; CON, control. |
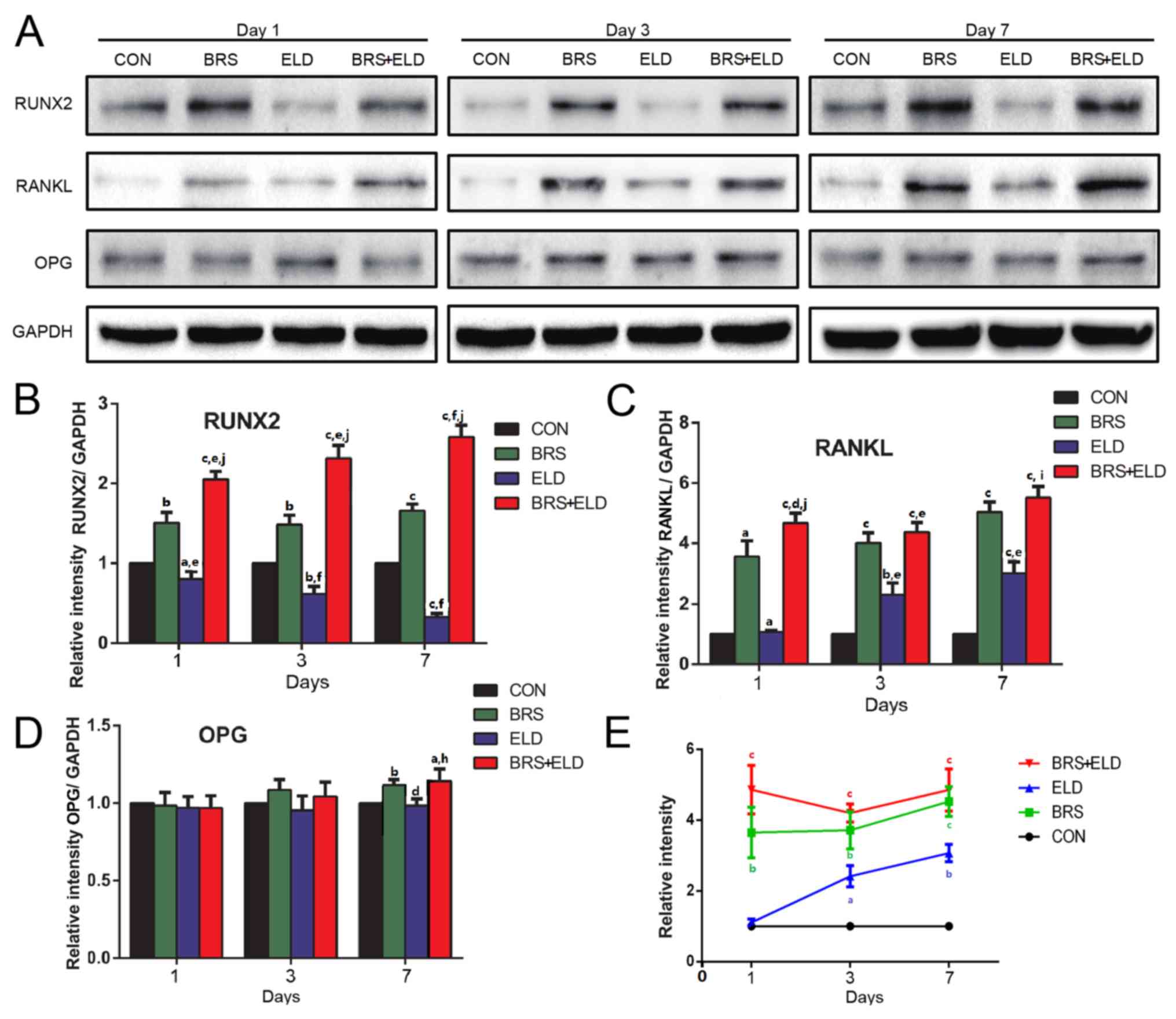 | Figure 6.Western blot analysis of
pre-osteoblast osteogenic markers and RANKL/OPG expression in
MC3T3-E1 cells treated with bone resorption culture supernatant and
ELD. (A) Western blot analysis of (A) RUNX2, RANKL, OPG and GADPH
expression with subsequent quantification of (B) RUNX2, (C) RANKL,
(D) OPG and (E) RANKL/OPG ratio in MC3T3-E1 cells treated with BRS,
ELD or BRS + ELD. aP<0.05, bP<0.01 and
cP<0.001 vs. CON; dP<0.05,
eP<0.01 and fP<0.001 vs. BRS;
hP<0.05, iP<0.01 and
jP<0.001 vs. ELD. ELD, eldecalcitol; RANKL, receptor
activator of NF-κB ligand; OPG, osteoprotegerin; RUNX2,
runt-related transcription factor 2; BRS, bone resorption culture
supernatant; CON, control. |
Discussion
Bone remodeling is based on the communication
between osteoblasts and osteoclasts and is a local process that can
occur anywhere on the bone surface throughout the lifespan of
humans (26). By removing old or
damaged bone and replacing it with new, stronger bone, the
structural integrity and strength of the bone are maintained.
Osteoblasts serve a pivotal role in bone metabolism but also
control and regulate the formation and activity of osteoclasts
(27). Osteoclasts, which develop
from hematopoietic cells of the monocyte-macrophage lineage, are
responsible for bone resorption that subsequently triggers the
differentiation and activation of osteoblasts (28). It has been shown that osteoblast and
osteoclast can communicate with each other through direct
cell-cell, cytokine or cell-bone matrix contact (3).
A previous study assessed c-fos deficient mice with
no osteoclasts and c-Src deficient mice with dysfunctional
osteoclasts, and it was histologically indicated that the presence
of osteoclasts is essential for osteoblastic activity (29). However, it is difficult to determine
how molecules secreted by osteoclasts and/or released by them from
the bone matrix affect osteoblasts in the absence of osteoclasts
in vivo. Therefore, in the current study, two distinct
osteoblast cell culture models were established in vitro for
the assessment of the osteogenic effects without direct osteoclast
contact. The aim of the present study was to investigate the
hypothesis that complex growth factors produced by active
osteoclasts during bone resorption exhibit the potential to
regulate the differentiation of osteoblast precursor cells.
The results indicated that RAW 264.7 osteoclast bone
resorption supernatant influenced the osteogenic activity of
osteoblast-like cells by inhibiting viability and promoting
differentiation. However, the RAW 264.7 OCS and ELD exhibited
opposite effects. RUNX2 has been demonstrated to promote the
expression of major bone matrix protein genes (30), and ALP is a marker of the early stage
of osteoblast differentiation (31).
A number of studies have indicated that direct effects are
exhibited by 1,25-(OH)2D3 on osteoblastic
cells in vitro (32–34). However, the effect on the viability
and differentiation of osteoblastic cells is undetermined. Kurihara
et al (33) demonstrated that
1,25-(OH)2D3 increases ALP activity in
MC3T3-E1 cells in the presence of serum (35). However, Majeska and Rodan (36) reported that in early ROS 17/2 cell
cultures, 1,25-(OH)2D3 elevates ALP activity,
but in later cultures, the steroid reduces ALP activity, indicating
that its effect may depend on the differentiation state of cells.
Jones (37) indicated that high
concentrations of 1,25-dihydroxyvitamin D3 induced the
production of analogous compounds, such as
24,25(OH)2D3 and
25,26(OH)2D3, which compete with
1,25-dihydroxyvitamin D3 to prevent the binding of
vitamin D to its receptors. Therefore, to increase the efficiency
of osteoblast differentiation, an adequate concentration of 1,
25-dihydroxyvitamin D3 must be maintained for an
appropriate time. It has been demonstrated that osteoclasts secrete
several potential factors that mediate cell-cell coupling. Kubota
et al (26) revealed that RAW
264.7 conditioned culture medium contained the B polypeptide chain
PDGF homodimer (PDGF BB), which may suppress osteoblast
differentiation in vitro. It has also been demonstrated that
PDGF increases osteoblast viability, but reduces ALP activity,
mineralized nodule formation and the expression of genes including
ALP, osteocalcin and type I collagen (38,39).
Sphingosine 1-phosphate (S1P) is produced by osteoclasts and is
associated with the S1P receptor expressed on osteoblasts to
enhance osteoblast migration and survival as well as RANKL
expression (40). It has been
demonstrated that molecules secreted from osteoclasts alone are
insufficient to initiate osteoblastogenesis (32).
In contrast to the OCS, the supernatant from the
osteoclast bone resorption model decreased the viability and
enhanced the differentiation of MC3T3-E1 cells (41). The results indicated that the effects
of OCS and BRS on the MC3T3-E1 pre-osteoblast viability and ALP
activity may be caused by the diversity of the molecules present in
these supernatants. Growth factors that are released from the bone
matrix, including transforming growth factor-β (TGF-β) and
insulin-like growth factors (IGF-1), have been considered to be
coupling factor candidates (42,43).
Bone remodeling depends on coordination between bone
resorption and subsequent bone formation. However, a study has
demonstrated that osteoclast bone resorptive activity is
dispensable for osteoblastic bone formation (44). Osteoclast ablation in M-CSF (45) or c-fos (46) deficient mice resulted in secondary
negative effects on bone formation, in contrast to mutations where
bone resorption is abrogated with sustained osteoclast numbers,
such as in c-src deficient mice (47). These data indicated that the presence
of osteoclasts, rather than osteoblastic bone resorption, is
important for the subsequent activation of osteoblasts during bone
remodeling (29). However, several
in vivo factors should be considered. For example, the
topography of the bones surface could affect the osteoblastic bone
formation process (48). The
systemic anabolic effect of parathyroid, Vitamin D3 and
calcium levels serve prominent roles in bone remodeling (49,50). The
coordination between osteoclasts and osteoblasts is a multifaceted
process, with numerous contributing regulator molecules (51,52). It
is unlikely that a single factor dominates during the entire
coupling process. Additional data is required to aid in the
understanding of the precise coordination mechanism of osteoclasts
and osteoblasts during bone remodeling.
The most prominent signals exhibited from
osteoblasts to osteoclasts mainly come from M-CSF and the RANKL/OPG
system (32). These signals are
essential and sufficient to drive the process of bone resorption
and formation and make them tightly coupled (1). In the current study, the effects of
medium containing OCS and BRS on the expressions of RANKL and OPG
on the mouse osteoblastic cell line MC3T3-E1 was assessed using
RT-qPCR and western blot analysis. OCS and BRS enhanced RANKL
expression. However, when compared with the CON group, OPG
expression of the OCS groups decreased, but was slightly increased
in the BRS groups. These discrepancies resulted in the
significantly different RANKL/OPG ratio between the OCS groups
(fold change >20) and BRS groups (fold change <20) when
compared with the CON group. The increased RANKL/OPG ratio
exhibited by the OCS group demonstrated a positive feedback loop,
through which osteoblastic cells attempted to increase the number
of osteoclasts in the absence of osteoclasts and a non-bone
resorption situation (53). The
aforementioned results also revealed that the osteoclastogenic
function is continuously being controlled and balanced for bone
remodeling, in case of increased bone resorption over bone
formation, which leads to bone loss disease.
Despite their osteoclastogenesis effect in
vitro, vitamin D3 analogs, including ELD, have been
used as therapeutic drugs for osteoporosis (13,54).
Currently, it has not been determined as to how vitamin
D3 increases bone mineral density via the suppression of
osteoclastic bone resorption in vivo. The differences in
culture environments without interference from hormones, including
PTH and estrogen, in vitro compared with in vivo may
provide an explanation for the substantial discrepancy between
in vitro and in vivo effects of vitamin D compounds
on bone resorption (55). In the
present study the conditions of the culture medium were applied to
determine whether the bone resorption environment influenced the
effect of ELD on osteoblasts. These in vitro studies
demonstrated that ELD decreases MC3T3-E1 pre-osteoblast viability
and differentiation markers ALP and RUNX2, and these results were
repeated in previous studies that used the same cell line (56,57).
Specific gene modification studies using mice, have revealed that
vitamin D3 is not a positive regulator of bone formation
(58–60). The positive effect on bone
mineralization in vivo, including the regulation of serum
calcium levels through the intestine, occurred outside the skeletal
tissues (61,62). Additionally, it has previously been
clarified that vitamin D3 induces the expression of a
variety of pro-osteoclastogenic cytokines, especially RANKL
(63). The present study
demonstrated that ELD downregulated OPG expression and upregulated
RANKL expression, leading to an increased RANKL/OPG ratio when
administrated to MC3T3-E1 cells alone or when combined with OCS/BRS
media. These results indicated that the culture environment may not
be the primary influence of the vitamin D3 effect.
However, further studies are required to assess the association of
ELD concentration and condition media in the action of other
osteoblastic cells.
The present study indicated that the molecules
secreted by osteoclasts and/or released from the bone matrix
exhibited important effects on osteoblast activity. In addition,
osteoclast bone resorption supernatant influenced the osteogenic
activity of osteoblast cells. However, RANKL expression and the
RANKL/OPG ratio of osteoblasts were increased by the treatment of
BRS, BRS + ELD and OCS + ELD. Eldecalcitol exhibited opposite
effects on osteoblastic differentiation and function in the
presence or absence of osteoclastic bone resorption. That is, ELD
inhibits osteoblastic differentiation in vitro. However, in
the presence of BRS, which mimics the local bone microenvironment
in vivo, the net effect on osteogenesis was positive.
Results observed in the presented study suggest that some
substances released in the surrounding microenvironment during bone
resorption serve an important role in the anti-osteoporosis effect
of ELD.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by The
National Nature Science Foundation of China (grant nos. 81470719
and 81611140133) to ML and The National Nature Science Foundation
of China (grant no. 81771108) to GJ.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and ML conceived and designed the current study.
JB and JD performed the experiments, analyzed and interpreted the
data; LS, WF and WW gathered data, prepared the figures and drafted
the manuscript. JG, TH and HL participated in performing the
experiments, edited and revised the manuscript. All authors read
and approved the final manuscript to be published.
Ethics approval and consent to
participate
Formal ethical approval and patient consent for this
study was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann N Y Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamma R and Zallone A: Osteoblast and
osteoclast crosstalks: From OAF to Ephrin. Inflamm Allergy Drug
Targets. 11:196–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno Y, Shinki T, Nagai Y, Murayama H,
Fujii K and Suda T: In vivo administration of 1,25-dihydroxyvitamin
D3 suppresses the expression of RANKL mRNA in bone of
thyroparathyroidectomized rats constantly infused with PTH. J Cell
Biochem. 90:267–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Driel M and van Leeuwen JP: Vitamin D
endocrine system and osteoblasts. Bonekey Rep. 3:4932014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pèrez A, Chen TC, Turner A, Raab R, Bhawan
J, Poche P and Holick MF: Efficacy and safety of topical calcitriol
(1,25-dihydroxyvitamin d3) for the treatment of psoriasis. Br J
Dermatol. 134:238–246. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guyton KZ, Kensler TW and Posner GH:
Cancer chemoprevention using natural vitamin D and synthetic
analogs. Annu Rev Pharmacol Toxicol. 41:421–442. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishii Y: Active vitamin D and its analogs
as drugs for the treatment of osteoporosis: Advantages and
problems. J Bone Miner Metab. 20:57–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishii Y and Okano T: History of the
development of new vitamin D analogs: Studies on 22-oxacalcitriol
(OCT) and 2beta- (3-hydroxypropoxy)calcitriol (ED-71). Steroids.
66:137–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishii Y: Rationale for active vitamin D
and analogs in the treatment of osteoporosis. J Cell Biochem.
88:381–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mano H, Nishikawa M, Yasuda K, Ikushiro S,
Saito N, Takano M, Kittaka A and Sakaki T: Development of novel
bioluminescent sensor to detect and discriminate between vitamin D
receptor agonists and antagonists in living cells. Bioconjug Chem.
26:2038–2045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto T, Miki T, Hagino H, Sugimoto T,
Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M
and Nakamura T: A new active vitamin D, ED-71, increases bone mass
in osteoporotic patients under vitamin D supplementation: A
randomized, double-blind, placebo-controlled clinical trial. J Clin
Endocrinol Metab. 90:5031–5036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto T, Takano T, Yamakido S,
Takahashi F and Tsuji N: Comparison of the effects of eldecalcitol
and alfacalcidol on bone and calcium metabolism. J Steroid Biochem
Mol Biol. 121:261–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hatakeyama S, Yoshino M, Eto K, Takahashi
K, Ishihara J, Ono Y, Saito H and Kubodera N: Synthesis and
preliminary biological evaluation of 20-epi-eldecalcitol
[20-epi-1alpha,25- dihydroxy-2beta-(3-hydroxypropoxy)vitamin D3:
20-epi-ED-71]. J Steroid Biochem Mol Biol. 121:25–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uchiyama Y, Higuchi Y, Takeda S, Masaki T,
Shira-Ishi A, Sato K, Kubodera N, Ikeda K and Ogata E: ED-71, a
vitamin D analog, is a more potent inhibitor of bone resorption
than alfacalcidol in an estrogen-deficient rat model of
osteoporosis. Bone. 30:582–588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z,
Zhao L, Nagy TR, Peng X, Hu J, et al: TGF-beta1-induced migration
of bone mesenchymal stem cells couples bone resorption with
formation. Nat Med. 15:757–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weivoda MM, Ruan M, Pederson L, Hachfeld
C, Davey RA, Zajac JD, Westendorf JJ, Khosla S and Oursler MJ:
Osteoclast TGF-β receptor signaling induces Wnt1 secretion and
couples bone resorption to bone formation. J Bone Miner Res.
31:76–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu
T, Crane J, Frassica F, Zhang L, Rodriguez JP, et al: Matrix IGF-1
maintains bone mass by activation of mTOR in mesenchymal stem
cells. Nat Med. 18:1095–1101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Engelholm LH, Melander MC, Hald A, Persson
M, Madsen DH, Jürgensen HJ, Johansson K, Nielsen C, Nørregaard KS,
Ingvarsen SZ, et al: Targeting a novel bone degradation pathway in
primary bone cancer by inactivation of the collagen receptor
uPARAP/Endo180. J Pathol. 238:120–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boeyens JC, Deepak V, Chua WH, Kruger MC,
Joubert AM and Coetzee M: Effects of ω3- and ω6-polyunsaturated
fatty acids on RANKL-induced osteoclast differentiation of RAW264.7
cells: A comparative in vitro study. Nutrients. 6:2584–2601. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YS, Kim YS, Lee SY, Kim GH, Kim BJ,
Lee SH, Lee KU, Kim GS, Kim SW and Koh JM: AMP kinase acts as a
negative regulator of RANKL in the differentiation of osteoclasts.
Bone. 47:926–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi SW, Son YJ, Yun JM and Kim SH:
Fisetin inhibits osteoclast differentiation via downregulation of
p38 and c-Fos-NFATc1 signaling pathways. Evid Based Complement
Alternat Med. 2012:8105632012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zauli G, Rimondi E, Nicolin V, Melloni E,
Celeghini C and Secchiero P: TNF-related apoptosis-inducing ligand
(TRAIL) blocks osteoclastic differentiation induced by RANKL plus
M-CSF. Blood. 104:2044–2050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim MH, Shim KS, Lee SU, Kim YS, Min YK
and Kim SH: Stimulatory effect of undecylenic acid on mouse
osteoblast differentiation. Phytother Res. 24:559–564.
2010.PubMed/NCBI
|
|
26
|
Kubota K, Sakikawa C, Katsumata M,
Nakamura T and Wakabayashi K: Platelet-derived growth factor BB
secreted from osteoclasts acts as an osteoblastogenesis inhibitory
factor. J Bone Miner Res. 17:257–265. 2010. View Article : Google Scholar
|
|
27
|
Tanaka Y, Nakayamada S and Okada Y:
Osteoblasts and osteoclasts in bone remodeling and inflammation.
Curr Drug Targets Inflamm Allergy. 4:325–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishiya Y and Sugimoto S: Effects of
various antihypertensive drugs on the function of osteoblast. Biol
Pharm Bull. 24:628–633. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toray H, Hasegawa T, Sakagami N, Tsuchiya
E, Kudo A, Zhao S, Moritani Y, Abe M, Yoshida T, Yamamoto T, et al:
Histochemical assessment for osteoblastic activity coupled with
dysfunctional osteoclasts in c-src deficient mice. Biomed Res.
38:123–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Komori T: Regulation of bone development
and extracellular matrix protein genes by RUNX2. Cell Tissue Res.
339:189–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoemann CD, El-Gabalawy H and McKee MD: In
vitro osteogenesis assays: Influence of the primary cell source on
alkaline phosphatase activity and mineralization. Pathol Biol
(Paris). 57:318–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Y, You X, Xing W, Zhang Z and Zou W:
Paracrine and endocrine actions of bone-the functions of secretory
proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res.
6:162018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurihara N, Ishizuka S, Kiyoki M, Haketa
Y, Ikeda K and Kumegawa M: Effects of 1,25-dihydroxyvitamin D3 on
osteoblastic MC3T3-E1 cells. Endocrinology. 118:940–947. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van den Bemd GJ, Pols HA, Birkenhäger JC,
Kleinekoort WM and van Leeuwen JP: Differential effects of
1,25-dihydroxyvitamin D3-analogs on osteoblast-like cells and on in
vitro bone resorption. J Steroid Biochem Mol Biol. 55:337–346.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haneji T, Kurihara N, Ikeda K and Kumegawa
M: 1 alpha, 25-Dihydroxyvitamin D3 and analogues of vitamin D3
induce alkaline phosphatase activity in osteoblastic cells derived
from newborn mouse calvaria. J Biochem. 94:1127–1132. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Majeska RJ and Rodan GA: The effect of
1,25(OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma
cells. J Biol Chem. 257:3362–3365. 1982.PubMed/NCBI
|
|
37
|
Jones G: Pharmacokinetics of vitamin D
toxicity. Am J Clin Nutr. 88:582S–586S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Centrella M, McCarthy TL, Kusmik WF and
Canalis E: Relative binding and biochemical effects of
heterodimeric and homodimeric isoforms of platelet-derived growth
factor in osteoblast-enriched cultures from fetal rat bone. J Cell
Physiol. 147:420–426. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu X, Hsieh SC, Bao W and Graves DT:
Temporal expression of PDGF receptors and PDGF regulatory effects
on osteoblastic cells in mineralizing cultures. Am J Physiol.
272:C1709–C1716. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y
and Kim HH: Sphingosine 1-phosphate as a regulator of osteoclast
differentiation and osteoclast-osteoblast coupling. EMBO J.
25:5840–5851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen LL, Wang K, Zhang J and Wu YM: Effect
of the bone resorption supernatant from RAW264.7 osteoclast on the
osteogenic activity of mouse MC3T3-E1 cell. Zhonghua Kou Qiang Yi
Xue Za Zhi. 47:32–37. 2012.(In Chinese). PubMed/NCBI
|
|
42
|
Howard GA, Bottemiller BL, Turner RT,
Rader JI and Baylink DJ: Parathyroid hormone stimulates bone
formation and resorption in organ culture: Evidence for a coupling
mechanism. Proc Natl Acad Sci USA. 78:3204–3208. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martin TJ and Sims NA: Osteoclast-derived
activity in the coupling of bone formation to resorption. Trends
Mol Med. 11:76–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Karsdal MA, Martin TJ, Bollerslev J,
Christiansen C and Henriksen K: Are nonresorbing osteoclasts
sources of bone anabolic activity? J Bone Miner Res. 22:487–494.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sakagami N, Amizuka N, Li M, Takeuchi K,
Hoshino M, Nakamura M, Nozawa-Inoue K, Udagawa N and Maeda T:
Reduced osteoblastic population and defective mineralization in
osteopetrotic (op/op) mice. Micron. 36:688–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Soriano P, Montgomery C, Geske R and
Bradley A: Targeted disruption of the c-src proto-oncogene leads to
osteopetrosis in mice. Cell. 64:693–702. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gray C, Boyde A and Jones SJ:
Topographically induced bone formation in vitro: Implications for
bone implants and bone grafts. Bone. 18:115–123. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kalu DN, Doyle FH, Pennock J,
Denys-Matrajt H and Foster GV: Anabolic effect of parathyroid
hormone on bone in the rat. Calcif Tissue Res. (Suppl):S721970.
View Article : Google Scholar
|
|
50
|
Baldock PA, Thomas GP, Hodge JM, Baker SU,
Dressel U, O'Loughlin PD, Nicholson GC, Briffa KH, Eisman JA and
Gardiner EM: Vitamin D action and regulation of bone remodeling:
Suppression of osteoclastogenesis by the mature osteoblast. J Bone
Miner Res. 21:1618–1626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schwarz P, Brixen KT and Mosekilde L:
Calcium homeostasis and normal bone remodeling. Ugeskr Laeger.
167:871–873. 2005.(In Danish). PubMed/NCBI
|
|
52
|
Sanchez-Fernandez MA, Gallois A, Riedl T,
Jurdic P and Hoflack B: Osteoclasts control osteoblast chemotaxis
via PDGF-BB/PDGF receptor beta signaling. PLoS One. 3:e35372008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lotinun S, Kiviranta R, Matsubara T,
Alzate JA, Neff L, Lüth A, Koskivirta I, Kleuser B, Vacher J,
Vuorio E, et al: Osteoclast-specific cathepsin K deletion
stimulates S1P-dependent bone formation. J Clin Invest.
123:666–681. 2013.PubMed/NCBI
|
|
54
|
Noguchi Y, Kawate H, Nomura M and
Takayanagi R: Eldecalcitol for the treatment of osteoporosis. Clin
Interv Aging. 8:1313–1321. 2013.PubMed/NCBI
|
|
55
|
Suda T, Takahashi F and Takahashi N: Bone
effects of vitamin D-Discrepancies between in vivo and in vitro
studies. Arch Biochem Biophys. 523:22–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi YC, Worton L, Esteban L, Baldock P,
Fong C, Eisman JA and Gardiner EM: Effects of continuous activation
of vitamin D and Wnt response pathways on osteoblastic
proliferation and differentiation. Bone. 41:87–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen YC, Ninomiya T, Hosoya A, Hiraga T,
Miyazawa H and Nakamura H: 1α,25-Dihydroxyvitamin D3 inhibits
osteoblastic differentiation of mouse periodontal fibroblasts. Arch
Oral Biol. 57:453–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tanaka H and Seino Y: Direct action of
1,25-dihydroxyvitamin D on bone: VDRKO bone shows excessive bone
formation in normal mineral condition. J Steroid Biochem Mol Biol.
89:343–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamamoto Y, Yoshizawa T, Fukuda T,
Shirode-Fukuda Y, Yu T, Sekine K, Sato T, Kawano H, Aihara K,
Nakamichi Y, et al: Vitamin D receptor in osteoblasts is a negative
regulator of bone mass control. Endocrinology. 154:1008–1020. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nakamichi Y, Udagawa N, Horibe K,
Mizoguchi T, Yamamoto Y, Nakamura T, Hosoya A, Kato S, Suda T and
Takahashi N: VDR in osteoblast-lineage cells primarily mediates
vitamin D treatment-induced increase in bone mass by suppressing
bone resorption. J Bone Miner Res. 32:1297–1308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Amling M, Priemel M, Holzmann T, Chapin K,
Rueger JM, Baron R and Demay MB: Rescue of the skeletal phenotype
of vitamin D receptor-ablated mice in the setting of normal mineral
ion homeostasis: Formal histomorphometric and biomechanical
analyses. Endocrinology. 140:4982–4987. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lieben L and Carmeliet G: The delicate
balance between vitamin D, calcium and bone homeostasis: Lessons
learned from intestinal- and osteocyte-specific VDR null mice. J
Steroid Biochem Mol Biol. 136:102–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee SK, Kalinowski J, Jastrzebski S and
Lorenzo JA: 1,25(OH)2 vitamin D3-stimulated osteoclast formation in
spleen-osteoblast cocultures is mediated in part by enhanced IL-1
alpha and receptor activator of NF-kappa B ligand production in
osteoblasts. J Immunol. 169:2374–2380. 2002. View Article : Google Scholar : PubMed/NCBI
|