Introduction
Severe traumatic brain injury (TBI) is a leading
cause of death and disability, which puts a heavy strain on the
global economy (1). It is reported
that ~5.48 million cases of TBI occur annually worldwide, most of
which are associated with road traffic injuries (1). TBI is classified into mild [Glasgow
Coma Scale (GCS score), 13–15], moderate (GCS score, 9–12) and
severe (GCS score, ≤8) TBI based on disease severity (2,3). The
survival and therapeutic outcomes for severe TBI are extremely
poor. According to statistical data of the Chinese population, in
7,145 cases of acute head trauma, the mortality rate for severe TBI
stood at 21.8% (4). In addition,
several factors have been reported to correlate with the outcome of
severe TBI, including intracranial pressure (ICP) (4–6), brain
hypoxia (5), cerebral perfusion
pressure (5) and quantitative
cerebral blood flow within 12 h following injury (7).
The diagnosis, treatment and prognosis prediction of
TBI is performed using a number of non-invasive neuroimaging
methods, including conventional non-contrast computerized
tomography (CT), CT perfusion, positron emission tomography and
perfusion magnetic resonance imaging (8,9).
However, the accuracy and precision of conventional CT is not
satisfactory (10). In relation to
cerebral injuries and hemodynamic changes before and after
treatment, CT perfusion provides increased sensitivity and more
valuable information compared with conventional non-contrast CT
(11). Additionally, more specific
information regarding circulatory disturbances, including regional
cerebral blood flow (rCBF), regional cerebral blood volume (rCBV)
and mean transit time (MTT), can also be obtained using CT
perfusion (12). However, data
regarding cerebral hemodynamics following severe TBI have yet to be
characterized using perfusion CT.
Accumulating evidence indicates that early cranial
decompression is a potential strategy for improving survival and
preventing disability in patients afflicted with severe TBI
(13–16). Cranial decompression supports the
reduction of ICP and is generally associated with favorable
clinical outcomes in patients with TB; however, the precise role of
cranial decompression in the treatment of TBI remains unclear, as
it also has been reported that decompression may result in
unfavorable outcomes and disabilities (17). In addition, the effects of
decompression on the cerebral hemodynamic changes after TBI have
yet to be fully described.
In the present study, cerebral hemodynamics in a
rabbit model of severe TBI induction was examined using CT
perfusion. In particular, the therapeutic outcomes following
treatment with either common or controlled decompression according
to cerebral hemodynamic changes were also investigated using this
technique. The findings presented in this study may provide
valuable insights into the use of CT perfusion to understand and
interpret intervention outcomes for severe TBI.
Materials and methods
Reagents
Ketamine was obtained from Jiangsu Hengrui Medicine
Co., Ltd. Droperidol was purchased from Shanghai Xudong Haipu
Pharmaceutical Co., Ltd. Iopromide 370 was purchased from Bayer AG.
Evans blue dye was purchased from Sigma-Aldrich (Merck KGaA).
Animals and experimental
assignment
A total of 20 male and 20 female adult healthy New
Zealand white rabbits (weight, 2.5–3.0 kg; age, 7 months) were
obtained from the Laboratory Animal Center, Jiangsu Institute of
Parasitic Diseases [Wuxi, China; license no. for use of
experimental animals: SXYK (Su) 2015-0023]. Animals were housed in
a temperature- (~25°C) and humidity- (50–60%) controlled room
maintained on a 10:14-h light-dark cycle with free access to food
and water. Animals were randomly assigned into four groups (n=10
rabbits/group): i) Control, ii) TBI, iii) TBI + common
decompression or iv) TBI + controlled decompression. The present
animal study was approved by the Animal Ethics Committee of Wuxi
Clinical College, Anhui Medical University (Wuxi, China).
Establishment of TBI model in rabbits
and interventions
To generate the TBI model in rabbits, compression
was induced in animals by epidural balloon catheter inflation
according to a previously performed protocol (18). Briefly, animals were given 25 mg/kg
ketamine by intraperitoneal injection and 1.0 mg/kg droperidol by
intramuscular injection (19).
Following anesthesia, rabbits were placed on an operating table in
the prone position with their heads fixed. A midline incision was
made in the parietal bone to expose the skull, and an aperture 5 mm
in diameter was made between the left interparietal bone and the
midline of the parietal bone using a bone drill (Guangzhou Senxuan
Medical Instrument Co., Ltd.). The dura mater was then stripped off
from the inner skull plate, and a balloon catheter (Haiyan Kangyuan
Medical Instrument Co., Ltd.), which is in turn connected to a
TCI–II micro-infusion pump (Guangxi VERYARK Technology Co., Ltd.),
was placed into the epidural space. A needle was then inserted into
the aperture of the dura mater, and a Codman intracranial pressure
sensor (Johnson & Johnson) was placed into the brain tissue at
a depth of ~1 cm. For TBI, approximately 0.5–1 ml of normal saline
was infused into the balloon catheter through the micro-infusion
pump at a rate of 10 ml/h until the ICP value reached 25 mmHg; this
infusion was sustained for 15 min.
For the TBI + common/controlled decompression
groups, the infusion of normal saline was performed as described in
the TBI group. In the TBI + common decompression group, normal
saline was immediately pumped out from the balloon catheter, before
animals were monitored for another 15 min. In the TBI + controlled
decompression group, normal saline was slowly pumped out from the
balloon catheter, stopping for 5 min when the ICP value had
declined to 20 15 and 10 mmHg before the saline was pumped out
completely. In the control group, the animals were not infused with
normal saline.
At the end of all treatment procedures, all rabbits
were examined using conventional CT scanning and CT perfusion
scanning.
Conventional CT and CT perfusion
scanning
CT scanning was performed using a 320-row spiral CT
scanner (Aquilion One; Toshiba Corp.). For conventional CT
scanning, rabbits under anesthesia were placed in the prone
position with their heads fixed. The coronal CT images were
acquired at 5 mm slice thickness. The parameters for CT scanning
were as follows: Tube voltage, 80 kV; current, 100 mA; matrix,
512×512 and field of view, 10×10 cm. After conventional CT
scanning, CT perfusion scanning was performed at the basal ganglia.
The non-ionic contrast media Iopromide 370 (370 mgI/ml; 1.5 ml/kg)
was injected through a binocular high-pressure injector into the
ear vein at a rate of 1.0 ml/sec. Normal saline was injected at the
same rate. After 5 sec, dynamic CT images were acquired at 0.5-mm
slice thickness. The parameters for CT perfusion scanning were as
follows: Voltage, 80 kV; current, 120 mA; matrix, 512×512; and
field of view, 10×10 cm. A total of 2,720 raw images were acquired
within 35 sec.
Image processing
Following data acquisition, images were uploaded
onto a GE ADW4.6 Workstation (GE Healthcare) and processed using
Stroke mode in the CTP-4D software (version 11.3) of the
Workstation. The cerebral middle cerebral artery and the superior
sagittal sinus were automatically set as the input artery and
output vein, respectively. A parametric diagram reflecting blood
perfusion in cerebral tissues was constructed. The diagram was
evaluated by a senior neuroradiologist, who elected each region of
interest (ROI) ~2 mm2 in size at the bilateral temporal
lobes and basal ganglion and measured the rCBF, rCBV and MTT. Areas
were selected to avoid vessels and damaged brain tissues. The
values for each parameter within the ROIs were recorded, from which
the average values were calculated.
Determination of BBB permeability
Animals were injected with 2 g/l Evans blue dye (2
ml/kg) through the ear vein 1 h prior to sacrifice. Animals were
perfused with 200–300 ml normal saline to remove Evans blue dye
from the blood vessels. Brain tissues from the bilateral temporal
lobes were carefully removed from the animals following sacrifice.
A total of 3 ml formamide was added to 0.5 g of the brain tissue
homogenate to dissolve the Evans blue dye. After incubation in a
37°C water bath for 48 h, the samples were centrifuged at 755 × g
for 5 min. The supernatant was then collected before absorbance
measurement at 632 nm was performed using a UV spectrophotometer
(Hitachi, Ltd.). Evans blue content was calculated according to the
standard curve.
Statistical analysis
Data were analyzed using IBM SPSS statistics version
23 (IBM Corp.). All experimental results are presented as the mean
± standard deviation. Data were compared using one-way analysis of
variance. In cases of variance non-homogeneity [in the rCBF, rCBV
and the MTT data in the basal ganglion (P<0.05)], Welch's
t-test was applied. Comparisons between groups were made using the
Games-Howell post-hoc test. Variance homogeneity was detected in
the MTT data of bilateral temporal lobes (P=0.245) and Evans blue
content data (P=0.074) P<0.05 was considered to indicate a
statistically significant difference.
Results
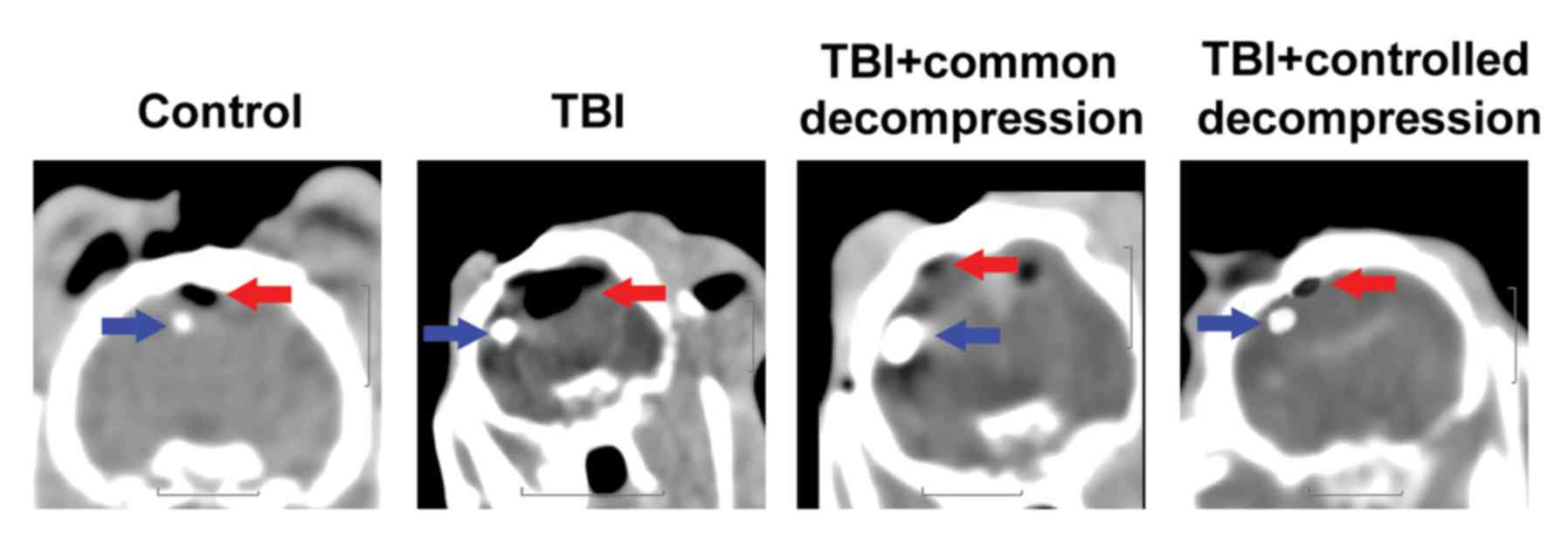
Conventional CT imaging
A clear cerebral sulcus and cerebral gyrus could be
observed in the control group (Fig.
1, Control); the CT value in the frontal lobe of the white
matter was calculated to be 29–30 HU. Following compression, the
cerebral sulcus was absent from the CT images, and the cerebral
gyrus was unclear (Fig. 1, TBI); the
CT value was observed to be 25–27 HU in the TBI group. After
decompression, the cerebral sulci of the bilateral hemispheres were
absent from the CT scanning images; the CT values were reduced
further to 18–20 HU and 20–22 HU in the TBI + common decompression
and TBI + controlled decompression groups, respectively (Fig. 1, TBI + common decompression and TBI +
controlled decompression).
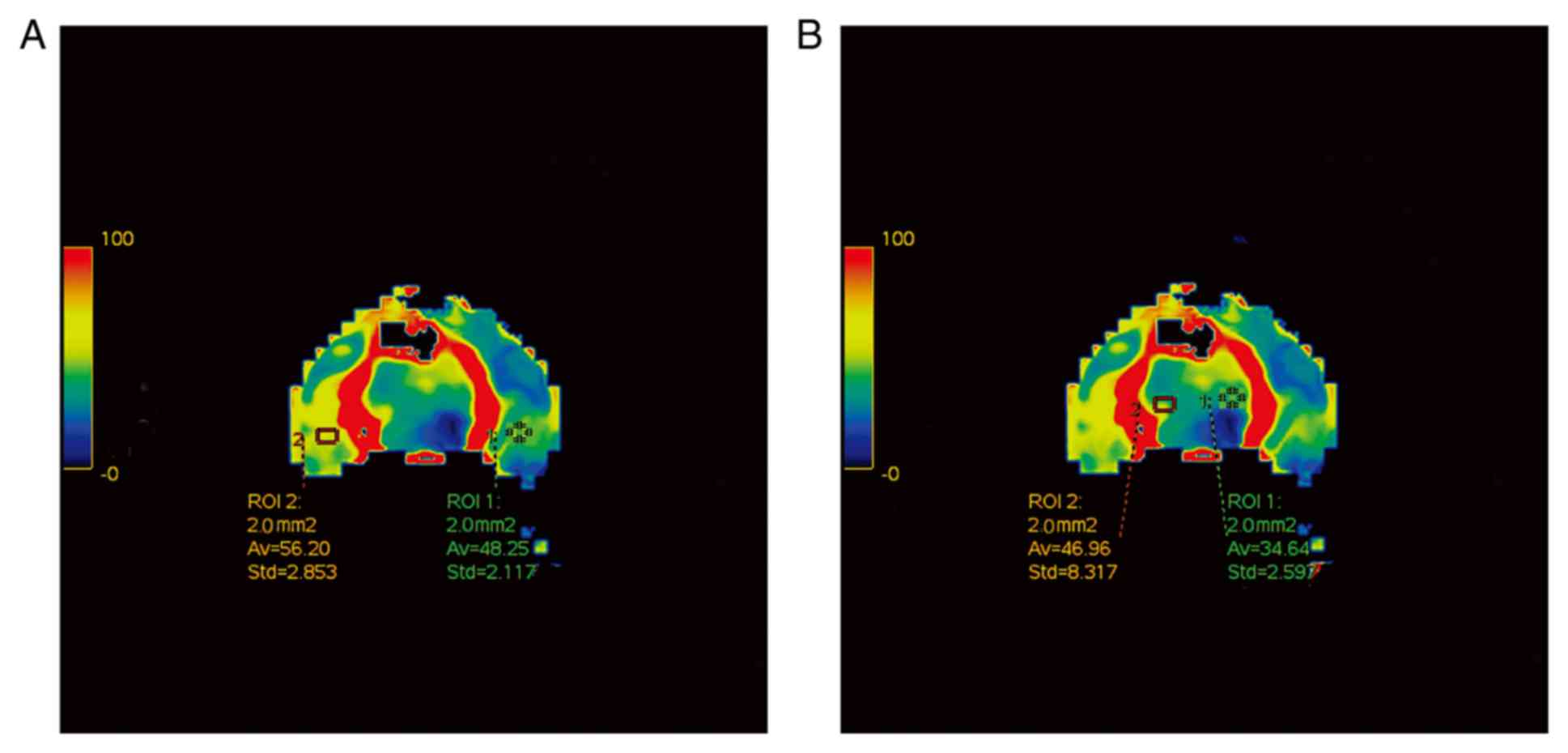
CT perfusion imaging
The regions of interest selected for the measurement
of rCBF in the temporal lobe and the basal ganglia in the control
group is shown in Fig. 2. Compared
with those in the control group, the local cerebral rCBF and rCBV
values were demonstratively decreased in the TBI, TBI + common
decompression and TBI + controlled decompression groups (Fig. 3), suggesting reduced blood flow to
the rabbit brain tissues. In addition, prolonged MTTs were observed
in the TBI, TBI + common decompression, and TBI + controlled
decompression groups, with the most notable MTT extension detected
in the common decompression group (Fig.
3). The time-CT value curves for the bilateral temporal lobes
and the basal ganglion were also presented in Fig. 3.
Comparison of CT perfusion parameters
between the four groups
The CT perfusion parameters between the four
experimental groups were compared in Table I. The rCBF and rCBV values of the
bilateral temporal lobes and basal ganglion in the TBI, TBI +
common decompression and TBI + controlled decompression groups were
significantly lower compared with the control group (P<0.01).
Comparison between the TBI, TBI + common decompression and TBI +
controlled decompression groups also revealed significant
differences in the rCBF and rCBV values of the bilateral temporal
lobes and basal ganglion (P<0.01). Notably, controlled
decompression slightly alleviated TBI-induced reductions of rCBF
and rCBV values in the bilateral temporal lobes and basal ganglion
compared with TBI group (P<0.01). Common decompression only
rescued the TBI-induced decrease in rCBV in the basal ganglion
compared with TBI group (P<0.01) and worsened the other
parameter index values (rCBF of bilateral temporal lobes and basal
ganglion; rCBV of bilateral temporal lobes). Compared with the
control group, the MTT values of the bilateral temporal lobes and
basal ganglion in the TBI, TBI + common decompression, and TBI +
controlled decompression groups were significantly increased
(P<0.01). Significant differences were also observed between the
TBI and TBI + intervention groups (P<0.01). In particular, the
TBI-induced extension of MTT in the bilateral temporal lobes
appeared to be partially reversed by controlled decompression
(P<0.01), but common decompression further prolonged the MTT
values in the bilateral temporal lobes and basal ganglion compared
with the TBI group (P<0.01).
 | Table I.Computed tomography perfusion
parameters in bilateral temporal lobes and basal ganglion and Evans
blue content in brain tissues. |
Table I.
Computed tomography perfusion
parameters in bilateral temporal lobes and basal ganglion and Evans
blue content in brain tissues.
|
| Bilateral temporal
lobes | Basal ganglion |
|
|---|
|
|
|
|
|
|---|
| Group (n=10
rabbits/group) | rCBF (ml 100
g−1/min) | rCBV (ml/100 g) | MTT (sec) | rCBF (ml 100
g−1/min) | rCBV (ml/100 g) | MTT (sec) | Evans blue content
(µg/ml) |
|---|
| Control | 58.75±4.86 | 2.79±0.30 | 2.56±0.34 | 46.36±3.29 | 1.52±0.23 | 2.15±0.08 | 0.43±0.05 |
| TBI |
5.23±1.71a |
0.51±0.19a |
6.36±0.42a |
3.42±0.28a |
0.09±0.03a |
2.98±0.23a |
1.52±0.11a |
| TBI + common
decompression |
2.41±0.96a,b |
0.04±0.02a,b |
8.09±0.62a,b |
2.57±0.21a,b |
0.28±0.03a,b |
5.92±0.32a,b |
2.23±0.15a,b |
| TBI + controlled
decompression |
7.80±1.19a–c |
0.56±0.15a,c |
4.14±0.35a–c |
4.61±0.30a–c |
0.43±0.05a–c |
5.31±0.45a–c |
1.88±0.10a–c |
| F-value | 18.91 | 15.30 | 289.41 | 19.01 | 19.07 | 16.946 | 478.602 |
Comparison of BBB permeability between
the four groups
The Evans blue dye content in the control group was
significantly lower compared with the other experimental groups
(Table I), representing intact BBB
in the control rabbit brain. Significantly increased BBB
permeability was detected in the TBI, TBI + common decompression
and TBI + controlled decompression groups compared with control
(P<0.01; Table I). However,
neither common decompression nor controlled decompression could
reduce the increase in BBB permeability induced by TBI.
Discussion
The use of CT perfusion for the diagnosis and
evaluation of treatment outcomes has not been widely applied. In
the present study, a TBI model was developed in rabbits through
epidural balloon catheter inflation. The efficacy of common
decompression or controlled decompression for alleviating changes
in cerebral hemodynamics induced by TBI in model animals was
determined using CT perfusion. Findings in the present study
demonstrated that controlled decompression contributed to improved
cerebral hemodynamics compared with common decompression for TBI
treatment in rabbits.
High ICP levels increase the risk of death, as a
previous study reported that the mortality rate for patients with
acute TBI and an ICP 20–40 mmHg is higher than that for patients
with an ICP <20 mmHg (21.4 vs. 6.3%) (4). In Sprague-Dawley rats, an ICP of 30
mmHg is known as the lower threshold for cerebrovascular
autoregulation (20). In a rabbit
model of acute intracranial hypertension, impaired cerebral
microcirculation occurs with an ICP ≥28.5 mmHg (21). In addition, Donnelly et al
(22) reported that cortical
perfusion and vascular reactivity are significantly decreased with
a 10 mmHg increase in ICP. The baseline ICP value for healthy
rabbits lie in the 5–9 mmHg range. As a consequence of these
findings, the ICP value of 25 mmHg was elected for the present
study. Indeed, ICP did reach 25 mmHg in the TBI group, which led to
increased BBB permeability in addition to abnormalities in the
cerebral hemodynamic parameters, indicating successful TBI
establishment in the rabbits.
Compared with conventional non-contrast CT, CT
perfusion directly reflects the dynamic changes in circulation,
providing values for important hemodynamic parameters including
rCBF, rCBV and MTT in the process (23–25).
Following TBI induction, the decline in rCBF may trigger
cerebrovascular autoregulation, which increases rCBV and maintains
blood flow by cerebral vasodilation (26). However, in the present study, a
significant decline in CBV was detected in early TBI, implying
dysfunction of cerebrovascular autoregulation in rabbits following
TBI.
Decompressive craniectomy is commonly applied for
the management of acute TBI in the clinic (27,28).
However, the efficacy of cranial decompression in improving outcome
and reducing disability after TBI therapy is controversial.
Although most of the existing evidence indicates that cranial
decompression is generally favorable in terms of TBI patient
survival (13–16). one previous study demonstrated that
such treatment may lead to an unfavorable outcome due to
complications caused by intraoperative brain extrusion (17). Controlled decompression differs from
common decompression in terms of the procedure and strategy applied
for the intraoperative release of ICP. It has been reported that
controlled decompression carries advantages in reducing the
incidence of ischemic reperfusion injuries and acute postoperative
cerebral infarction (29). In the
present study, the changes in cerebral hemodynamics between common
decompression (rapid release of ICP) and controlled decompression
(step-by-step gradual release of ICP) in TBI animals were compared.
It was found that neither common nor controlled decompression could
reverse the reduced blood flow in cerebral tissues or the increase
in BBB permeability following TBI induction. This phenomenon might
be attributed to dysfunction in the automatic cerebrovascular
adjustment system. Under normal conditions, decreases in CBF
triggers the vasodilatation of cerebral vessels to increase CBV to
maintain adequate blood flow in brain tissues (30). In the present study, a decline in CBV
after TBI was observed, which suggested a dysfunctional
cerebrovascular adjustment system in early TBI. By contrast,
damages to the BBB may also result in defective cerebrovascular
adjusting and neurovascular coupling (31). Compared with common decompression,
the use of controlled decompression was more effective for
reversing low perfusion and extending MTT, but not to baseline
levels. As the MTT value is affected by CBF, both common and
controlled decompression induced reductions in CBF, which may lead
to the extension of MTT. It is possible that controlled
decompression may prevent circulatory abnormalities by improving
cerebrovascular autoregulation in rabbits following TBI injury. In
addition, data from the present study revealed that the rCBF value
was further reduced in the bilateral temporal lobes after common
decompression in the TBI-model animals. However, the change in rCBV
in the bilateral temporal lobe and the basal ganglion varied
between the TBI and TBI + common decompression groups. The basal
ganglion is localized deep in the brain and has an abundant blood
supply. In this study, intracranial hypertension was induced in
rabbits by epidural compression, which exhibited more notable
effects on the hemodynamic changes in the frontal and temporal
lobes, while the influence on basal ganglion hemodynamics might lag
behind or be relatively small. Although the underlying mechanisms
remain unclear, this phenomenon may explain why decompressive
craniectomy reduced ICP but did not improve the poor overall
prognosis in patients with severe TBI (27,28). A
previous preliminary study compared the therapeutic outcomes of
common decompression and controlled decompression in 128 patients
afflicted with severe head injury (29). According to those findings,
controlled decompression is more potent than common decompression
for preventing the incidence of intraoperative acute brain
swelling. Nevertheless, the application of controlled decompression
in animal studies and patients is a relatively novel concept, and
the efficacy of this therapeutic strategy requires further
exploration
This study does carry some limitations. Firstly, a
local TBI injury model was used here, and therefore the possibility
that the cerebral hemodynamics may vary in other TBI models could
not be ruled out. Secondly, the indices of cerebral hemodynamics
were measured 30 min after injury, and the changes in these indices
had not yet been evaluated for a more prolonged time period.
Thirdly, in this study, TBI was induced by increasing the ICP to
over 25 mmHg, and the changes in cerebral hemodynamics under
different ICP values have not been determined. Lastly, future
studies are still required for exploring the mechanism by which
controlled decompression alleviates the circulatory abnormalities
induced by TBI injury.
In conclusion, the present study demonstrated that
CT perfusion could be used to analyze local changes in cerebral
hemodynamics after TBI induction in rabbits. TBI induced
significant reductions in rCBF and rCBV, prolonged MTT and greatly
increased BBB permeability in rabbits. Controlled decompression was
more effective compared with common decompression at preventing
these abnormalities in cerebral hemodynamics following TBI injury
induction. These findings provide valuable insights for
understanding the use of CT perfusion to evaluate therapeutic
outcomes and cerebral hemodynamics in patients with TBI.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science
Foundation of Wuxi Municipal Commission of Health and Family
Planning in China (grant no. MS201527 to KFC).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KFC and YHW designed the experiments; KFC and FHD
performed the experiments; GXL and JRD analyzed the data; KFC wrote
the paper.
Ethics approval and consent to
participate
The animal study was approved by the Animal Ethics
Committee of Wuxi Clinical College, Anhui Medical University (Wuxi,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iaccarino C, Carretta A, Nicolosi F and
Morselli C: Epidemiology of severe traumatic brain injury. J
Neurosurg Sci. 62:535–541. 2018.PubMed/NCBI
|
|
2
|
Blennow K, Brody DL, Kochanek PM, Levin H,
McKee A, Ribbers GM, Yaffe K and Zetterberg H: Traumatic brain
injuries. Nat Rev Dis Primers. 2:160842016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teasdale G, Murray G, Parker L and Jennett
B: Adding up the glasgow coma score. Acta Neurochir Suppl (Wien).
28:13–16. 1979.PubMed/NCBI
|
|
4
|
Jiang JY; Chinese Head Trauma Study
Collaborators: Head trauma in China, : Injury. 44:1453–1457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oddo M, Levine JM, Mackenzie L, Frangos S,
Feihl F, Kasner SE, Katsnelson M, Pukenas B, Macmurtrie E,
Maloney-Wilensky E, et al: Brain hypoxia is associated with
short-term outcome after severe traumatic brain injury
independently of intracranial hypertension and low cerebral
perfusion pressure. Neurosurgery. 69:1037–1045. 2011.PubMed/NCBI
|
|
6
|
Farahvar A, Gerber LM, Chiu YL, Härtl R,
Froelich M, Carney N and Ghajar J: Response to intracranial
hypertension treatment as a predictor of death in patients with
severe traumatic brain injury. J Neurosurg. 114:1471–1478. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaloostian P, Robertson C, Gopinath SP,
Stippler M, King CC, Qualls C, Yonas H and Nemoto EM: Outcome
prediction within twelve hours after severe traumatic brain injury
by quantitative cerebral blood flow. J Neurotrauma. 29:727–734.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rostami E, Engquist H and Enblad P:
Imaging of cerebral blood flow in patients with severe traumatic
brain injury in the neurointensive care. Front Neurol. 5:1142014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim D, Lee SH, Kim DH, Choi DS, Hong HP,
Kang C, Jeong JH, Kim SC and Kang TS: The possibility of
application of spiral brain computed tomography to traumatic brain
injury. Am J Emerg Med. 32:1051–1054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Servadei F, Nasi MT, Giuliani G, Cremonini
AM, Cenni P, Zappi D and Taylor GS: CT prognostic factors in acute
subdural haematomas: The value of the ‘worst’ CT scan. Br J
Neurosurg. 14:110–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Douglas DB, Chaudhari R, Zhao JM, Gullo J,
Kirkland J, Douglas PK, Wolin E, Walroth J and Wintermark M:
Perfusion imaging in acute traumatic brain injury. Neuroimaging
Clin N Am. 28:55–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bendinelli C, Bivard A, Nebauer S, Parsons
MW and Balogh ZJ: Brain CT perfusion provides additional useful
information in severe traumatic brain injury. Injury. 44:1208–1212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brazinova A, Mauritz W, Leitgeb J,
Wilbacher I, Majdan M, Janciak I and Rusnak M: Outcomes of patients
with severe traumatic brain injury who have Glasgow Coma Scale
scores of 3 or 4 and are over 65 years old. J Neurotrauma.
27:1549–1555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bell RS, Mossop CM, Dirks MS, Stephens FL,
Mulligan L, Ecker R, Neal CJ, Kumar A, Tigno T and Armonda RA:
Early decompressive craniectomy for severe penetrating and closed
head injury during wartime. Neurosurg Focus. 28:E12010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gouello G, Hamel O, Asehnoune K, Bord E,
Robert R and Buffenoir K: Study of the long-term results of
decompressive craniectomy after severe traumatic brain injury based
on a series of 60 consecutive cases. ScientificWorldJournal.
2014:2075852014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Charry JD, Rubiano AM, Nikas CV, Ortíz JC,
Puyana JC, Carney N and Adelson PD: Results of early cranial
decompression as an initial approach for damage control therapy in
severe traumatic brain injury in a hospital with limited resources.
J Neurosci Rural Pract. 7:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quintard H, Lebourdon X, Staccini P and
Ichai C: Decompression surgery for severe traumatic brain injury
(TBI): A long-term, single-centre experience. Anaesth Crit Care
Pain Med. 34:79–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen WL, Yang LK and Kuang H:
Establishment of posttraumatic acute diffuse brain swelling with
sinus balloon compression method in rabbits. Chin J Trauma.
8:753–757. 2015.
|
|
19
|
Gungormus M and Kaya O: Evaluation of the
effect of heterologous type I collagen on healing of bone defects.
J Oral Maxillofac Surg. 60:541–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bragin DE, Statom GL, Yonas H, Dai X and
Nemoto EM: Critical cerebral perfusion pressure at high
intracranial pressure measured by induced cerebrovascular and
intracranial pressure reactivity. Crit Care Med. 42:2582–2590.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan LZ, Sheng LY and Bing C: Changes of
the transcranial Doppler spectrum wave form in the model of acute
intracranial hypertension in rabbits. Bulletin Hunan Medical Univ.
27:441–444. 2002.
|
|
22
|
Donnelly J, Czosnyka M, Harland S, Varsos
GV, Cardim D, Robba C, Liu X, Ainslie PN and Smielewski P: Cerebral
haemodynamics during experimental intracranial hypertension. J
Cereb Blood Flow Metab. 37:694–705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Honda M, Ichibayashi R, Yokomuro H,
Yoshihara K, Masuda H, Haga D, Seiki Y, Kudoh C and Kishi T: Early
cerebral circulation disturbance in patients suffering from severe
traumatic brain injury (TBI): A Xenon CT and perfusion CT study.
Neurol Med Chir (Tokyo). 56:501–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan J, Zhang J, Huang W, Cheng X, Ling Y,
Dong Q and Geng D: Value of perfusion computed tomography in acute
ischemic stroke: Diagnosis of infarct core and penumbra. J Comput
Assist Tomogr. 37:645–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thierfelder KM, Sommer WH, Baumann AB,
Klotz E, Meinel FG, Strobl FF, Nikolaou K, Reiser MF and von
Baumgarten L: Whole-brain CT perfusion: Reliability and
reproducibility of volumetric perfusion deficit assessment in
patients with acute ischemic stroke. Neuroradiology. 55:827–835.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powers WJ, Grubb RL Jr and Raichle ME:
Physiological responses to focal cerebral ischemia in humans. Ann
Neurol. 16:546–552. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barthelemy EJ, Melis M, Gordon E, Ullman
JS and Germano IM: Decompressive craniectomy for severe traumatic
brain injury: A systematic review. World Neurosurg. 88:411–420.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cooper DJ, Rosenfeld JV, Murray L, Arabi
YM, Davies AR, D'Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly
P, et al: Decompressive craniectomy in diffuse traumatic brain
injury. N Engl J Med. 364:1493–1502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Wang C, Yang L, Cai S, Cai X, Dong
J, Zhang J and Zhu J: Controlled decompression for the treatment of
severe head injury: A preliminary study. Turk Neurosurg.
24:214–220. 2014.PubMed/NCBI
|
|
30
|
Toth P, Szarka N, Farkas E, Ezer E,
Czeiter E, Amrein K, Ungvari Z, Hartings JA, Buki A and Koller A:
Traumatic brain injury-induced autoregulatory dysfunction and
spreading depression-related neurovascular uncoupling:
Pathomechanisms, perspectives, and therapeutic implications. Am J
Physiol Heart Circ Physiol. 311:H1118–H1131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moretti R, Pansiot J, Bettati D,
Strazielle N, Ghersi-Egea JF, Damante G, Fleiss B, Titomanlio L and
Gressens P: Blood-brain barrier dysfunction in disorders of the
developing brain. Frontiers Neuroscience. 9:402015. View Article : Google Scholar
|