Introduction
Cervical cancer is one of the most frequently
diagnosed malignancies among females, and results in >10,000 new
cases and >4,000 mortalities in the United States every year
(1). Cervical cancer is considered
the leading cause of cancer-associated deaths in certain African
and Asian countries (2). In spite of
the efforts made to improve the treatment of cervical cancer, the
5-year survival rate in China remains <50% (3). Cervical squamous cell carcinoma (CSCC)
is the most common type of cervical cancer and accounts for >90%
of the cases (4). Human
papillomavirus (HPV) infection is the major cause of CSCC (5). Incidence of HPV-positive CSCC has been
significantly reduced with the HPV infection screening program
(6). However, HPV-negative CSCC is
more aggressive than HPV-positive CSCC and has an increasing
incidence rate (7).
Previous studies revealed that long non-coding
(lnc)RNAs serve important roles in cancer biology (8,9). lncRNAs
have potential diagnostic and therapeutic efficacy in certain types
of malignant tumors, including lung and liver cancer (10). lncRNA HAND2 antisense RNA 1
(HAND2-AS1) exhibited tumor suppressor effects in several types of
cancer including osteosarcoma (11)
and endometrioid endometrial carcinoma (12). A previous study revealed that the
HAND2-AS1 expression level in HPV-positive patients with CSCC was
negatively correlated with that of Rho-associated protein kinase 1
(ROCK1), which serves an oncogenic role in cancer biology (13). The present study demonstrated that
lncRNA HAND2-AS1 may inhibit cancer cell proliferation, migration
and invasion by downregulating ROCK1 in HPV-positive and -negative
CSCC.
Materials and methods
Patients and serum specimens
A total of 122 patients with CSCC were admitted to
Heping Hospital Affiliated to Changzhi Medical College (Changzhi,
China) between May 2015 and January 2018. The present study
included 22 HPV-16 positive cases, 18 HPV-18-positive cases and 20
HPV-negative cases. Patients were diagnosed based on HPV infection
detection results obtained using sensitive polymerase chain
reaction (PCR) techniques (14). The
inclusion criteria were as follow: i) CSCC diagnosis by
pathological examinations; ii) normal function in other organs;
iii) stage I and II CSCC; and iv) patients willing to participate
in the current study and who signed informed consent forms. The
exclusion criteria were as follows: i) Severe co-morbidities; ii)
treatment prior to admission; iii) multiple HPV strain infections;
and iv) patients who could not fully understand the experimental
protocol. The age of the 22 HPV-16-positive patients ranged between
23 and 67 years, with a mean age of 46.1±7.1 years. The age of the
18 HPV-18 positive patients ranged between 24 and 68 years, with a
mean age of 46.3±6.6 years. The age of the 20 HPV negative patients
ranged between 24 and 65 years, with a mean age of 45.9±5.8 years.
The present study included 24 healthy volunteers who received
examinations during the same time to serve as the control group.
The age of healthy volunteers ranged between 22 and 68 years, with
a mean age of 45.4±6.9 years. There were no significant differences
in age among the groups. The Ethics Committee of Peace Hospital of
Changzhi Medical College approved the current study. Blood was
extracted from the elbow vein of each participant prior to
breakfast the day following admission to prepare serum through
centrifugation at 1,400 × g for 20 min at room temperature.
Serum was stored in liquid nitrogen until required.
Reverse transcription (RT)-qPCR
Total RNA was extracted from serum and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the SuperScript III Reverse
Transcriptase kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. qPCR was subsequently performed using the
SYBR® Green PCR Master mix (Thermo Fisher Scientific,
Inc.) according to manufacturer's instructions. The following
primers pairs were used for the qPCR: lncRNA HAND2-AS1 forward,
5′-GGGTGTTTACGTAGACCAGAACC-3′ and reverse,
5′-CTTCCAAAAGCCTTCTGCCTTAG-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The following thermocycling conditions were used: Initial
denaturation at 95°C for 1 min, followed by 40 cycles of 95°C for
15 sec and 56°C for 35 sec. mRNA levels were quantified using the
2−ΔΔCq method and normalized to the internal reference
gene β-actin (15).
ELISA
Serum levels of ROCK1 were measured using an ELISA
kit purchased from Abcam (cat. no. ab211175) according to
manufacturer's instructions. The sensitivity of this kit was 0.1
pg/ml and the assay range was 2.5–80 ng/ml.
Cell culture and transfection
C-33 A (HPV negative) and SiHa (HPV positive) human
CSCC cell lines, and HCvEpC (HPV negative) and Ect1/E6E7 (HPV
positive) human cervical epithelial cell lines were purchased from
the American Type Culture Collection (ATCC). The cells were
cultured in Eagle's Minimum Essential Medium (EMEM; ATCC)
containing 10% FBS (ATCC). Cell culture conditions were 37°C and 5%
CO2. lncRNA HAND2-AS1 and ROCK1 pIRSE2 expression
vectors as well as empty pIRSE2 vectors were purchased from
GeneCopoeia, Inc. Lipofectamine® 2000 reagent (cat no.
11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect 10 nM of the vectors into the cancer cell lines (10 nM
for each vector in case of double transfections). Cancer cells
transfected with empty vectors served as the negative control (NC)
group. Cells without transfection were used as control (C) cells.
An overexpression rate >180% (range, 180–225%) was demonstrated
by RT-qPCR at 24 h post-transections prior to subsequent
experiments.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay
(Sigma-Aldrich; Merck KGaA) was performed to measure cell
proliferation following transfection. Briefly, cells were harvested
and resuspended to a cell density of 4×104 cells/ml.
Each well of a 96-well plate was filled with 0.1 ml cell suspension
and incubated at 37°C in a 5% CO2 incubator. A total of
10 µl CCK-8 cell solution was added into each well following 24,
48, 72 and 96 h of incubation. Cells were cultured for an
additional 4 h, followed by measurement of optical density values
at a wavelength of 450 nm using a microplate reader.
Transwell migration and invasion
assay
Transwell migration and invasion assays were
performed to investigate cell migration and invasion following
transfection. Briefly, cells were harvested and cell suspensions
(4×104 cells/ml) were prepared using serum-free EMEM. A
total of 4×103 cells in 0.1 ml cell suspension were
seeded in the upper chamber of the insert, while the lower chamber
was filled with RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
containing 20% fetal calf serum (Sigma-Aldrich; Merck KGaA). The
upper chamber was coated with Matrigel® (cat no. 356234;
EMD Millipore) prior to the invasion assay at 37°C for 6 h. Cells
were cultured at 37°C in a 5% CO2 incubator for 12 h.
Migrated and invaded cells were stained with 0.5% crystal violet
(Sigma-Aldrich, Merck KGaA) for 20 min at room temperature. Cells
were observed and counted under a light microscope. Five visual
fields were selected for each sample to count cell number
(magnification, ×50).
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) and quantified using a bicinchoninic acid assay. Following
denaturation in boiling (100°C) water for 5 min, 20 µg protein/lane
was separated via SDS-PAGE on a 10% gel. The separated proteins
were subsequently transferred onto a polyvinylidene difluoride
membrane. Following incubation in 5% skimmed milk for 2 h at room
temperature, the membranes were incubated with antibodies against
ROCK1 (1:1,200; cat no. ab97592; Abcam) and GAPDH (1:1,200; cat no.
ab37168; Abcam) overnight at 4°C. The membranes were subsequently
incubated with a horseradish peroxidase-labeled secondary
antibodies (1:1,000; cat no. MBS435036; MyBioSource, Inc.) at room
temperature for 2 h. Signal development was performed using BM
Chemiluminescence ELISA substrate (Sigma-Aldrich, Merck KGaA). Data
normalization was performed using ImageJ software (version 1.6;
National Institutes of Health).
Statistical analysis
SPSS software (version 19; IBM Corp) was used for
statistical analysis. Data were expressed as mean ± standard
deviation. Comparisons among multiple groups were performed using
the one way analysis of variance followed by the Tukey test.
Correlations between the serum levels of lncRNA HAND2-AS1 and ROCK1
were analyzed by the Pearson's correlation test. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic value of serum lncRNA HAND2-AS1 for CSCC. P<0.05 was
considered to indicate a statistical significant difference.
Results
HAND2-AS1 is downregulated and ROCK1
is upregulated in HPV-positive and negative patients with CSCC
compared with healthy controls
Serum levels of HAND2-AS1 and ROCK1 in all subjects
were measured using RT-qPCR and ELISA, respectively. Compared with
the control group, HAND2-AS1 was downregulated (P<0.05; Fig. 1A), while ROCK1 (P<0.05; Fig. 1B) was upregulated in all three groups
of patients with CSCC. However, no significant differences in the
serum levels of HAND2-AS1 and ROCK1 were found among the three
groups of patients with CSCC.
Serum levels of HAND2-AS1 and ROCK1
were negatively correlated in HPV-positive and -negative CSCC
patients but not in healthy controls
Pearson correlation coefficient analysis revealed a
reverse correlation between serum levels of HAND2-AS1 and ROCK1 in
HPV-16-positive CSCC patients (Fig.
2A), HPV-18-positive CSCC patients (Fig. 2B) and HPV-negative CSCC patients
(Fig. 2C) but not in healthy
controls (Fig. 2D).
Downregulation of lncRNA HAND2-AS1
distinguished CSCC patients from healthy controls
ROC curve analysis was performed to evaluate the
diagnostic value of serum lncRNA HAND2-AS1 for different types of
CSCC. For HPV-16-positive CSCC, the area under the curve (AUC) was
0.8930, with standard error of 0.05144 and 95% confidence interval
of 0.7922–0.9938 (P<0.001; Fig.
3A). For HPV-18-positive CSCC, the AUC was 0.8866, with
standard error of 0.05295 and 95% confidence interval of
0.7828–0.9904 (P<0.001; Fig. 3B).
For HPV-negative CSCC, the AUC was 0.8781, with standard error of
0.05311 and 95% confidence interval of 0.7740–0.9822 (P<0.001;
Fig. 3C). For all types of CSCC, the
AUC was 0.8861, with standard error of 0.04901 and 95% confidence
interval of 0.79000–0.9822 (P<0.001; Fig. 3D).
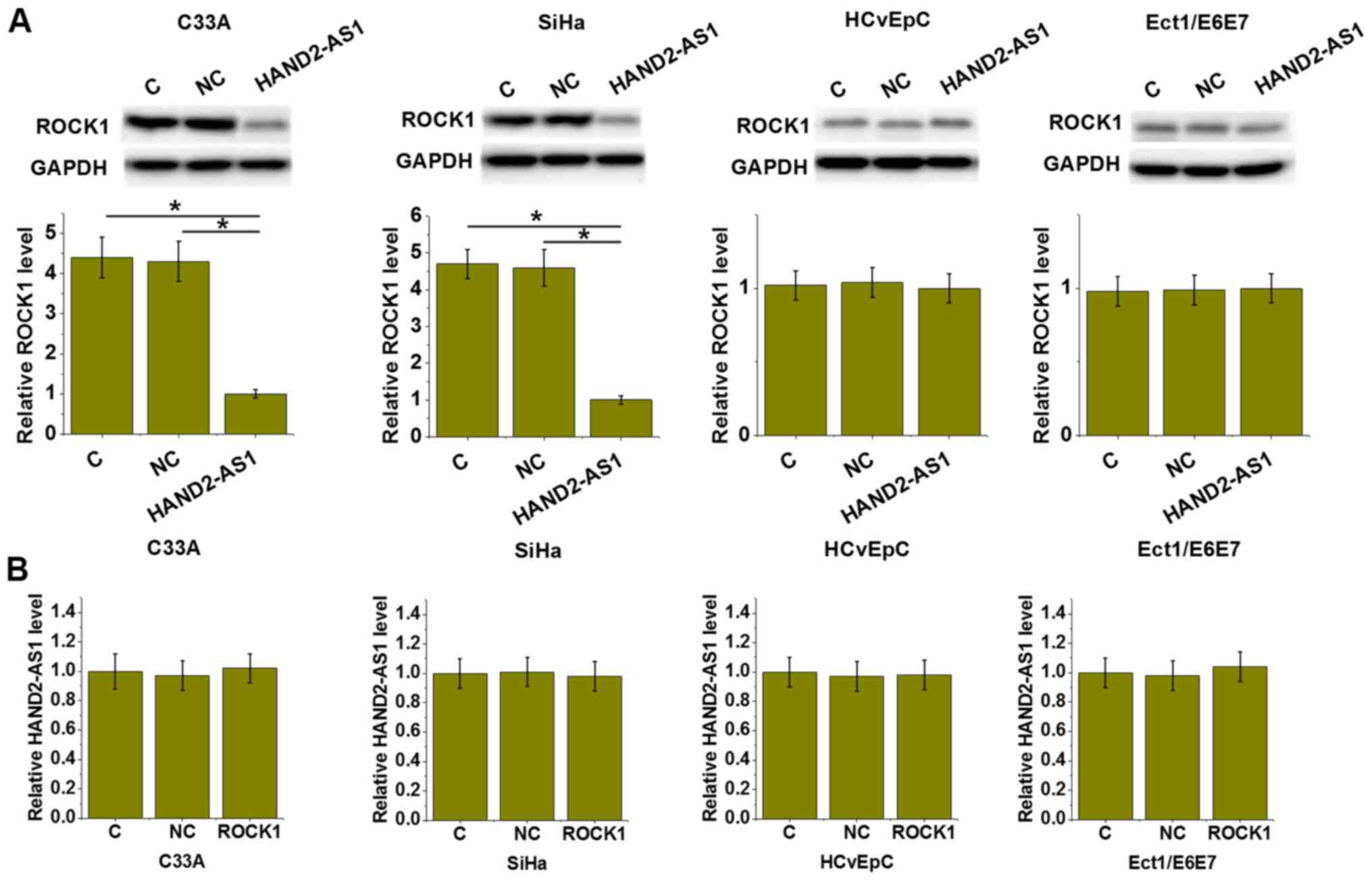
lncRNA HAND2-AS1 downregulated ROCK1
in both HPV-positive and -negative CSCC cells but not in normal
cells
To further investigate the interactions between
lncRNA HAND2-AS1 and ROCK1, lncRNA HAND2-AS1 and ROCK1 expression
vectors were transfected into cells. The expression of lncRNA
HAND2-AS1 and ROCK1 were detected by RT-qPCR and western blot,
respectively. Compared with cells in the C and NC groups, lncRNA
HAND2-AS1 overexpression led to the downregulation of ROCK1
expression in C-33 A (HPV negative) and SiHa (HPV positive) CSCC
cell lines (P<0.05), but not in HCvEpC (HPV negative) and
Ect1/E6E7 (HPV positive) human cervical epithelial cell lines
(Fig. 4A). By contrast, ROCK1
overexpression resulted in no significant effects on the expression
of lncRNA HAND2-AS1 in all four cell lines (Fig. 4B).
lncRNA HAND2-AS1 and ROCK1 serve
opposing roles in the proliferation, migration and invasion of CSCC
cells
Compared with cells in the C group, lncRNA HAND2-AS1
overexpression significantly inhibited, while ROCK1 overexpression
significantly promoted the proliferation (Fig. 5A), migration (Fig. 5B) and invasion (Fig. 5C) of C-33 A (HPV negative) and SiHa
(HPV positive) human CSCC cell lines (P<0.05), but not of HCvEpC
(HPV negative) and Ect1/E6E7 (HPV positive) human cervical
epithelial cell lines (data not shown). In addition, ROCK1
overexpression significantly attenuated the inhibitory effects of
lncRNA HAND2-AS1 overexpression on the proliferation (Fig. 5A), migration (Fig. 5B) and invasion (Fig. 5C) of cancer cells (P<0.05).
 | Figure 5.lncRNA HAND2-AS1 and ROCK1 serve
opposite roles in proliferation, migration and invasion of CSCC
cells. HAND2-AS1 overexpression significantly inhibited, while
ROCK1 overexpression significantly promoted the (A) proliferation
(B) migration and (C) invasion of C-33 A and SiHa human CSCC cell
lines. In addition, ROCK1 overexpression significantly attenuated
the inhibitory effects of lncRNA HAND2-AS1 overexpression on
proliferation, migration and invasion of cancer cells. *P<0.05.
The relative cell migration and invasion were calculated by
normalizing cell number to control group, which was set to 100%.
lncRNA, long non-coding RNA; HAND2-AS1, HAND2 antisense RNA 1;
ROCK1, rho associated coiled-coil containing protein kinase 1; HPV,
human papillomavirus; CSCC, cervical squamous cell carcinoma; C,
control; NC, negative control. |
Discussion
The role of lncRNA HAND2-AS1 has been previously
characterized in two types of human malignancies including
osteosarcoma (11) and endometrioid
endometrial carcinoma (12). The
present study revealed that lncRNA HAND2-AS1 serves a tumor
suppressor role in HPV-negative and HPV-positive CSCC cells,
possibly by downregulating ROCK1.
Downregulation of lncRNA HAND2-AS1 was previously
reported in osteosarcoma (11) and
endometrioid endometrial carcinoma (12). Overexpression of lncRNA HAND2-AS1
inhibited the progression of the aforementioned malignancies,
indicating that lncRNA HAND2-AS1 may be a potential therapeutic
target for osteosarcoma (11) and
endometrioid endometrial carcinoma (12). The current study revealed that serum
lncRNA HAND2-AS1 was downregulated in HPV-positive and -negative
patients with CSCC compared with healthy controls. In addition,
lncRNA HAND2-AS1 overexpression inhibited the proliferation,
migration and invasion of HPV-positive and -negative human CSCC
cells in vitro. HAND2-AS1 overexpression resulted in no
significant effects on the biological behaviors of normal human
cervical epithelial cells. The data obtained in the present study
suggested that lncRNA HAND2-AS1 may serve as a potential
therapeutic target for CSCC.
Tumor metastasis leads to high mortality rate among
cancer patients. Patients with metastatic CSCC usually have a poor
prognosis even following treatment (16,17).
Therefore, early diagnosis and treatment are required to improve
the overall survival rate of patients with CSCC. The present study
enrolled patients with CSCC at stages I and II, which are the two
early stages of CSCC. ROC curve analysis revealed that upregulated
plasma lncRNA HAND2-AS1 effectively distinguished HPV-16-positive,
HPV-18-positive and HPV-negative patients from healthy controls.
Therefore, plasma lncRNA HAND2-AS1 may have diagnostic value for
HPV-positive and -negative CSCC. However, lncRNA HAND2-AS1
expression is also altered in osteosarcoma (11) and endometrioid endometrial carcinoma
(12). Therefore, multiple
biomarkers may improve the diagnostic specificity for CSCC.
ROCK1 is a protein serine/threonine kinase that
serves important roles in cancer development and progression,
particularly in cell motility, metastasis and angiogenesis
(18,19). Inhibition of ROCK1 may be a promising
therapeutic target for the treatment of cancer (18,19).
ROCK1 interacts with lncRNAs to participate in the pathogenesis of
certain types of malignancies, such as osteosarcoma (20,21). The
current study revealed that lncRNA HAND2-AS1 may be an upstream
inhibitor of ROCK1 in HPV-positive and -negative CSCC. The present
study suggests that disease-associated factors may mediate the
interaction between lncRNA HAND2-AS1 and ROCK1: i) No significant
correlation between plasma lncRNA HAND2-AS1 and ROCK1 were observed
in normal controls; and ii) lncRNA HAND2-AS1 overexpression did not
significantly affect ROCK1 in normal human cervical epithelial
cells. ROCK1 overexpression only partially attenuated the
inhibitory effects of lncRNA HAND2-AS1 on CSCC cell proliferation,
migration and invasion. Therefore, lncRNA HAND2-AS1 may also
interact with other pathways to achieve this regulatory role.
Furthermore, the data obtained in the current study suggested that
HPV-positive and -negative CSCC may share similar pathological
pathways.
To the best of the authors' knowledge, the current
study is the first to investigate the role of HAND2-AS1 in CSCC.
The current study systemically investigated the role of HAND2-AS1
in different types of CSCC and evaluated the diagnostic value of
HAND2-AS1 for CSCC. The current study provided new insights into
the pathogenesis of CSCC. In conclusion, lncRNA HAND2-AS1 was
downregulated and ROCK1 was upregulated in HPV-positive and
-negative patients with CSCC. lncRNA HAND2-AS1 may inhibit cancer
cell proliferation, migration and invasion by downregulating ROCK1
in HPV-positive and -negative CSCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ designed experiments. LJ and JJ performed
experiments. LS, SJ and LP analyzed data. LJ drafted the
manuscript. All authors have given final approval of the version to
be published.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of the Peace Hospital of Changzhi Medical College
(Changzhi, China). All patients and healthy volunteers provided
written informed consent prior to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Siegel RL, Sauer AG, Miller
KD, Fedewa SA, Alcaraz KI and Jemal A: Cancer statistics for
African Americans, 2016: Progress and opportunities in reducing
racial disparities. CA Cancer J Clin. 66:290–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng R, Zeng H, Zhang S, Chen T and Chen
W: National estimates of cancer prevalence in China, 2011. Cancer
Lett. 370:33–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hildesheim A, Gonzalez P, Kreimer AR,
Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M,
Sidawy M, Schiller JT, et al: Impact of human papillomavirus (HPV)
16 and 18 vaccination on prevalent infections and rates of cervical
lesions after excisional treatment. Am J Obstet Gynecol.
215:212.e1–212.e15. 2016. View Article : Google Scholar
|
|
7
|
Rodríguez-Carunchio L, Soveral I,
Steenbergen RD, Torné A, Martinez S, Fusté P, Pahisa J, Marimon L,
Ordi J and del Pino M: HPV-negative carcinoma of the uterine
cervix: A distinct type of cervical cancer with poor prognosis.
BJOG. 122:119–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Y, Zhu X, Xu Y, Tang Q, Huang Z, Zhao
Z, Lu J, Song G, Xu H, Deng C and Wang J: Energy stress-induced
lncRNA HAND2-AS1 represses HIF1α-mediated energy metabolism and
inhibits osteosarcoma progression. Am J Cancer Res. 8:526–537.
2018.PubMed/NCBI
|
|
12
|
Yang X, Wang CC, Lee WYW, Trovik J, Chung
TKH and Kwong J: Long non-coding RNA HAND2-AS1 inhibits invasion
and metastasis in endometrioid endometrial carcinoma through
inactivating neuromedin U. Cancer Lett. 413:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei L, Surma M, Shi S, Lambert-Cheatham N
and Shi J: Novel insights into the roles of Rho kinase in cancer.
Arch Immunol Ther Exp (Warsz). 64:259–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kardani K, Agi E and Bolhassani A:
Diagnosis of HPV Infections, HPV Testing in Patients. HPV
Infections: Diagnosis, Prevention, and Treatment. Bolhassani:
Bentham Science Publishers Ltd. (Sharjah). 129–158. 2018.
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedlander M and Grogan M; U.S.
Preventative Services Task Force, : Guidelines for the treatment of
recurrent and metastatic cervical cancer. Oncologist. 7:342–347.
2002.PubMed/NCBI
|
|
17
|
Symonds RP, Gourley C, Davidson S, Carty
K, McCartney E, Rai D, Banerjee S, Jackson D, Lord R, McCormack M,
et al: Cediranib combined with carboplatin and paclitaxel in
patients with metastatic or recurrent cervical cancer (CIRCCa): A
randomised, double-blind, placebo-controlled phase 2 trial. Lancet
Oncol. 16:1515–1524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whatcott CJ, Ng S, Barrett MT, Hostetter
G, Von Hoff DD and Han H: Inhibition of ROCK1 kinase modulates both
tumor cells and stromal fibroblasts in pancreatic cancer. PLoS One.
12:e01838712017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chin VT, Nagrial AM, Chou A, Biankin AV,
Gill AJ, Timpson P and Pajic M: Rho-associated kinase signalling
and the cancer microenvironment: Novel biological implications and
therapeutic opportunities. Expert Rev Mol Med. 17:e172015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui M, Wang J, Li Q, Zhang J, Jia J and
Zhan X: Long non-coding RNA HOXA11-AS functions as a competing
endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p
in osteosarcoma. Biomed Pharmacother. 92:437–444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Zeng X, Wang N, Zhao W, Zhang X,
Teng S, Zhang Y and Lu Z: Long noncoding RNA DANCR, working as a
competitive endogenous RNA, promotes ROCK1-mediated proliferation
and metastasis via decoying of miR-335-5p and miR-1972 in
osteosarcoma. Mol Cancer. 17:892018. View Article : Google Scholar : PubMed/NCBI
|