Introduction
Economic growth and improvement of living standards
has led to an increase in the prevalence of diabetes (1). Coronary artery disease causing
myocardial infarction is one of the main complications associated
with diabetes (2). The mortality
rate of diabetic patients following myocardial infarction is
significantly increased, when compared with nondiabetic patients
(3). Previous studies showed some
key mechanisms involved in the sensitivity of the diabetic heart to
myocardial infarction, such as extracellular signal regulated
kinase (ERK)1/2 signaling (4),
inflammatory (5) and
phosphatidylinositol 3 kinase (PI3K)/protein kinase B
(AKT)/glycogen synthase kinase (GSK)-3β (6). Hyperglycemia is the major
characteristic of diabetes, which causes reactive oxygen species
overproduction and exacerbates myocardial infarction (7). Therefore, excessive autophagy is a
major mechanism of myocardial ischemia reperfusion injury in
diabetes (8). In ischemic patients,
reperfusion to the ischemic heart mitigates the mortality rate
(9); however, reperfusion also
causes further myocardial dysfunction (10). It is therefore urgent to explore
effective drugs against diabetic myocardial ischemia/reperfusion
(I/R) injury.
Resveratrol (RVS), otherwise known as
3,4′,5-trihydroxy- transstilbene, a polyphenolic compound present
in grapes, wines, peanuts and several other fruits and vegetables,
has been associated with increased longevity and shown to have a
positive effect in the treatment of cancer and cardiovascular
disease (11). The targets of RSV
include various pathways and molecules, such as sirtuins, FOXOs,
and autophagy and inflammatory cytokines (12). RSV has been shown to protect against
cerebral I/R injury by inhibiting NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) inflammasome activation,
revealing the relationship between RSV and inflammatory response
(13). Autophagy is a self-clearing
process that removes dying or unwanted cells and is accompanied by
the activation of autophagy markers Beclin 1 and light chain 3-II
(LC3-II) (14). It has been reported
that autophagy significantly contributes to the degree of
myocardial I/R injury (15),
suggesting that therapeutic manipulation of autophagy in the
myocardium may benefit post-infarction cardiac healing and
remodeling. Previous studies have shown that RSV reduces the
severity of myocardial I/R injury by inducing autophagy (16,17).
Therefore, RSV has been proposed as an effective therapy for the
prevention of myocardial injury (18). It has also been reported that RSV
alleviates myocardial dysfunction in diabetes and can serve as a
potent pharmacological agent in reducing diabetic-induced I/R
injury (3). However, its molecular
mechanism remains unclear.
In the present study, the diabetic rat model was
constructed through a high-fat diet combined with a streptozotocin
(STZ) injection. Diabetic rats were subjected to 30 min of ischemia
and 2 h of reperfusion to stimulate diabetic-induced myocardial I/R
injury. It was found that the injection of RSV mitigated the
ischemia- or I/R injury-induced myocardial damage on hemodynamic
function and infarct size, but the autophagy inhibitor
3-methyladenine (3-MA) significantly blocked the function of RSV.
Furthermore, the application of RSV significantly enhanced the
expression of Beclin-1 and LC-3II but blocked the serum levels of
cytokines tumor necrosis factor-α (TNF)-α and interleukin (IL)-6.
In addition, the application of 3-MA suppressed the function of
RSV. In conclusion, RSV alleviates I/R injury of the diabetic
myocardium via inducing the expression of autophagy markers
Beclin-1 and LC-3II and inhibiting expression of TNF-α and IL-6.
These findings revealed an alleviating effect of RSV on
diabetic-induced myocardial I/R injury, as well as its molecular
basis, providing a novel strategy for diabetic myocardial I/R
injury therapy.
Materials and methods
Animals and grouping
A total of 100 male Sprague-Dawley rats [Certificate
no. SCXK(Zhe) 2013-0003] aged 6–8 weeks and weighing 200±20 g were
purchased from the Experimental Animal Center of Zhejiang Province
(Taizhou, Zhejiang, China). Rats were housed at 24±1°C with 40–50%
humidity in a clean environment kept to a 12-h light/dark cycle.
All animals had unlimited access to food and water. All
experimental procedures were approved by the Experimental Animal
Ethics Committee of Municipal Hospital of Taizhou. Rats were given
a high-fat diet (20% sugar, 10% lard, 10% egg yolk powder, 2%
cholesterol and 58% common chow). After 2 months, rats were
intraperitoneally injected with STZ (30 mg/kg; Enzo Life Sciences,
Inc.) dissolved in 0.1 mM citrate buffer (pH 4.5) according to a
previous study (19). The fasting
blood glucose (based on tail vein samples) was detected once a week
for 4 weeks. Rats with a fasting blood glucose of ≥16.7 mM were
selected as diabetic animals for follow-up experiments. Diabetic
rats were randomly divided into four groups (n=20 per group), the
Control, Model, RSV (Merck KGaA) and 3-MA groups. Diabetic rats in
the RSV group were intragastrically administered RSV (10 mg/kg/d).
Rats in the 3-MA group were intravenously administered 3-MA (15
mg/kg/d), based on the RSV injection. The Control and Model groups
were injected with the same amount of physiological saline.
Animal model of myocardial I/R
Rats were anesthetized with 10% chloral hydrate (300
mg/kg) and exhibited no signs of peritonitis. A thoracotomy was
performed exposing the heart. The left anterior descending (LAD)
coronary artery was ligated to stimulate myocardial infarction for
30 min. Electrocardiogram (ECG) ST Segment elevation signified a
successful infarction. After 30 min, the ligation was removed to
restore blood flow for an additional 2 h, to stimulate reperfusion.
Rats in the Model, RSV and 3-MA groups were treated according to
the above procedure, and the control group underwent a sham
surgery.
Measurement of hemodynamic function of
the heart
Hemodynamic function was assessed prior to modeling
(baseline), during trachea cannula (before ischemia) and during
ischemia and I/R. The left ventricular systolic pressure (LVSP) and
time derivatives of the pressure change were measured during
contraction (+dP/dt) and relaxation (-dP/dt) using a polygraph, as
previously described (16). A MedLab
bio-signal system (Nanjing Medease Science and Technology Co.,
Ltd.) was used to monitor electrical activity in the heart through
an ECG throughout the experiment.
Measurement of myocardial infarct
size
A total of six rats in each group were randomly
selected for the measurement of myocardial infarct size. The left
ventricle was rapidly excised from the anesthetized rat, weighed
and cut into 1–2 mm thick sections. Viable tissues were stained in
red with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 20 min at
37°C. Images were captured of the stained sections and the
pale-appearing infarct area was calculated using Image Pro Plus 4.5
software (Media Cybernetics, Inc.). The infarct ratio (%) was
calculated by dividing the infarct volume by the total volume of
the sections.
Determination of the content of TNF-a
and IL-6
A total of 200 µl of blood were collected from the
tails veins. After centrifugation at 4,000 × g for 8 min at 4°C,
sera were collected for follow-up experiments. Serum levels of
TNF-α (cat. no. JM-01587R1) and IL-6 (cat. no. JM-01597R1) were
assessed with enzyme-linked immunosorbent assay (ELISA) kits
(Jingmei Biological Technology, Co., Ltd.).
Western blotting
Isolated rat cardiomyocytes were homogenized into
pieces for protein extraction. Proteins were extracted using RIPA
buffer (Sigma-Aldrich; Merck KGa) supplemented with 1 mM PMSF
(Sigma-Aldrich; Merck KGaA). The concentration was determined with
bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.). The same amount of protein (10 µg/lane) was separated by 12%
SDS-PAGE using a constant voltage of 80 V for 120 min. The proteins
were transferred into polyvinylidene fluoride membrane (EMD
Millipore). The membrane was blocked with 5% non-fat milk in
Tris-buffered saline (TBS) for 1 h at room temperature. After the
blockage, the membrane was incubated with the primary antibodies
(all Abcam), including rabbit monoclonal anti-PI3Kγ (ab32089;
1:1,000), anti-Beclin 1 (ab207612; 1:2,000), anti-LC-3B (ab192890;
1:2,000) and anti-GAPDH (ab181602; 1:10,000) antibodies overnight
at 4°C. The next morning, the membrane was washed with TBS
containing 0.05% Tween-20 (TBST) and probed with horseradish
peroxidase-conjugated goat anti-rabbit IgG H&L (ab6721;
1:10,000; Abacm) at 37°C for 1 h. After being washed with TBST, the
membranes were developed using Enhanced chemiluminescence kit
(PerkinElmer, Inc.) and visualized using an Odyssey Infrared
Imaging System (LI-COR Biosciences). The bands were quantified
using ImageJ software (v1.51; National Institutes of Health) by
measuring the target gene band intensity normalized to the internal
control GAPDH.
Statistical analysis
All data are presented as the mean ± standard
deviation for three independent experiments and analyzed by SPSS
software v16.0 (SPSS, Inc.). The statistical significance among the
four experimental groups was determined by a multivariate analysis
of variance followed by Tukey post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
The myocardial I/R rat model is
successfully constructed
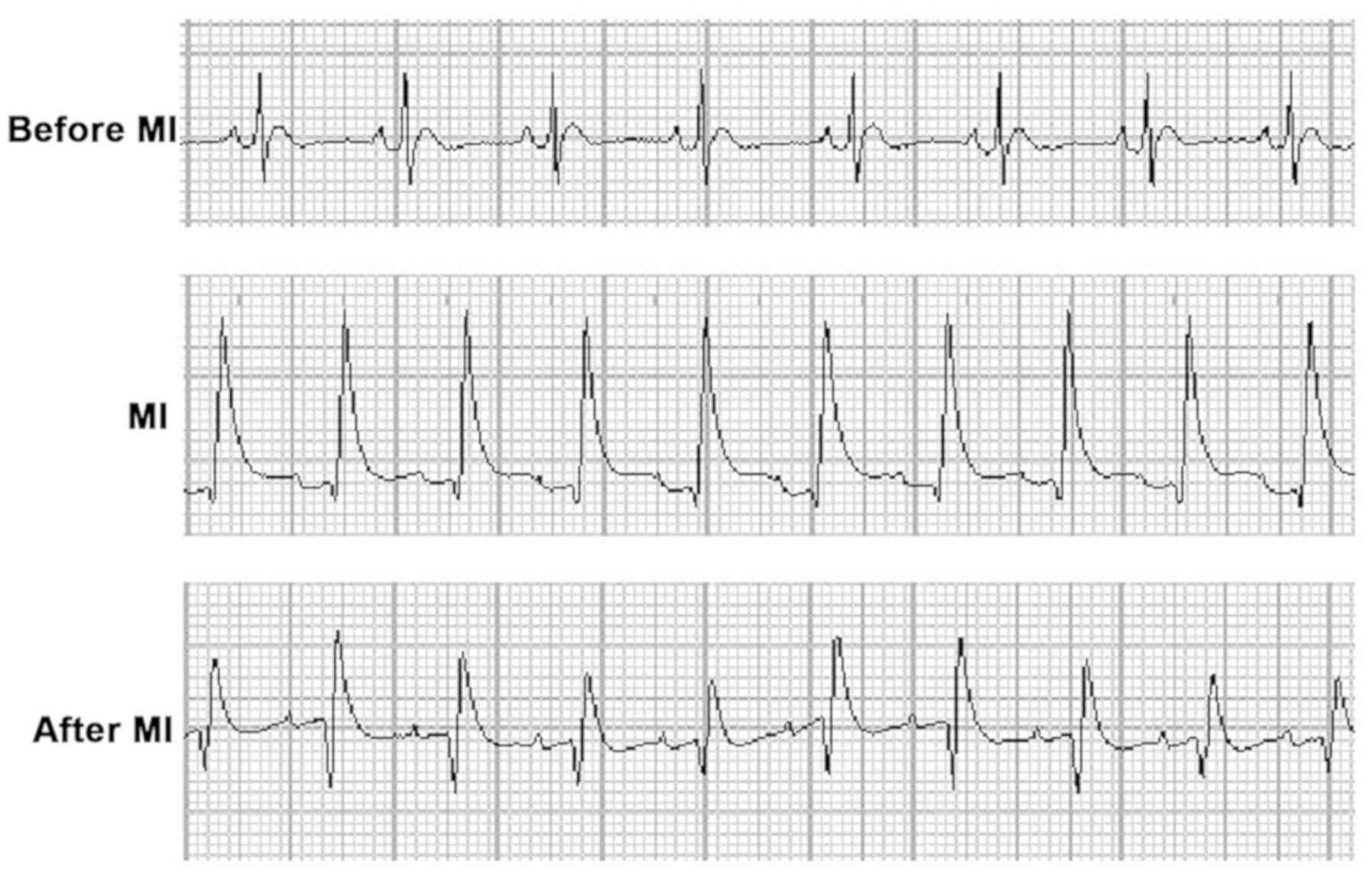
In order to examine the myocardial I/R rat model, a
MedLab bio-signal system was used to monitor the heart's electrical
activity through an ECG throughout the experiment (Fig. 1). Prior to myocardial ischemia, ECG
results appeared normal with a normal ST segment. Following LAD
coronary artery ligation for 30 min, A significant ST elevation was
observed, suggesting successful ischemia. Following blood flow
recovery for 2 h, the ST elevation was reduced to ~30–40% of its
baseline values, indicating successful I/R injury. These data
indicated the successful construction of the myocardial I/R rat
model for follow-up experiments.
RSV mitigates ischemia and I/R injury
on heart function
To investigate the effect of RSV on myocardial
function following I/R injury, hemodynamic parameters were measured
in all groups throughout the experiments (Fig. 2). No differences were identified in
the baseline, LVSP, +dP/dt and -dP/dt values among the four groups
(P>0.05, Fig. 2A-C). Prior to
myocardial ischemia, no difference was observed in the LVSP, +dP/dt
and -dP/dt values between the Control and model groups (P>0.05,
Fig. 2A-C). However, when compared
with the Model group, pretreatment with RSV significantly
upregulated LVSP, +dP/dt and -dP/dt (P<0.05, Fig. 2A-C). During ischemia, LVSP, +dP/dt
and -dP/dt in the Model group were significantly downregulated, as
compared with the control group (P<0.05, Fig. 2A-C). The addition of RSV mitigated
the blockage induced by ischemia, whereas the application of 3-MA
suppressed the function of RSV on LVSP, +dP/dt and -dP/dt (Fig. 2A-C). Similar trends were observed
during I/R damage. These data suggest that RSV pretreatment
mitigated the ischemia and I/R injury on heart function, but 3-MA
suppressed that function of RSV.
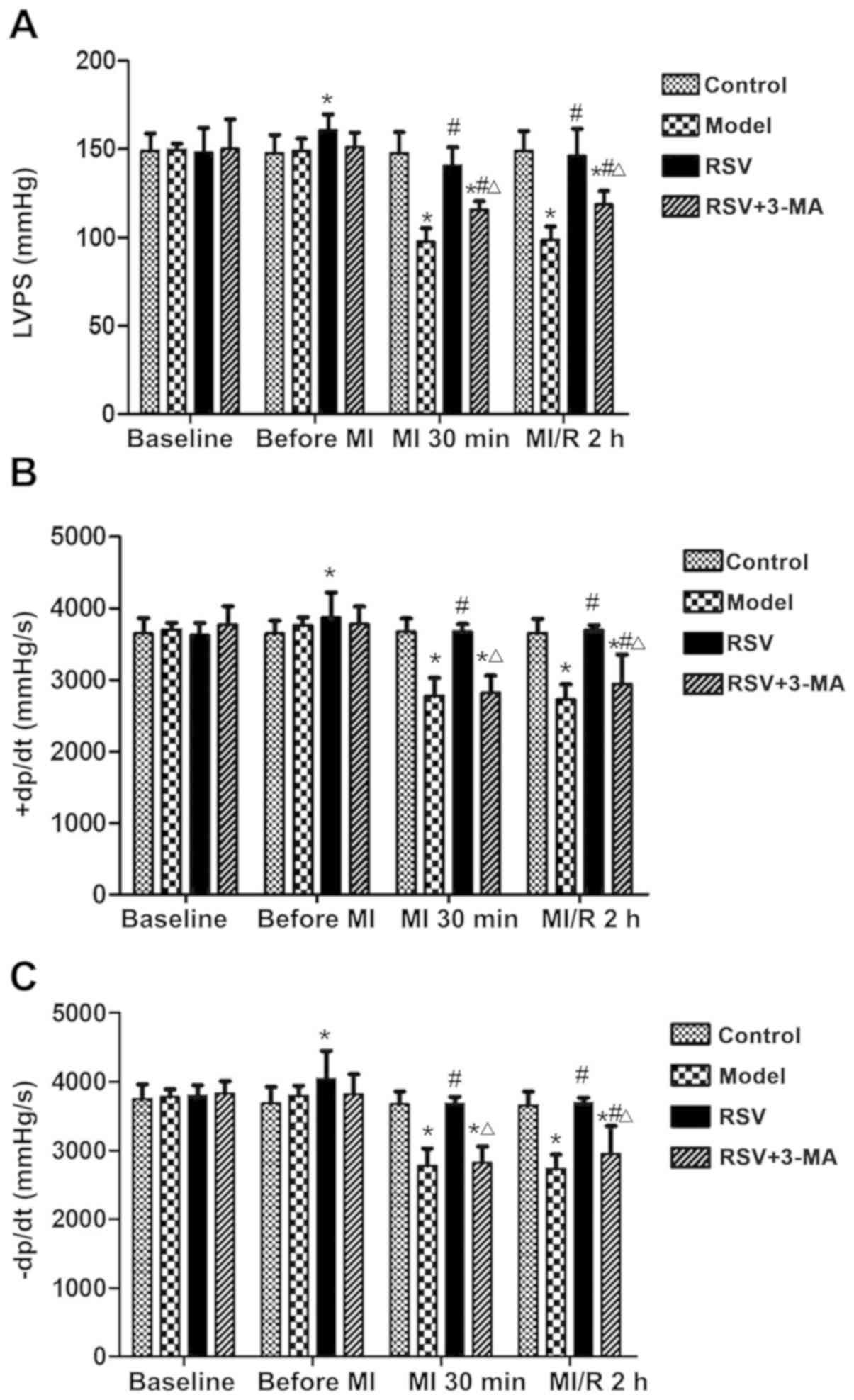 | Figure 2.RSV and 3-MA affects the myocardial
function via heart hemodynamic function examination. RSV and 3-MA
modulated the levels of (A) LVSP, (B) +dP/dt and (C) -dP/dt.
Diabetic rats in the RSV group were given RSV (10 mg/kg/d) by
intragastric administration. Rats in the 3-MA (RSV+3-MA) group were
given an intravenous injection of 3-MA (0.015 g/kg/d) based on the
RSV (10 mg/kg/d) injection. The Control and Model groups were
injected with the same amount of physiological saline. After 7
days, LAD coronary artery was performed on rats in the Model, RSV
and RSV+3-MA groups to stimulate myocardial infarction. The control
group underwent a sham surgery without LAD coronary artery
ligation. The hemodynamic parameters were measured prior to
modeling (baseline), during trachea cannula (before ischemia) and
during ischemia and I/R. *P<0.05 vs. the control group;
#P<0.05 vs. the Model group; ΔP<0.05
vs. the RSV group. RSV, resveratrol; 3-MA, 3-methyladenine; LVSP,
left ventricular systolic pressure; -dP/dt, time derivatives of the
pressure change during contraction; -dP/dt, time derivatives of the
pressure change during relaxation; LAD, left anterior descending;
I/R, ischemia/reperfusion; MI, myocardial ischaemia. |
RSV mitigates the myocardial infarct
size caused by ischemia and I/R
The myocardial infarct size was measured following
different treatments. Fig. 3A shows
the typical morphological characteristics of myocardial sections
following TTC staining. An extensive pale infarct area was observed
in the myocardial sections of rats in the Model group, which was
not there in the control group. Pretreatment with RSV significantly
decreased the infarct area (P<0.05). However, the pale infarct
area in the myocardial sections of the 3-MA group was significantly
increased, as compared with that in the RSV group sections
(P<0.05). Fig. 3B shows the
infarct size rate (%). These data indicated that RSV mitigated the
myocardial infarct size caused by ischemia and I/R, but 3-MA
suppressed the role of RSV.
 | Figure 3.RSV and 3-MA affects the infarct size.
(A) Visualization of the typical morphological characteristics of
myocardial sections by TTC staining. Diabetic rats in the RSV group
were given RSV (10 mg/kg/d). Rats in the 3-MA (RSV+3-MA) group were
administered 3-MA (0.015 g/kg/d), based on the RSV (10 mg/kg/d)
injection. The Control and Model groups were injected with the same
amount of physiological saline. After 7 days, LAD coronary artery
was performed on rats in the Model, RSV and RSV+3-MA groups to
stimulate myocardial I/R injury. The control group underwent a sham
surgery. The left ventricles from 6 rats in each group were
extracted and cut into 1–2-mm thick sections for TTC staining. Red
represents normal tissue and pale stands for infarct tissue. (B)
The infarct ratio (%) was calculated by dividing the infarct volume
by the total volume of the sections. *P<0.05 vs. the control
group; #P<0.05 vs. the Model group;
&P<0.05 vs. the RSV group. RSV, resveratrol;
3-MA, 3-methyladenine; TTC, 2,3,5-triphenyltetrazolium chloride;
LAD, left anterior descending; I/R, ischemia/reperfusion. |
RSV protects against I/R injury by
inducing autophagy and blocking cytokines
A previous study revealed the relationship between
ischemia and I/R and autophagy (20). To explore the mechanism by which RSV
mitigates ischemia and I/R, the expression levels of autophagy
markers Beclin 1 and LC-3II were mainly detected. The protein
levels of PI3K, Beclin 1 and LC-3II were significantly upregulated
in the Model group compared with the Control (P<0.01; Fig. 4A and B). Furthermore, the addition of
RSV further enhanced those levels. However, treatment with the
autophagy inhibitor 3-MA significantly blocked the upregulation
induced by RSV (P<0.01). The role of RSV on cytokines was
further investigated. The serum level of TNF-α in the Model group
was significantly increased compared with the control group
(P<0.01; Fig. 4C). However, the
addition of RSV blocked the increase of TNF-α and 3-MA application
inhibited the function of RSV. A similar trend was observed in
serum IL-6 levels (Fig. 4D). These
data suggest that RSV promoted the expression of Beclin 1 and
LC-3II but blocked the serum levels of TNF-α and IL-6.
 | Figure 4.RSV and 3-MA affects the expression of
Beclin 1 and LC3-II and the serum levels of TNF-α and IL-6. (A) RSV
promoted the expression of PI3K, Beclin 1 and LC-3II, as detected
by western blotting. Diabetic rats in the RSV group were given RSV
(10 mg/kg/d). Rats in the 3-MA (RSV + 3-MA) group were administered
3-MA (0.015 g/kg/d), based on the RSV (10 mg/kg/d) injection. The
Control and Model groups were injected with the same amount of
physiological saline. After 7 days, LAD coronary artery was
performed on rats in the Model, RSV and 3-MA groups to stimulate
myocardial I/R injury. The control group underwent a sham surgery.
Following I/R for 2 h, proteins were extracted for western
blotting. (B) The relative expression of targets was normalized to
GAPDH. RSV inhibited serum (C) TNF-α and (D) IL-6, as detected by
ELISA. aP<0.05 and bP<0.01 vs. the
control group; cP<0.01 vs. the Model group;
dP<0.05 and eP<0.01 vs. the RSV group.
RSV, resveratrol; 3-MA, 3-methyladenine; LC3-II, light chain 3-II;
LAD, left anterior descending; I/R, ischemia/reperfusion; TNF,
tumor necrosis factor; IL, interleukin; PI3K, phosphatidylinositol
3 kinase. |
Discussion
Cardiovascular disease, including I/R injury, has
been associated with high mortality worldwide. Microvascular
complications are considered to play a major role in diabetic
complications (21) and diabetes has
been reported to cause an enhanced vulnerability of the myocardium
to myocardial I/R injury (22). The
aim of the present study was to explore the function of RSV against
myocardial I/R injury in diabetes and to reveal the underlying
mechanisms. During the heart hemodynamic function examination, the
levels of LVSP, +dP/dt and -dP/dt were blocked by LAD and
reperfusion. The RSV injection mitigated that blockage. However,
the addition of 3-MA suppressed the function of RSV. In addition,
the I/R injury-induced increase in infarct size was blocked by RSV
and 3-MA reversed the function of RSV. These data provided new
evidence on the function of RSV on myocardial I/R injury in
diabetes. Furthermore, it was found that I/R injury enhanced the
expression of Beclin 1 and LC3II and serum levels of TNF-α and
IL-6. The application of RSV promoted the elevation of Beclin 1 and
LC-3II but decreased the serum levels of TNF-α and IL-6. However,
3-MA reversed the effect of RSV. These results showed that RSV
ameliorates diabetes-induced I/R injury through inducing autophagy
and blocking cytokines TNF-α and IL-6.
Dietary phytochemicals are becoming popular as
functional foods and nutritional supplements. RSV is a naturally
occurring polyphenol (23), now
considered a potent cardioprotective compound (24). A previous study showed that the
beneficial effects of RSV on myocardial I/R injury might have a
multifactorial basis (25). Certain
reports have revealed that RSV is involved in cardioprotection via
regulating the expression of thioredoxin-1 (26), hemeoxygenase-1 (27) and vascular endothelial growth factor
(28), among others. Despite
significant advances in the understanding of the function and
mechanism of RSV against myocardial I/R injury, the effects and
molecular basis of RSV on myocardial I/R injury under diabetic
conditions remain unclear. In the present study, it was found that
the application of RSV clearly elevated the levels of LVSP, +dP/dt
and -dP/dt, and ameliorated the infarct size in diabetic myocardial
I/R injury. These results indicated that RSV plays a role in the
mechanisms of resistance to I/R injury in diabetic rats, which was
consistent with a previous report (29).
Autophagy has been reported to be activated
following myocardial I/R injury in cardiomyocytes (30,31). The
regulation of autophagy provided a new strategy for the treatment
of myocardial ischemia and heart failure (32). The blockage of autophagy is commonly
achieved through the inhibition of class III PI3K via a
pharmacological agent 3-MA (33). In
this study, the administration of 3-MA improved the infarct size,
as compared with the RSV-treated group, suggesting the close
association between RSV and autophagy. A previous study showed that
RSV attenuates cardiac dysfunction associated with the regulation
of autophagic flux in diabetic mice (17), which was consistent with the results
of the present study. Beclin-1 is the mammalian homologue of the
yeast Atg6 (34) and the activation
of Beclin-1 at the site of injury denotes the induction of
autophagy (35). Similar to
Beclin-1, LC3-II, a mammalian homologue of yeast Atg8p, is also a
credible marker for autophagy (30).
It was found herein that the application of RSV significantly
promoted the expression of Beclin 1 and LC3-II, and that the
inhibition of autophagy achieved by 3-MA significantly blocked the
RSV-induced upregulation of Beclin 1 and LC3-II. These findings
were consistent with those of a previous study showing that RSV
induced autophagy, as evidenced by the abilities to activate
autophagic markers LC3-II and Beclin-1 (36,37). It
has been reported that RSV attenuated I/R-induced NLRP3
inflammasome-derived inflammation through SIRT1-dependent autophagy
activity (13). A number of studies
have shown that the upregulation of TNF-α and IL-6 directly leads
to organ damage by aggravating the inflammatory response (38–40). In
this study, it was found that RSV mitigated the I/R-induced release
of TNF-α and IL-6, which was in accordance with the above study. In
combination, these findings provided new evidence that RSV induced
autophagy and modulated inflammatory response in myocardial I/R
injury in diabetes.
The successful application of RSV lies in
understanding its mechanisms of action through direct and indirect
interactions with pathways. In the present study, RSV decreased the
levels of LVSP, +dP/dt and -dP/dt, as well the I/R injury-induced
infarct size of the diabetic myocardium. The effects of RSV on I/R
injury were reversed by 3-MA. RSV induced the activation of
autophagy via the upregulation of Beclin 1 and LC3-II and inhibited
inflammatory response via the blockage of TNF-α and IL-6. In
conclusion, RSV was found to alleviate I/R injury of the diabetic
myocardium via inducing autophagy and blocking inflammatory
response.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
science and Technology bureau of Taizhou (grant no. 162yw04).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XQ constructed the animal model of myocardial I/R,
analyzed the data and wrote the original manuscript; XC and QS
performed the measurement of hemodynamic function of the heart and
myocardial infarct size; XW and DW carried out the ELISA assays and
western blot analyzes. LY designed the study and corrected the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Experimental Animal Ethics Committee of Municipal Hospital of
Taizhou (Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Franz MJ, Boucher JL, Rutten-Ramos S and
VanWormer JJ: Lifestyle weight-loss intervention outcomes in
overweight and obese adults with type 2 diabetes: A systematic
review and meta-analysis of randomized clinical trials. J Acad Nutr
Diet. 115:1447–1463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Almeida Barreto FK, Montenegro RM Jr,
Fernandes VO, Oliveira R, de AraUjo Batista LA, Hussain A and de
Góes Cavalcanti LP: Chikungunya and diabetes, what do we know?
Diabetol Metab Syndr. 10:322018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thirunavukkarasu M, Penumathsa SV, Koneru
S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK and Maulik N:
Resveratrol alleviates cardiac dysfunction in
streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin,
and heme oxygenase. Free Radic Biol Med. 43:720–729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sotníková R, Nedelčevová J, Navarová J,
Nosáĺová V, Drábiková K, Szöcs K, Křenek P, Kyseĺová Z, Bezek S,
Knezl V, et al: Protection of the vascular endothelium in
experimental situations. Interdiscip Toxicol. 4:20–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bulhak AA, Jung C, Ostenson CG, Lundberg
JO, Sjoquist PO and Pernow J: PPAR-alpha activation protects the
type 2 diabetic myocardium against ischemia-reperfusion injury:
Involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol
Heart Circ Physiol. 296:H719–H727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J and Li J: Activated protein C: A
potential cardioprotective factor against ischemic injury during
ischemia/reperfusion. Am J Transl Res. 1:381–392. 2009.PubMed/NCBI
|
|
7
|
Korkmaz-Icöz S, Lehner A, Li S, Vater A,
Radovits T, Hegedűs P, Ruppert M, Brlecic P, Zorn M, Karck M and
Szabó G: Mild type 2 diabetes mellitus reduces the susceptibility
of the heart to ischemia/reperfusion injury: Identification of
underlying gene expression Changes. J Diabetes Res 2015.
3964142015.
|
|
8
|
Wang S, Wang C, Yan F, Wang T, He Y, Li H,
Xia Z and Zhang Z: N-acetylcysteine attenuates diabetic myocardial
ischemia reperfusion injury through inhibiting excessive autophagy.
Mediators Inflamm 2017. 92572912017.
|
|
9
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion-injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2015. View Article : Google Scholar
|
|
10
|
Feyzizadeh S and Badalzadeh R: Application
of ischemic postconditioning's algorithms in tissues protection:
Response to methodological gaps in preclinical and clinical
studies. J Cell Mol Med. 21:2257–2267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabe AA, Elmadhun NY, Dalal RS, Robich MP
and Sellke FW: Resveratrol regulates autophagy signaling in
chronically ischemic myocardium. J Thorac Cardiovasc Surg.
147:792–799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernandes GFS, Silva GDB, Pavan AR, Chiba
DE, Chin CM and Dos Santos JL: Epigenetic regulatory mechanisms
induced by resveratrol. Nutrients. 9(pii): E12012017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Q, Li Z, Wang Y, Hou Y, Li L and Zhao
J: Resveratrol alleviates cerebral ischemia/reperfusion injury in
rats by inhibiting NLRP3 inflammasome activation through
Sirt1-dependent autophagy induction. Int Immunopharmacol.
50:208–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizushima N: A brief history of autophagy
from cell biology to physiology and disease. Nat Cell Biol.
20:521–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gottlieb RA, Finley KD and Mentzer RM Jr:
Cardioprotection requires taking out the trash. Basic Res Cardiol.
104:169–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Liu YY, Liu XY, Jia SW, Zhao J,
Cui D and Wang L: Resveratrol protects neurons and the myocardium
by reducing oxidative stress and ameliorating mitochondria damage
in a cerebral ischemia rat model. Cell Physiol Biochem. 34:854–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Yang Q, Sun YY, Xing YF, Wang YB,
Lu XT, Bai WW, Liu XQ and Zhao YX: Resveratrol-enhanced autophagic
flux ameliorates myocardial oxidative stress injury in diabetic
mice. J Cell Mol Med. 18:1599–1611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mukhopadhyay P, Pacher P and Das DK:
MicroRNA signatures of resveratrol in the ischemic heart. Ann N Y
Acad Sci 1215. 109–116. 2011. View Article : Google Scholar
|
|
19
|
Zhang S, Yang J, Li H, Li Y, Liu Y, Zhang
D, Zhang F, Zhou W and Chen X: Skimmin, a coumarin, suppresses the
streptozotocin-induced diabetic nephropathy in wistar rats. Eur J
Pharmacol. 692:78–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu H, Ye M, Liu D, Yang J, Ding JW, Zhang
J, Wang XA, Dong WS and Fan ZX: UCP2 protect the heart from
myocardial ischemia/reperfusion injury via induction of
mitochondrial autophagy. J Cell Biochem. May 13–2019.(Epub ahead of
print). View Article : Google Scholar
|
|
21
|
Joshi MS, Williams D, Horlock D,
Samarasinghe T, Andrews KL, Jefferis AM, Berger PJ, Chin-Dusting JP
and Kaye DM: Role of mitochondrial dysfunction in
hyperglycaemia-induced coronary microvascular dysfunction:
Protective role of resveratrol. Diab Vasc Dis Res. 12:208–216.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donahoe SM, Stewart GC, McCabe CH,
Mohanavelu S, Murphy SA, Cannon CP and Antman EM: Diabetes and
mortality following acute coronary syndromes. JAMA. 298:765–775.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabe AA, Elmadhun NY, Robich MP, Dalal RS
and Sellke FW: Does resveratrol improve insulin signaling in
chronically ischemic myocardium? J Surg Res. 183:531–536. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho S, Namkoong K, Shin M, Park J, Yang E,
Ihm J, Thu VT, Kim HK and Han J: Cardiovascular protective effects
and clinical applications of resveratrol. J Med Food. 20:323–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Penumathsa SV and Maulik N: Resveratrol: A
promising agent in promoting cardioprotection against coronary
heart disease. Can J Physiol Pharmacol. 87:275–286. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Welsh SJ, Bellamy WT, Briehl MM and Powis
G: The redox protein thioredoxin-1 (Trx-1) increases
hypoxia-inducible factor 1alpha protein expression: Trx-1
overexpression results in increased vascular endothelial growth
factor production and enhanced tumor angiogenesis. Cancer Res.
62:5089–5095. 2002.PubMed/NCBI
|
|
27
|
Penumathsa SV, Koneru S, Samuel SM, Maulik
G, Bagchi D, Yet SF, Menon VP and Maulik N: Strategic targets to
induce neovascularization by resveratrol in hypercholesterolemic
rat myocardium: Role of caveolin-1, endothelial nitric oxide
synthase, hemeoxygenase-1, and vascular endothelial growth factor.
Free Radic Biol Med. 45:1027–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakamoto H, Nakamura T, Akuzawa N, Masuda
H, Sumino H, Saito Y, Ohyama Y, Kurashina T, Tamura J and
Kurabayashi M: Reciprocal expression of vascular endothelial growth
factor and nitric oxide synthase by coronary arterial wall cells
during chronic inhibition of nitric oxide synthesis in rats.
Nephron. 92:472–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Q, Wang HC, Liu Y, Gao C, Sun L and
Tao L: Resveratrol cardioprotection against myocardial
ischemia/reperfusion injury involves upregulation of adiponectin
levels and multimerization in type 2 diabetic mice. J Cardiovasc
Pharmacol. 68:304–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martinet W, Knaapen MW, Kockx MM and De
Meyer GR: Autophagy in cardiovascular disease. Trends Mol Med.
13:482–491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gustafsson AB and Gottlieb RA: Autophagy
in ischemic heart disease. Circ Res. 104:150–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishida K, Yamaguchi O and Otsu K:
Crosstalk between autophagy and apoptosis in heart disease. Circ
Res. 103:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lekli I, Ray D, Mukherjee S, Gurusamy N,
Ahsan MK, Juhasz B, Bak I, Tosaki A, Gherghiceanu M, Popescu LM and
Das DK: Co-ordinated autophagy with resveratrol and γ-tocotrienol
confers synergetic cardioprotection. J Cell Mol Med. 14:2506–2518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zalckvar E, Berissi H, Eisenstein M and
Kimchi A: Phosphorylation of Beclin 1 by DAP-kinase promotes
autophagy by weakening its interactions with Bcl-2 and Bcl-XL.
Autophagy. 5:720–722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xuan W, Wu B, Chen C, Chen B, Zhang W, Xu
D, Bin J and Liao Y: Resveratrol improves myocardial ischemia and
ischemic heart failure in mice by antagonizing the detrimental
effects of fractalkine*. Crit Care Med. 40:3026–3033. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen-Scarabelli C, Agrawal PR, Saravolatz
L, Abuniat C, Scarabelli G, Stephanou A, Loomba L, Narula J,
Scarabelli TM and Knight R: The role and modulation of autophagy in
experimental models of myocardial ischemia-reperfusion injury. J
Geriatr Cardiol. 11:338–348. 2014.PubMed/NCBI
|
|
38
|
Liu D, Jin X, Zhang C and Shang Y:
Sevoflurane relieves hepatic ischemia-reperfusion injury by
inhibiting the expression of Grp78. Biosci Rep. 38(pii):
BSR201805492018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Li X, Yang J, Chai J, Yu Y, Duan H,
Song H, Feng R, Wang T, Yin H, et al: Comparison of systemic
inflammation response and vital organ damage induced by severe
burns in different area. Int J Clin Exp Pathol. 8:6367–6376.
2015.PubMed/NCBI
|
|
40
|
Zhang S, Xin H, Li Y, Zhang D, Shi J, Yang
J and Chen X: Skimmin, a coumarin from hydrangea paniculata, slows
down the progression of membranous glomerulonephritis by
anti-inflammatory effects and inhibiting immune complex deposition.
Evid Based Complement Alternat Med 2013. 8192962013.
|