Introduction
Cardiovascular disease has the highest rates of
morbidity and mortality worldwide (1), as it results in cardiac remodeling
which is associated with the potential pathological changes
observed in most heart diseases, including heart failure,
myocardial infarction (MI), atrial fibrillation, atherosclerosis
(AS) and ischemia/reperfusion (IR) (2). MI is one of the main factors that
threatens human health and causes cardiac cell death (3). During MI, myocardial cells suffer
ischemia and hypoxia for a long period of time, which can lead to
irreversible cell death or apoptosis and cardiac dysfunction
(4). Reducing apoptosis of
myocardial cells can improve cardiac function and cardiac
remodeling process after MI (5).
Oxidative stress and apoptosis play a crucial role
in the development of cardiovascular diseases (6). The balance between oxidants and
antioxidants plays a vital role in maintaining normal biological
function. Excessive levels of reactive oxygen species cause serious
damage to cardiac myocytes, which can damage the
oxidation-antioxidant equilibrium system (7). In addition, further damage can lead to
apoptosis (8). Therefore, mitigation
of oxidative stress and direct intervention in inhibiting apoptotic
pathways can provide potential molecular targets for treatment
(9,10).
MicroRNAs (miRs/miRNAs) are a class of non-coding
RNA molecules, 18–24 nucleotides long, that are endogenous,
conserved and can degrade or inhibit the translation of their
target mRNAs, thereby regulating gene expression and playing an
important role in a wide range of biological processes (11–13).
There is an increasing body of evidence that has indicated the
important role of miRNAs in a number of types of cardiac diseases
(14–16). Although the functions of miR-370 in
different diseases are controversial, miR-370 has always been
considered to be a tumor suppressor in a number of types of human
cancers. For example, miR-370 was downregulated in endometrioid
ovarian cancer by regulating endoglin to suppress cell
proliferation and induce cell apoptosis (17). Feng et al (18) illustrated that there was significant
downregulation of miR-370 in gastric cancer tissues and cells
targeting progestin and AdipoQ receptor family member 4. It has
been reported that the expression of miR-370 was decreased in AS
models and upregulation of miR-370 could inhibit vascular
inflammation and oxidative stress (19). Furthermore, the expression of miR-370
in the myocardium was reported to decrease after MI (20). However, the expression of miR-370 in
cardiac myocytes after induction with hydrogen peroxide
(H2O2) and the corresponding mechanisms on
oxidative stress and apoptosis have not been studied.
H2O2 has been extensively used
to induce an apoptotic response in different cell types and the
H9C2 cell line is frequently used to study oxidative stress induced
cardiomyocyte apoptosis (21).
Therefore, the aim of this present study was to investigate the
effect of miR-370 on H2O2-induced myocardial
damage in H9C2 cells via oxidative stress and apoptosis.
Materials and methods
Cell culture and treatment
The H9C2 cardiomyocytes were purchased from the
American Type Culture Collection. Cells were cultivated in 10%
fetal bovine serum (FBS; Beyotime Institute of Biotechnology) and
1% penicillin/streptomycin DMEM (Beyotime Institute of
Biotechnology) and incubated at 37°C in an atmosphere containing 5%
CO2. The cells were subjected to
H2O2 (50, 100 and 200 µM; Merck KGaA)
solution in DMEM without FBS treatment for 4 h.
Cell transfection
H9C2 cells were seeded into 12-well plates with
1×106 cells/well and cultured in serum-free DMEM at 37°C
with 5% CO2 continuously to ensure that cell confluence
reached 80% before transfection. According to the manufacturer's
protocol, miR-370 mimics (cat. no. miR10000722-1-5), negative
control (NC) mimic (cat. no. miR1190315051351), small interfering
RNA (siR-/siRNA)-Forkhead box O1 (FOXO1)-1
(5′-GGACAACAACAGTAAATTT-3′), siR-FOXO1-2
(5′-GCACCGACTTTATGAGCAA-3′) and NC siRNA (cat. no. siT0000003-1-5)
(Each, 100 nM; all, Guangzhou RiboBio Co., Ltd.) were transfected
into H9C2 cells for 48 h using Lipofectamine 2000™ reagent
(Invitrogen; Thermo Fisher Scientific, Inc.).
Cell viability assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to assess cardiomyocyte viability.
After H9C2 cells were treated with H2O2, 10
µl CCK-8 solution was added to each well and the cells were
incubated at 37°C for 4 h. Finally, the optical density of each
well was measured at a wavelength of 490 nm using a plate reader
(Infinite® 200 PRO NanoQuant; Tecan Group, Ltd.).
ELISA
The concentrations of plasma superoxide dismutase
(SOD; cat. no. ml077379), malondialdehyde (MDA; cat. no. ml077384)
and lactate dehydrogenase (LDH, ml003416) were measured using
commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co.,
Ltd.) according to the manufacturer's protocols. The experimental
groups were as follows: Control group, H9C2 cells untreated with
H2O2; H2O2 group, H9C2
cells treated with H2O2; NC mimic
+H2O2 group, H9C2 cells treated with NC mimic
after H2O2; miR-370 mimic+
H2O2 group, H9C2 cells treated with miR-370
mimic after H2O2; NC siRNA+
H2O2 group, H9C2 cells treated with NC siRNA
after H2O2; and siR-FOXO1-1+
H2O2 group, H9C2 cells treated with
siR-FOXO1-1 after H2O2.
Flow cytometry assay
Cell apoptosis was evaluated using an Annexin V-FITC
Apoptosis Detection kit (BD Biosciences; Becton, Dickson and
Company) in accordance with the manufacturer's protocol. The cells
were treated as described above. All adhering and floating cells
were harvested, washed twice with PBS, transferred into sterile
centrifuge tube and then stained successively with propidium iodide
(10 µl) and Annexin-V-FITC (10 µl; Nanjing KeyGen Biotech Co.,
Ltd.) for 15 min at 37°C. A flow cytometer (BD Biosciences; Becton,
Dickson and Company) was used to assess apoptotic cells and data
were analyzed using CellQuest software (version 3.1, BD
Biosciences). Experiments were repeated three times
independently.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.). Then, the total RNA was
used to synthesize cDNA using a PimeScript RT master mix kit
(Takara Biotechnology Co., Ltd.) following the manufacturer's
protocol. The RT conditions were as follows: 10 min at 25°C, 30 min
at 45°C and 5 min at 95°C. qPCR was performed with the following
thermocycling condition: 95°C for an initial 10 min followed by 40
cycles of denaturation for 15 sec at 95°C, annealing at 60°C for 30
sec and extension at 72°C for 30 sec with the SYBR Premix Ex Taq
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Fold-changes
of miR-370 and mRNA levels were calculated using the
2−ΔΔCq method (22). U6
and GAPDH were used as the endogenous controls. The following
primers were used: GAPDH forward, 5′-AGACAGCCGCATCTTCTTGT-3′ and
reverse, 5′-TGATGGCAACAATGTCCACT-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′, and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
miR-370 forward, 5′-TACTCAGGATCCTGTGCAAGGCGGGCTACT-3′ and reverse,
5′-TACTCAAAGCTTCCCTCCCTCACCCAAATC-3′; FOXO1 forward,
5′-AGGATCCGATGTCACCATGGCCG-3′ and reverse,
5′-AAAGGATCCACCATGGCCG-3′.
Western blot analysis
The total proteins from cells were isolated using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and the
concentration of protein was measured with a bicinchoninic acid kit
(Beyotime Institute of Biotechnology). Subsequently, an equal
quantity of proteins (20 µg) was separated by 10% SDS-PAGE and then
transferred onto polyvinylidene fluoride membranes (EMD Millipore).
Then the membranes were blocked with 5% non-fat dry milk for 1 h at
room temperature. After washing, the membranes were incubated with
primary antibodies against FOXO1 (1:1,000; cat. no. 2880) and GAPDH
(1:2,000; cat. no. 5174) from Cell Signaling Technology, Inc. at
4°C overnight and washed with 0.1% Tris-Buffered Saline with Tween
20, followed by incubation with horseradish peroxidase conjugated
secondary antibodies (1:3,000; cat. no. ab214880; Abcam) for 1 h at
room temperature. Blots were developed using an enhanced
chemiluminescence film kit (Perkin Elmer, Inc.) and the images were
analyzed using ImageJ software (version 1.8.0; National Institutes
of Health). All experiments were repeated three times
independently.
Dual luciferase reporter assays
The online bioinformatics tool, TargetScan
(http://www.targetscan.org/vert_71/),
was used to predict the possible binding targets of miR-370 and
FOXO1. A fragment of the FOXO1-3′ untranslated region (UTR)
containing the miR-370 predicted seed region (wild-type; WT) was
amplified from the cDNA of cells and inserted into pGL-3 plasmids
(Promega Corporation). The H9C2 cells were co-transfected with the
WT 3′UTR of FOXO1 containing the miR-370 binding sites and mutant
FOXO1 3′UTR with NC mimics or miR-370 mimics using Lipofectamine
2000™ (Thermo Fisher Scientific, Inc.). Luciferase activity was
measured by the Dual Luciferase Reporter Assay system (Promega
Corporation), normalizing to Renilla luciferase activity.
All experiments were repeated three times independently.
Statistical analysis
All data were presented as the mean ± standard
deviation. SPSS v20.0 software (IBM Corp.) was used to analyze the
data. Additionally, a Student's t-test was used to determine
differences between two groups, while one-way analysis of variance
followed by the Dunnett's post hoc test were used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell viability and the expression of
miR-370 are inhibited by H2O2 in
cardiomyocytes
The H9C2 cells were treated with
H2O2 at different concentrations (0, 50, 100
and 200 µmol/l) for 4 h. Then, the cell viability was detected
using a CCK-8 assay. As shown in Fig.
1, when compared with the control (0 µmol/l
H2O2) group, the activity of H9C2 cells
treated with oxidative stress was concentration dependent and cell
viability significantly decreased with the increasing concentration
(P<0.01). A dose of 100 µmol/l (cell viability, 50%) was
selected for subsequent experiments.
miR-370 overexpression attenuates
H2O2-induced oxidative stress and apoptosis
in H9C2 cells
The H2O2-induced H9C2 cells
were transfected with the miR-370 mimic or NC mimic vectors.
Subsequently, RT-qPCR was performed to detect the expression levels
of miR-370. As shown in Fig. 2A, the
expression of miR-370 was significantly decreased in the
H2O2-induced H9C2 cells when compared with
the normal H9C2 cells (P<0.001); however, the expression of
miR-370 was significantly increased in H9C2 cells transfected with
miR-370 mimic when compared with the NC mimic group (P<0.001;
Fig. 2A). The results indicated that
miR-370 expression could be inhibited by H2O2
in H9C2 cells, which in turn suggested that miR-370 may be
downregulated in myocardial cells with ischemia and hypoxia.
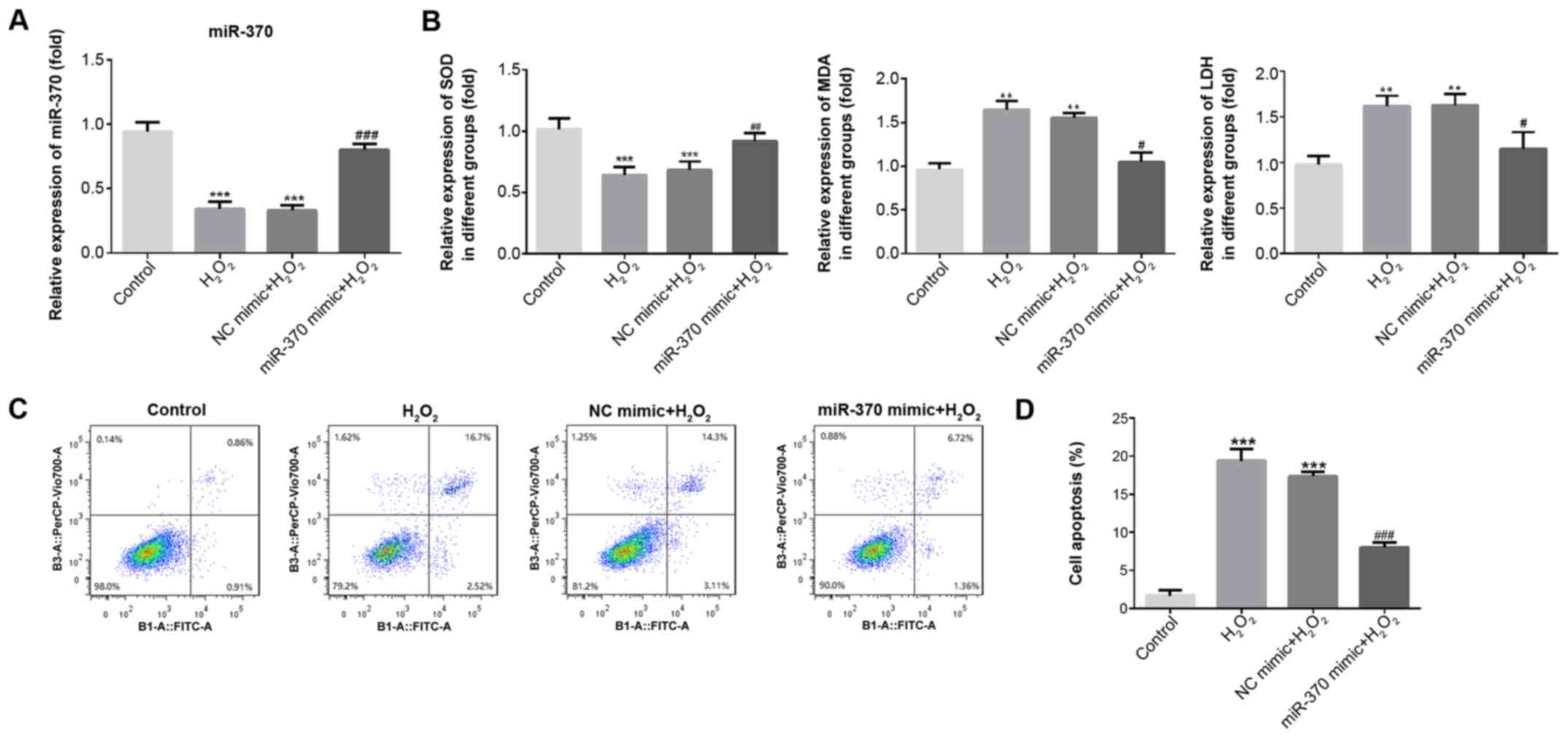 | Figure 2.miR-370 overexpression attenuates
H2O2-induced oxidative stress and apoptosis
in H9C2 cells. (A) The expression of miR-370 was detected by
reverse transcription-quantitative PCR upon transfection of
H2O2-induced H9C2 cells with NC and miRNA-370
mimics. (B) The activity of SOD, LDH and MDA in
H2O2-induced H9C2 cells was determined by
ELISA. (C) After transfection with miR-370 or NC mimics, H9C2 cells
were determined by Annexin-V-FITC/propidium iodide staining. (D)
Percentage of apoptotic cell death. Three independent experiments
were carried out. Error bars represent the mean ± standard
deviation of at least three independent experiments. **P<0.01
and ***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2 group. H2O2,
hydrogen peroxide; miR-370, microRNA-370; NC, negative control;
SOD, superoxide dismutase; LDH, lactate dehydrogenase; MDA,
malondialdehyde; FITC, fluorescein isothiocyanate. |
To verify miR-370 function in oxidative stress and
apoptosis, an ELISA was used to detect SOD, MDA and LDH levels in
the supernatant, and a flow cytometry assay was used to detect
apoptosis. As exhibited in Fig. 2B,
when compared with the control group, SOD expression significantly
decreased (P<0.001) and MDA and LDH expression significantly
increased in the H2O2 and NC mimic groups
(P<0.01). Compared with the H2O2 and NC
mimic groups, SOD expression increased, and MDA and LDH expression
reduced in the miR-370 mimics group. Furthermore, the results of
flow cytometry revealed that the rate of apoptosis was
significantly increased in the H2O2 and NC
mimic groups when compared with the control group (P<0.001;
Fig. 2C and D). Compared with the
H2O2 and NC mimic groups, the rate of
apoptosis was decreased in the miR-370 mimics group. These findings
suggested that the overexpression of miR-370 could markedly
suppress the oxidative stress and apoptosis induced by
H2O2.
Luciferase reporter assay for target
verification
The results of the online bioinformatics tool
TargetScan suggested that FOXO1 was predicted to be a possible
target gene of miR-370. A luciferase reporter assay was conducted
to verify this prediction. As shown in Fig. 3, the 3′-UTR of the gene FOXO1 was
shown to contain the binding sequences for miR-370, suggesting that
FOXO1 may be a downstream target gene of miR-370. Luciferase
activity was significantly reduced in the FOXO1 3′UTR WT group
transfected with miR-370-3p mimics (P<0.001), whereas there was
no variation in the mutant-type FOXO1 3′UTR, therefore also
suggesting that FOXO1 may be a direct target gene of miR-370.
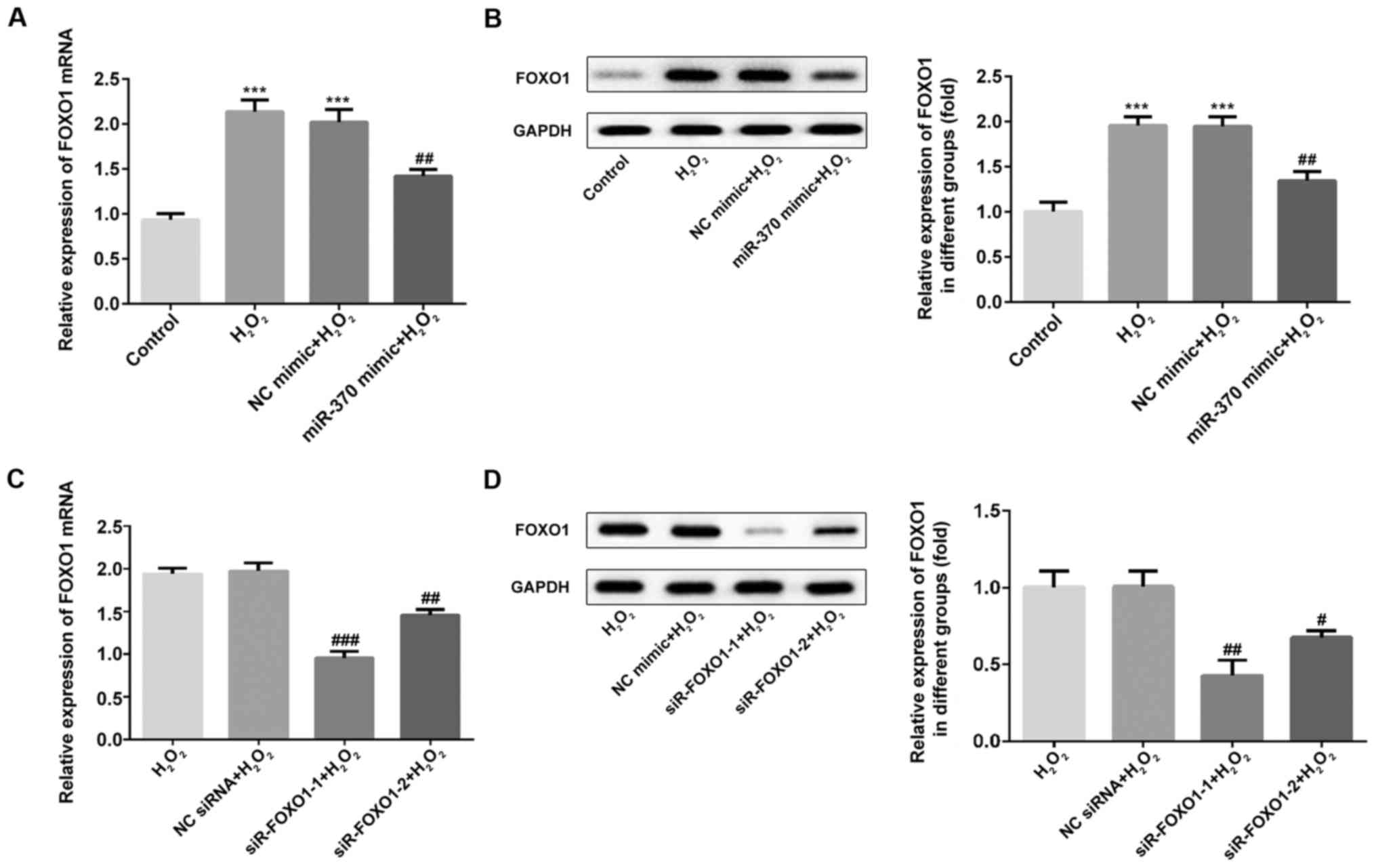
FOXO1 expression is increased in
H2O2-induced H9C2 cells
The mRNA and protein levels of FOXO1 were measured
by RT-qPCR and western blotting assay. As shown in Fig. 4A and B, the expression of FOXO1 was
markedly increased in the H2O2 and NC mimic
groups when compared with the control group. Compared with the
H2O2 and NC mimic groups, FOXO1 expression
was decreased in the miR-370 mimic group. Then, the interference
efficiency of FOXO1 siRNA plasmids was detected using RT-qPCR and
western blot assays. As shown in Fig. 4C
and D, the expression of FOXO1 was significantly reduced in the
siR-FOXO-1 and siR-FOXO1-2 groups when compared with the NC siRNA
group (P<0.001). The siR-FOXO1-1 plasmid with the best
interference effects was selected for subsequent experiments.
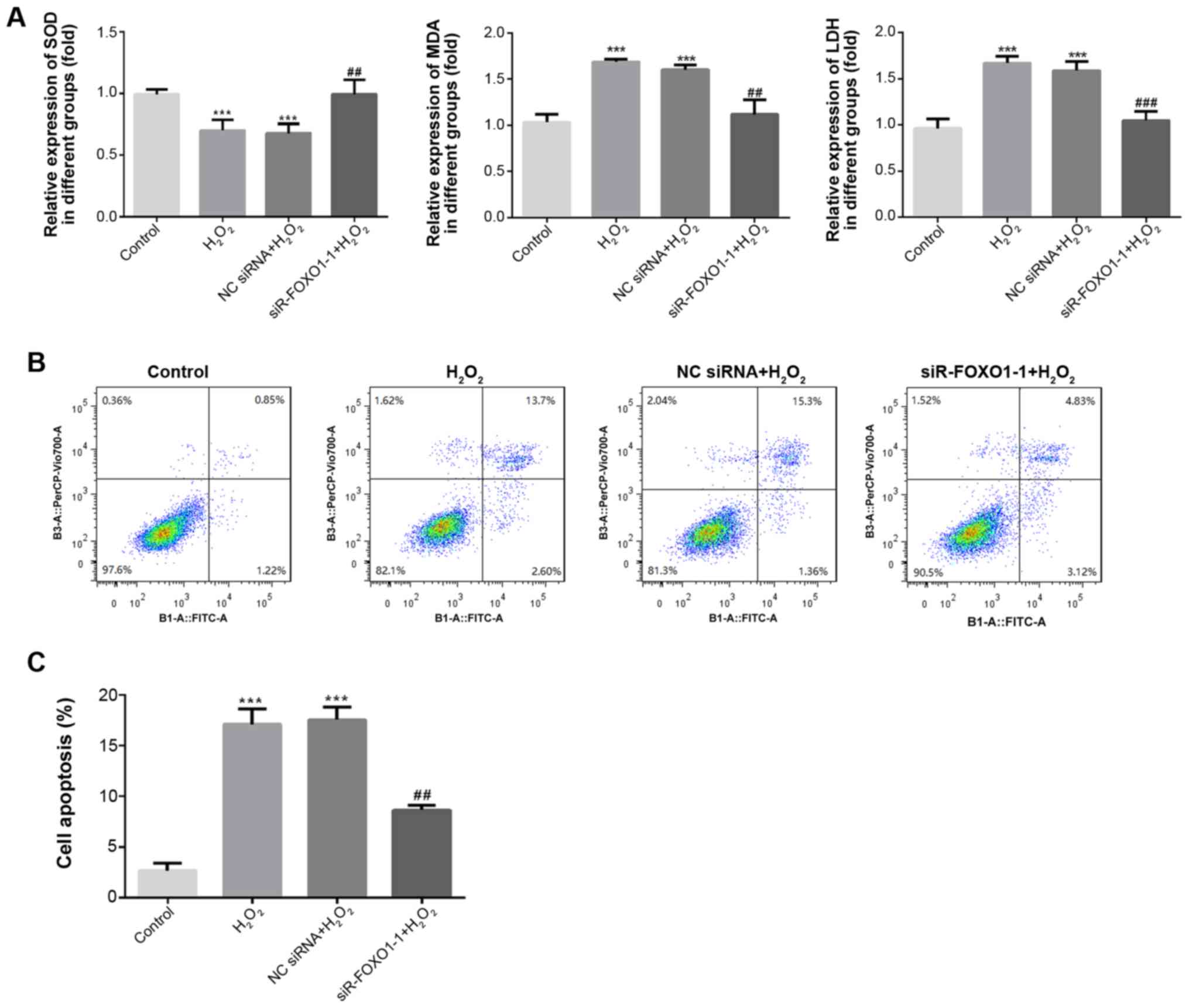
FOXO1 silencing inhibits oxidative
stress and apoptosis in H2O2-induced H9C2
cells
ELISA was used to detect the levels of SOD, MDA and
LDH in the supernatant, and a flow cytometry assay was used to
detect apoptosis in H2O2-induced H9C2 cells.
As shown in Fig. 5A, compared with
the control group, the level of SOD significantly decreased
(P<0.001) and the levels of MDA and LDH were significantly
increased in the H2O2 and NC siRNA groups
(P<0.001). However, compared with the H2O2
and NC siRNA groups, SOD expression significantly increased
(P<0.01), and MDA and LDH expression significantly decreased in
the siR-FOXO1-1 group (P<0.01). The results of flow cytometry
revealed that the rate of apoptosis was significantly increased in
the H2O2 and NC siRNA groups when compared
with the control group (P<0.001; Fig.
5B and C). In addition, compared with the
H2O2 and NC siRNA groups, the rate of
apoptosis was significantly decreased in the siR-FOXO1-1 group
(P<0.01). These results suggested that knockdown of FOXO1 could
markedly suppress the oxidative stress and apoptosis of H9C2 cells
induced by H2O2.
Discussion
The results of the present study revealed that the
expression of miR-370 was decreased in
H2O2-induced H9C2 cells. In addition, miR-370
overexpression attenuated H2O2-induced
oxidative stress and apoptosis in H9C2 cells. Nevertheless, the
underlying mechanism of miR-370 in ischemic heart disease remains
largely unknown. In vitro experiments are required to
further explore the effect of miR-370, in order to provide the
theoretical basis for the research.
After maturation, miRNA can regulate gene expression
via complementation with target mRNA and regulating cell
differentiation proliferation, metabolism, and apoptosis (23–26). It
has been indicated that miR-370 is involved in various types of
heart diseases (27,28). A number of studies have published
their results on miR-370 in cardiovascular diseases; however, the
results are controversial. A previous study reported that miR-370
was significantly higher in patients with coronary artery disease
(29). Additionally, miR-370 was
reported to be upregulated in peripheral blood mononuclear cells of
coronary AS patients (30). By
contrast, miR-370 has also been found to have a positive effect in
different diseases. Consistent with a previous study, the
expression level of miR-370 markedly decreased in AS mouse models
and oxidized low-density lipoprotein incubated THP-1 cells, and
miR-370 overexpression inhibited vascular inflammation and
oxidative stress (19). In
myocardial remodeling, the expression of miR-370 in the infarct
border area was decreased after MI, but myocardial fibrosis was
suppressed through the intervention of miR-370 (20). The positive role of miR-360 is
consistent with the results of the present study, the expression of
miR-370 was markedly decreased in H9C2 cells induced by
H2O2.
In the present study, H2O2 had
proapoptotic effects on H9C2 cells. Hypoxia is the main cause of
ischemic heart disease which leads to autophagy and apoptosis
through the activation of a caspase cascade via the release of
cytochrome c from the mitochondria to the cytoplasm (31). H2O2 activated
oxidative stress through the downregulation of SOD levels and
upregulation of MDA and LDH levels in the present study. In
addition, H2O2 induced apoptosis by
downregulating Bcl-2 and upregulating the expression of
proapoptotic proteins, such as Bax, caspase-8, and
cleaved-caspase-3 (32). Oxidative
stress and apoptosis are involved in cardiovascular diseases. For
example, the overexpression of miRNA-132 increased the
anti-oxidative stress and anti-apoptotic abilities of H9C2 cells in
heart failure (33). The present
study demonstrated that overexpression of miR-370 suppressed the
oxidative stress and apoptosis induced by
H2O2.
FOXO1 is an essential regulator of endothelial cell
proliferation and can be modulated by a number of signaling
pathways (34). In osteoblasts,
FOXO1 expression was increased when induced by
H2O2 (35).
Therefore, the present study speculated that FOXO1 may also be
involved in the apoptosis of cardiac myocytes induced by oxidative
stress. TargetScan analysis suggested that transcription factor
FOXO1 was a possible target gene of miR-370. As the results
demonstrated, FOXO1 expression was increased in H9C2 cells induced
by H2O2. Furthermore, FOXO1 silencing
suppressed H2O2-induced oxidative stress and
apoptosis in H9C2 cells. These results indicated that FOXO1 was
involved in H2O2-induced oxidative stress and
apoptosis, which was consistent with the hypothesis of the present
study.
However, some limitations should be considered in
the present study, the H9C2 cells are not mature cardiomyocytes and
only an in vitro model, which does not entirely explain the
results in physiological and pathological processes. Therefore,
future studies will be needed to confirm the present results in IR
with in vivo models.
In conclusion, the results of the present study
indicate that FOXO1 was a target gene of miR-370, and miR-370
reduced H2O2-induced oxidative stress and
apoptosis in H9C2 cells by targeting FOXO1, which provides a
theoretical basis for the treatment of ischemic heart disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong
Medical and Health Science and Technology Development Plan Project
(grant no. 2018WS193), Science and the Technology Development Plan
Project of Jinan (grant no. 201602184), and the Shandong Province
TCM Science and Technology Development Plan (grant no.
2015-091).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
ZJQ, LW, LNW and YGC designed the research. ZJQ, LW,
HYM, FX and BS performed the experiments. ZJQ, LW, XBL, JLW and FK
analyzed the data. ZJQ, LW, HYM and FX drafted the manuscript and
analyzed data. LNW and YGC interpreted data and revised the final
manuscript. ZJQ and LW wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poole-Wilson P: The prevention of
cardiovascular disease worldwide: Whose task and WHO's task? Clini
Med (Lond). 5:379–384. 2005. View Article : Google Scholar
|
|
2
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiu H, Liu JY, Wei D, Li N, Yamoah EN,
Hammock BD and Chiamvimonvat N: Cardiac-generated prostanoids
mediate cardiac myocyte apoptosis after myocardial ischaemia.
Cardiovasc Res. 95:336–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katz AM and Messineo FC: Lipid-membrane
interactions and the pathogenesis of ischemic damage in the
myocardium. Circ Res. 48:1–16. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abbate A and Narula J: Role of apoptosis
in adverse ventricular remodeling. Heart Fail Clin. 8:79–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Navarro-Yepes J, Burns M, Anandhan A,
Khalimonchuk O, del Razo LM, Quintanilla-Vega B, Pappa A,
Panayiotidis MI and Franco R: Oxidative stress, redox signaling,
and autophagy: Cell death versus survival. Antioxid Redox Signal.
21:66–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morales CR, Pedrozo Z, Lavandero S and
Hill JA: Oxidative stress and autophagy in cardiovascular
homeostasis. Antioxid Redox Signal. 20:507–518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogura S and Shimosawa T: Oxidative stress
and organ damages. Curr Hypertens Rep. 16:4522014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farias JG, Molina VM, Carrasco RA, Zepeda
AB, Figueroa E, Letelier P and Castillo RL: Antioxidant therapeutic
strategies for cardiovascular conditions associated with oxidative
stress. Nutrients. 9(pii): E9662017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown DI and Griendling KK: Regulation of
signal transduction by reactive oxygen species in the
cardiovascular system. Circ Res. 116:531–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baek D, Villen J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orenes-Pinero E, Montoro-Garcia S, Patel
JV, Valdes M, Marin F and Lip GY: Role of microRNAs in cardiac
remodelling: New insights and future perspectives. Int J Cardiol.
167:1651–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joladarashi D, Thandavarayan RA, Babu SS
and Krishnamurthy P: Small engine, big power: microRNAs as
regulators of cardiac diseases and regeneration. Int J Mol Sci.
15:15891–8911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen C, Ponnusamy M, Liu C, Gao J, Wang K
and Li P: MicroRNA as a therapeutic target in cardiac remodeling.
Biomed Res Int. 2017:12784362017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XP, Chen YG, Lan JY and Shen ZJ:
MicroRNA-370 suppresses proliferation and promotes endometrioid
ovarian cancer chemosensitivity to cDDP by negatively regulating
ENG. Cancer Lett. 353:201–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng Y, Sun T, Yu Y, Gao Y, Wang X and
Chen Z: MicroRNA-370 inhibits the proliferation, invasion and EMT
of gastric cancer cells by directly targeting PAQR4. J Pharmacol
Sci. 138:96–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian D, Sha Y, Lu JM and Du XJ: MiR-370
inhibits vascular inflammation and oxidative stress triggered by
oxidized low-density lipoprotein through targeting TLR4. J Cell
Biochem. 119:6231–6237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan H and Gao J: The role of miR370 in
fibrosis after myocardial infarction. Mol Med Rep. 15:3041–3027.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H, Gao H, Gao S, Lei Z, Dai L, Wang X,
Han Y, Wang Z and Han L: A Chinese 4-herb formula, Yiqi-Huoxue
granule, alleviates H2O2-induced apoptosis by
upregulating uncoupling protein 2 in H9c2 cells. Phytomedicine.
53:171–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu G, Zhu H, Zhang M and Xu J: Histone
deacetylase 3 is associated with gastric cancer cell growth via the
miR-454-mediated targeting of CHD5. Int J Mol Med. 41:155–163.
2018.PubMed/NCBI
|
|
24
|
Qi R, Huang J, Wang Q, Liu H, Wang R, Wang
J and Yang F: MicroRNA-224-5p regulates adipocyte apoptosis induced
by TNFα via controlling NF-κB activation. J Cell Physiol.
233:1236–1246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, He D, Yue B, Zhang C, Fang X and
Chen H: miR-101-1 expression pattern in Qinchuan cattle and its
role in the regulation of cell differentiation. Gene. 636:64–69.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang K and Guo L: MiR-767 promoted cell
proliferation in human melanoma by suppressing CYLD expression.
Gene. 641:272–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian D, Sha Y, Lu JM and Du XJ: MiR-370
inhibits vascular inflammation and oxidative stress triggered by
oxidized low-density lipoprotein through targeting TLR4. J Cell
Biochem. 119:6231–6237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan H and Gao J: The role of miR-370 in
fibrosis after myocardial infarction. Mol Med Rep. 15:3041–3047.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu H, Yang N, Fei Z, Qiu J, Ma D, Liu X,
Cai G and Li S: Analysis of plasma miR-208a and miR-370 expression
levels for early diagnosis of coronary artery disease. Biomed Rep.
5:332–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoekstra M, van der Lans CA, Halvorsen B,
Gullestad L, Kuiper J, Aukrust P, van Berkel TJ and Biessen EA: The
peripheral blood mononuclear cell microRNA signature of coronary
artery disease. Biochem Biophys Res Commun. 394:792–797. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu J, Chu Z, Han J, Zhang Q, Zhang D, Dang
Y, Ren J, Chan HC, Zhang J and Huang Y: Phosphorylation-dependent
mitochondrial translocation of MAP4 is an early step in
hypoxia-induced apoptosis in cardiomyocytes. Cell Death Dis.
5:e14242014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang H, Li C, Huo K, Wang Q, Lu L, Zhang
Q, Wang Y and Wang W: Luteolin prevents H2O2-induced apoptosis in
H9C2 cells through modulating Akt-P53/Mdm2 signaling pathway.
Biomed Res Int. 2016:51258362016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Tong Z, Chen K, Hu X, Jin H and Hou
M: The role of miRNA-132 against apoptosis and oxidative stress in
heart failure. Biomed Res Int. 2018:34527482018.PubMed/NCBI
|
|
34
|
Wilhelm K, Happel K, Eelen G, Schoors S,
Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger
T, et al: FOXO1 couples metabolic activity and growth state in the
vascular endothelium. Nature. 529:216–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z, Ji G, Wu Q, Feng S, Zhao Y, Cao Z
and Tao C: Integrated microarray meta-analysis identifies miRNA-27a
as an oncogene in ovarian cancer by inhibiting FOXO1. Life Sci.
210:263–270. 2018. View Article : Google Scholar : PubMed/NCBI
|