Introduction
Polycythemia vera (PV) is a myeloproliferative
disease characterized by clonal proliferation of hematopoietic stem
cells, leading to abnormal increases in circulating red blood
cells, white blood cells and platelets, resulting in an increase of
blood viscosity and reduction of blood flow velocity (1). These disorders of hemorheology are all
factors of thrombosis. In addition, high blood cell specific
capacity may lead to vascular endothelial injury, increasing
susceptibility to vascular disease (2–4). The
platelet dysfunction in PV patients is mainly manifested as the
impairment of aggregation function, which leads to hemostasis
disorder, therefore platelet dysfunction is an important factor in
bleeding events (5). High hematocrit
(HCT) in PV patients can quickly lead to vascular endothelial
injury, and the continuous high blood volume may cause the
exudation from local blood vessels (3). Dysfunctions in platelet aggregation and
the prolongation of activated partial prothrombin time (aPPT) may
cause cerebral hemorrhage (6).
Several studies have reported that PV is frequently combined with
cerebral infarction or cerebral hemorrhage (7–9). In the
current study, the authors reported that one patient with acute
cerebral infarction and multiple cerebral microhemorrhage combined
with PV, and analyzed imaging features and the pathogenesis.
Case report
Case presentation
A 60-year-old female patient was admitted to the
department of Neurology Inspection, People's Hospital of Liaoning
Province (Shenyang, China) on the 13th of November 2017. She
complained about sudden impairment of right limb movement for 1 day
and slurred speech for 12 h. This patient had been taking
anti-psychotic drugs, including chlorpromazine 25 mg/day,
alprazolam 0.4 mg/day and trihexyphenidyl 2 mg/day for six months
due to schizophrenia. The patient had poor general self-care
ability due to mental disorders, who was taken care of by her
husband. The patients husband told the authors that she had been
hypertensive for 5 years without any systematic diagnosis and
treatment. In recent years, large ecchymosis frequently appeared in
the limbs after bumping into a table. At six months previously, the
patient was reported to operate slowly, be unresponsive and fall
easily, and had therefore been hospitalized for six months in a
mental health center, and treated with chlorpromazine 25 mg/day,
alprazolam 0.4 mg/day and trihexyphenidyl 2 mg/day, the symptoms
were relieved. A complete routine laboratory blood analysis
revealed that the patient had no abnormalities, e.g.
erythrocytosis, in May 2017. At 1 week prior to admission, the
patient developed epistaxis and visited the otolaryngology
department to stop the bleeding.
Examination
Physical examination revealed that the blood
pressure was 220/120-148-87 mmHg during hospitalization. The
patients consciousness was fluctuating and dominated by fuzzy
states (associated with anti-psychotic drugs). In the awake state,
the speech was slurred, and the skin of the head, face and palm
were dark red. The left nasolabial fold was shallow. The sensation
of pain in left facial was lost. The muscle strength was grade 0 in
the right limbs, grade 3 in the left upper limb and grade 0 in the
lower left limb. The muscular tension did not increase or reduce in
the limbs. The patient had a Babinski sign (L+, R+) and cervical
ankylosis. The lower mandible was three horizontal fingers from the
anterior chest.
Laboratory examination revealed that the complete
blood count and coagulation were obviously abnormal (Table I). Hemoglobin and HCT were elevated
in this patient, and aPPT was prolonged. Through the treatment of
bloodletting and Hydroxycarbamide, the patients HCT reduced a
little and the number of white cell returned to normal. At multiple
time-points, the blood glucose and glycosylated hemoglobin levels
were also abnormal. The maximum blood glucose level reached 19.22
mmol/l. Renal function was abnormal. BUN levels were 13.78 mmol/l,
creatinine levels were 155.4 µmol/l (multiple abnormality) and
potassium levels were 5.68 mmol/l. Regarding blood lipids,
high-density lipoprotein was normal, triglycerides were 2.62
mmol/l, cholesterol was 7.88 mmol/l and low-density lipoprotein was
5.51 mmol/l. Erythropoietin levels were 3.86 mIU/ml (5.4–31). Genes
were not detected. The patient underwent bone marrow puncture and
blood smear examination one week after hospitalization. The results
indicated that granulocytes, erythrocytes and megakaryocytes
proliferated.
 | Table I.Blood routine parameters and
indicators of coagulation function. |
Table I.
Blood routine parameters and
indicators of coagulation function.
|
| RBC
(×1012/l) | Hb (g/l) | HCT (%) | WBC
(×109/l) | PLT
(×109/l) | PT (sec) | aPPT (sec) | FIB (g/l) |
|---|
|
|
|
|---|
| Normal ranges | 3.8–5.1 | 115–150 | 35–45 | 3.5–9.5 | 125–350 | 9–13 | 21.1–36.5 | 1.8–3.5 |
|---|
| Day after
admission |
|
|
|
|
|
|
|
|
| 1 | 7.56 | 231 | 72.7 | 22.18 | 340 | 4.8 | 50.2 | 4.50 |
| 2 | 7.17 | 215 | 71.5 | 18.63 | 279 | 14.3 | 44.1 | 4.26 |
| 3 | 6.57 | 201 | 66.0 | 22.43 | 301 | 13.6 | 42.4 | 3.99 |
| 5 | 7.71 | 231 | 74.3 | 23.26 | 352 | 21.3 | 65.4 | 3.84 |
|
6a | 7.30 | 221 | 68.6 | 24.70 | 349 | 25.0 | 63.5 | 2.49 |
| 9 | 7.40 | 223 | 71.0 | 24.59 | 450 | 29.1 | 56.9 | 4.14 |
| 11a | 7.57 | 228 | 72.5 | 19.97 | 480 | 45.8 | 82.2 | 4.14 |
| 16b | 6.92 | 208 | 63.7 | 14.33 | 327 | 16.2 | 39.4 | 5.28 |
| 20 | 7.34 | 217 | 70.3 | 7.31 | 265 | 16.7 | 56.9 | 4.63 |
Ultrasound of the liver, gallbladder and spleen
revealed fatty liver and splenomegaly (thick diameter, 4.7 cm; long
diameter, 11.4 cm). Ultrasound of the heart indicated concentric
hypertrophy in the left ventricular wall, while no mural thrombosis
or obvious valvular disease were present. Ultrasound of the carotid
artery revealed no stenosis and occlusive changes had occurred in
the extracranial carotid artery, vertebral artery and subclavian
artery. On computed tomography of the head, multiple dot-like and
flaky low-density changes in the left occipital lobe, the site
adjacent to the body of the lateral ventricle, basal ganglia, right
frontal lobe and parietal lobe were observed (Fig. 1).
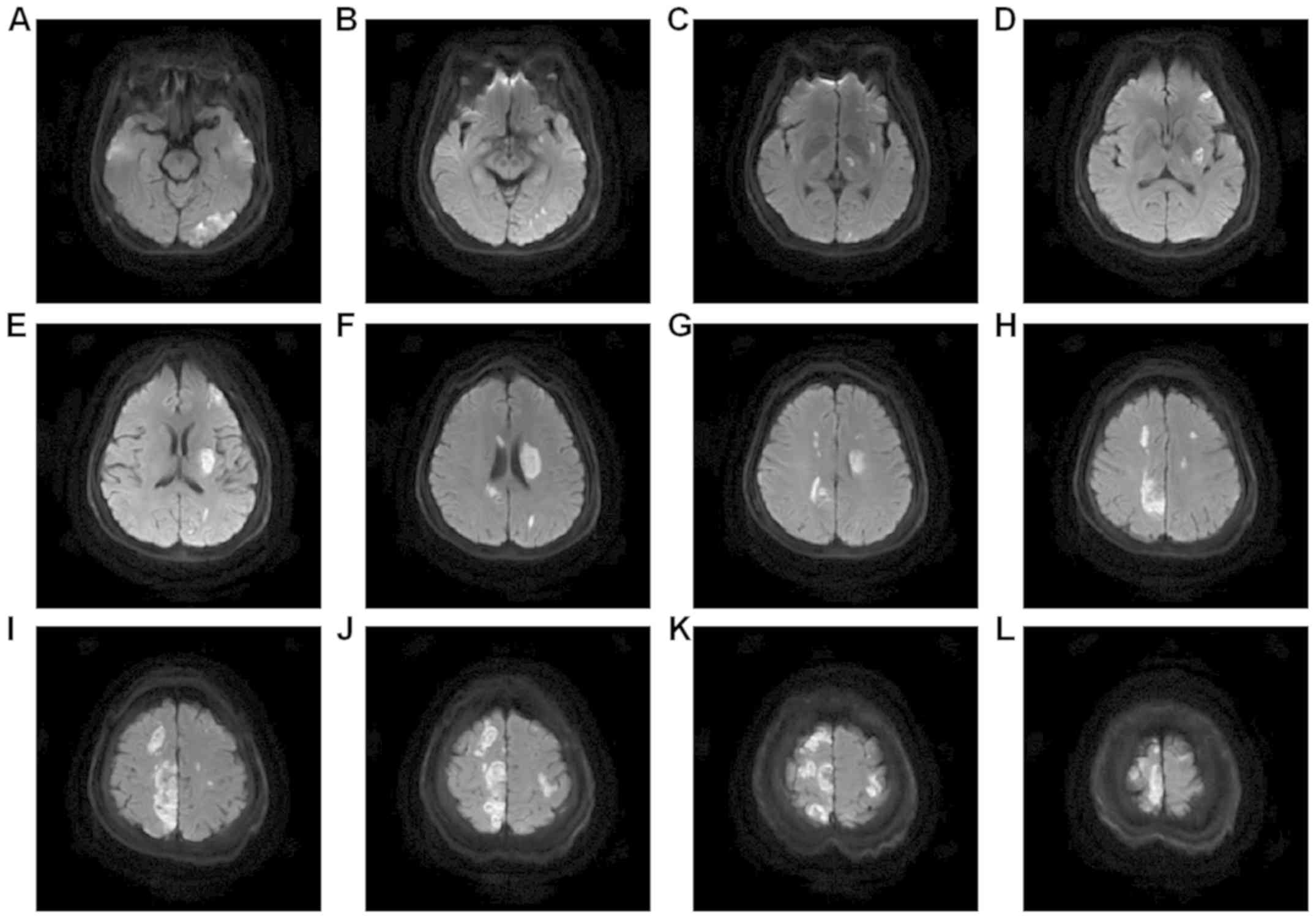
Magnetic resonance images (MRI) of the head were
shown in different figures: Fig. 2
was T1 weight imaging, Fig. 3 was T2
weight imaging, Fig. 4 was
fluid-attenuated inversion recovery, Fig. 5 was diffusion-weighted image,
Fig. 6 was enhanced magnetic
resonance imaging and Fig. 7 was
susceptibility weighted imaging. Fig.
2 showed the mixed signal intensities, mainly low signal
intensity, in the round lesions adjacent to the left body of the
lateral ventricle, in line with the changes in the hemorrhagic
transformation (type HI-1) post-infarction. Fig. 3 showed multiple intracranial cerebral
infarctions, and that round high-signal intensity in the site
adjacent to the left body of the lateral ventricle had low signal
changes, which was in line with the changes in the hemorrhagic
transformation (type HI-1) post infarction. Fig. 4 was fluid-attenuated inversion
recovery, which showed the lesions of the right frontal and
parietal lobes presented as multiple mixed signals. Fig. 5 was diffusion-weighted image, which
showed the acute cerebral infarction, and infarcts with
supratentorial, infratentorial and bilateral multiple high-signal
intensities. The enhanced magnetic resonance imaging in Fig. 6 had a faint dot-like enhanced signal
in the right frontal parietal lobe and the right frontal lobe.
Fig. 7 demonstrated multiple
dot-like, striped and lumpy low-signal changes.
MRI of the head revealed that the left posterior
cerebral artery was occlusive, and that the right anterior cerebral
artery and left vertebral artery were thinned. No distinct flow
void signal was observed in the conventional sequence, suggesting
multiple intracranial microhemorrhage in this area.
Diagnosis
The patient was diagnosed with acute multiple
cerebral infarction combined with cerebral microhemorrhage and
hemorrhagic transformation. Left posterior cerebral artery
occlusion was likely and the right anterior cerebral artery and the
left vertebral artery were thin. The patient was also diagnosed
with PV and splenomegaly, stage 3 hypertension (very high-risk
group), type 2 diabetes and renal insufficiency, abnormal blood
lipids, electrolyte disturbance, and abnormally high potassium.
Treatment
After admission, the patient was treated with 30 mg
nifedipine for hypertension and intravenously administrated with 60
mg furosemide for the abnormally high potassium. The patient was
injected intravenously with 20 ml 10% glucose to treat
hypoglycemia. Due to renal insufficiency in the compensatory
period, no clinical medication was given. Medication was
administrated immediately after admission to the hospital. Blood
pressure was controlled by intravenous injection of urapidil and
sodium nitroprusside. Two or three oral hypertensive medications
were combined and administered at the same time, including
amlodipine besylate, benidipine, irbesartan and metoprolol. The
patient endured the cerebral infarction combined with multiple
cerebral microhemorrhage and hemorrhagic transformation, so that no
anti-thrombotic drugs were administered. Bloodletting therapy was
performed twice, and 380 ml of venous blood was taken out in two
sessions 5 days apart. Due to the increasing erythrocyte count and
platelet count, and prolonged prothrombin time and activated
partial thromboplastin time, stimulation of bone marrow hyperplasia
after bloodletting could not be excluded. Thus, the bloodletting
therapy was terminated. Hydroxycarbamide was orally administered
for 3 days. Conventional blood analysis indicated an obvious
improvement. Furthermore, the skin color of the head, face and both
hands tended to be more like the normal color than before and
stable. The blood pressure and the intracranial condition were
stable. At 13 days after admission, the patient was administered
hydroxycarbamide (10 mg/kg/day), leading to a reduction in the red
blood cell count and inhibition of the hematopoietic ability of the
bone marrow, which is a typical effect of hydroxycarbamide
treatment. The patients consciousness changed from fuzzy to awake;
the condition was stable and the patient was discharged. The
patient was prescribed 30 mg of the anti-hypertensive drug
nifedipine sustained release tablets per day after discharge, and
the blood pressure was controlled within the normal range. The
patient did not receive any anti-coagulant or anti-platelet
therapy.
Discussion
PV is a myeloproliferative neoplasm characterized by
clonal erythrocytosis (1). PV is
characterized by amplification of one or more blood cell lineages,
resulting in increased mature blood components in the peripheral
blood, including erythrocytosis, leukocytosis and/or thrombocytosis
(1). According to the diagnostic
criteria set by the World Health Organization in 2016, the
diagnosis of PV is mainly based on a comprehensive assessment of
clinical and laboratory characteristics (10). Hemoglobin and HCT were elevated in
this patient. Bone marrow aspiration and blood smear demonstrated
the proliferation of granulocytes, erythrocytes and megakaryocytes,
and reduction of serum erythropoietin. Various laboratory and
imaging examinations excluded other causes for polycythemia, so the
diagnosis of PV was confirmed.
PV may include unique thrombosis and hemorrhage
(5,11). The incidence of hemorrhage was
reported to be 2–20% (12–15). Hemorrhage is usually observed in the
skin mucosa, and epistaxis, gingival bleeding and gastrointestinal
bleeding may also occur (16).
Massive epistaxis had occurred in the patient of the present study
at one week prior to admission. In recent years, unexplained
bruising has frequently occur in the patient, which may be
associated with PV. However, there was no abnormality in blood
tests performed six months previously, which may indicate an
insidious onset of PV. The incidence of thrombotic complications in
PV patients was reported to be 12–39% (5,12,17).
Thrombosis is commonly detected in the extremities, mesentery,
cerebral blood vessels and coronary arteries. In the patient of the
present study, cerebral blood vessels were mainly involved.
According to MRI of the head, this patient was diagnosed with acute
multiple cerebral infarction combined with multiple cerebral
microhemorrhage and hemorrhagic transformation.
According to a previous study, PV was mainly
combined with bilateral multiple cerebral infarction, mainly in the
basal ganglia, thalamus, coronaradiate and subcortical lobe, but
large-area cerebral infarction was rare (17). In the current study, the patient
hemorrhaged in the hypothalamus, base arum and cerebral; the
patient also exhibited cerebral microhemorrhage, which has not yet
been reported, to the best of our knowledge.
There are numerous mechanisms for ischemic events in
PV patients. Leukocytosis and erythrocytosis are risk factors for
thrombosis (14–16). Excessive erythrocytosis may
significantly increase the HCT and blood viscosity and reduce the
cerebral blood flow velocity (15).
Theses hemorheologic changes are all thrombophilic factors.
Furthermore, chronic excess red blood cells and persistent high HCT
may cause vascular endothelial damage, similar to an inflammatory
response process that ultimately increases the susceptibility to
vascular disease (16). In this
case, the erythrocyte HCT and white blood cells of the patient were
significantly increased, which are risk factors for thrombosis. An
important factor associated with bleeding events in PV patients is
abnormal in platelet function, which is also an important cause of
clinical manifestations of hemorrhage and ischemia in PV patients.
Platelet dysfunction in PV patients is mainly manifested in
impaired aggregation function, including reduced reactivity to
adrenaline and ADP14, resulting in disorders of hemostasis and
prolonged aPPT. High HCT in PV patients easily causes vascular
endothelial injury, and a constantly high blood volume causes local
oozing of blood vessels, defects in platelet aggregation function
and prolonged aPPT, which are all possible causes of the brain
microbleeds in the patient of the present study. In addition,
platelet dysfunction may also manifest as spontaneous aggregation
(17), suggesting a mechanism for
regulating platelet activation. This aggravation of hypofunction
and spontaneous platelet aggregation frequently occur in the same
patient, having a role in disease-associated processes.
The effects of atherosclerosis-associated risk
factors on the risk or condition of thromboembolic disease in PV
patients are not sufficiently outlined in current guidelines.
Hypertension, hyperlipidemia, diabetes and congestive heart failure
are risk factors for embolic events in PV patients, and it is
suggested that these risk factors should be controlled (18). In the patient of the present study,
granulocytes, erythrocytes and megakaryocytes had obviously
proliferated, and there were further risk factors for multiple
stroke, which increased the chance of occurrence of thrombotic
diseases. The blood pressure was high and difficult to control,
which was associated with the increased blood volume due to
increased blood constituents caused by PV. The pathogenesis of
cerebral microhemorrhage and hemorrhagic transformation was
considered to be associated with abnormal coagulation function and
persistent and obvious hypertension, which further increased the
risk of bleeding.
The patient had no history of heart disease. There
was no arrhythmia or heart valve disease, including atrial
fibrillation after continuous electrocardiogram (ECG) monitoring,
repeated ECG and echocardiography. The presence of multiple infarct
foci did not support the diagnosis of cardiogenic brain embolism.
However, the patient suffered from multiple intracranial lesions
and hemorrhagic transformation, and it was important to identify
those. MRI indicated that the case was combined with anterior
cerebral artery stenosis, which should be distinguished from
atherosclerotic cerebral infarction. However, the infarcts were
supratentorial, infratentorial, bilateral and multiple; the infarct
focus in the same artery-dominated region was also multiple, which
does not conform to the characteristics of atherosclerotic cerebral
infarction. Taken together, only cerebral venous system involvement
cannot fully explain the mechanism of infarction, but it does not
exclude the possibility of simultaneous involvement of the cerebral
arteries and cortical veins.
In the present case, the erythrocyte, leukocyte and
platelet levels were obviously increased, and the blood fluidity
was significantly reduced, leading to the hypercoagulable state of
the blood. Although an increase in platelets was obvious, the
platelet function was defective, and the PT and the aPPT were
prolonged. It was suggested that there is a risk of thrombotic
disease as well as a tendency to bleed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during the current
study are included in this published article.
Authors contributions
All the authors analyzed the case and data, and
wrote the paper. LL was responsible for the treatment of the
patient and collecting patient clinical data. XJ and XHC were in
charge of analyzing and interpreting the patient data. DL performed
the magnetic resonance examination of brain and provided MRI
reports. NW collected patient clinical data and was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The patients signed the informed consent form.
Patient consent for publication
Written informed consent was obtained from the
patient prior to participation in the present case report and the
patient consented to publication of images.
Competing interests
The authors declare that they have no competing
interests regarding the publication of this article.
References
|
1
|
Spivak JL: Polycythemia Vera. Curr Treat
Options Oncol. 19:122018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landolfi R, Di Gennaro L, Barbui T, De
Stefano V, Finazzi G, Marfisi R, Tognoni G and Marchioli R;
European Collaboration on Low-Dose Aspirin in Polycythemia Vera
(ECLAP), : Leukocytosis as a major thrombotic risk factor in
patients with polycythemia vera. Blood. 109:2446–2452. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim Y, Lee JO, Kim SH, Kim JW, Kim YJ, Lee
KW, Lee JS and Bang SM: Prediction of thrombotic and hemorrhagic
events during polycythemia vera or essential thrombocythemia based
on leukocyte burden. Thromb Res. 135:846–851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marchioli R, Finazzi G, Specchia G,
Cacciola R, Cavazzina R, Cilloni D, De Stefano V, Elli E, Iurlo A,
Latagliata R, et al: Cardiovascular events and intensity of
treatment in polycythemia vera. N Engl J Med. 368:22–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falanga A and Marchetti M: Thrombotic
disease in the myeloproliferative neoplasms. Hematology Am Soc
Hematol Educ Program. 2012:571–581. 2012.PubMed/NCBI
|
|
6
|
Zhang H, Sun L, Tang Y, Wang M and Wu J:
Effects of Naomaitai Capsule on cerebral blood flow and apoptosis
of hippocampus neuron in rats with vascular dementia induced by
chronic forebrain ischemia. J Int Neurol Neurosurgery. 36:290–293.
2009.
|
|
7
|
Arboix A, Besses C, Massons J and Titus F:
Cerebral infarction as the first manifestation of polycythemia
vera. Med Clin (Barc). 101:398–399. 1993.(In Spanish). PubMed/NCBI
|
|
8
|
Yazdi R and Côté C: Watershed infarction
in a case of polycythemia vera. Clin Nucl Med. 11:665–666. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xin CH, Xu JQ, Sui JR and Wang XL:
Analysis on 71 patients with polycythemia vera. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 20:667–670. 2012.PubMed/NCBI
|
|
10
|
Zou JM, Pan ZJ and Wang SL:
Pharmacodynamic and toxicologic research on nao mai kang capsule.
China J Traditional Chin Med Pharm. 18:408–413. 2003.
|
|
11
|
Zhang HN, Sun L, Tang YY, Wang MY and Wu
J: Effects of Naomaitai Capsule on cerebral blood flow and
apoptosis of hippocampus neuron in rats with vascular dementia
induced by chronic forebrain ischemia. J Int Neurol Neurosurgery.
36:290–293. 2009.
|
|
12
|
Elliott MA and Tefferi A: Thrombosis and
haemorrhage in polycythaemia vera and essential thrombocythaemia.
Br J Haematol. 128:275–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kander EM, Raza S, Zhou Z, Gao J, Zakarija
A, McMahon BJ and Stein BL: Bleeding complications in BCR-ABL
negative myeloproliferative neoplasms: Prevalence, type, and risk
factors in a single-center cohort. Int J Hematol. 102:587–593.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barbui T, Carobbio A, Rumi E, Finazzi G,
Gisslinger H, Rodeghiero F, Randi ML, Rambaldi A, Gisslinger B,
Pieri L, et al: In contemporary patients with polycythemia vera,
rates of thrombosis and risk factors delineate a new clinical
epidemiology. Blood. 124:3021–3023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marchioli R, Finazzi G, Landolfi R, Kutti
J, Gisslinger H, Patrono C, Marilus R, Villegas A, Tognoni G and
Barbui T: Vascular and neoplastic risk in a large cohort of
patients with polycythemia vera. J Clin Oncol. 23:2224–2232. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landolfi R, Cipriani MC and Novarese L:
Thrombosis and bleeding in polycythemia vera and essential
thrombocythemia: Pathogenetic mechanisms and prevention. Best Pract
Res Clin Haematol. 19:617–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tefferi A: Polycythemia vera and essential
thrombocythemia: 2012 update on diagnosis, risk stratification, and
management. Am J Hematol. 87:285–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cerquozzi S, Barraco D, Lasho T, Finke C,
Hanson CA, Ketterling RP, Pardanani A, Gangat N and Tefferi A: Risk
factors for arterial versus venous thrombosis in polycythemia vera:
A single center experience in 587 patients. Blood Cancer J.
7:6622017. View Article : Google Scholar : PubMed/NCBI
|