Introduction
In spinal cord injury (SCI), in addition to direct
injury caused by primary trauma, the secondary pathological changes
continue to expand around it; these include ischemia, free radical
damage, inflammatory response, excitotoxicity, neuronal
degeneration and necrosis, as well as apoptosis (1). Lipid peroxidation is one of the
earliest biochemical changes after SCI, which is a continuation of
free radical damage, and it is considered to be an important factor
leading to the exacerbation of the original injury area. The
inflammatory response acts as double-edged sword in
SCI-inflammatory cells are extensively accumulated in the early
stage, and secrete a large number of toxic cytokines and free
radicals so as to aggravate SCI, while protective cytokines are
synthesized in the late stage (2,3).
SCI may arise from high falls and traffic accidents,
and is common in the clinic (4).
After SCI, primary injury and secondary injury (including
apoptosis) of spinal cord neurons are the main causes of spinal
nerve dysfunction, but the secondary injury of neurons may have
more serious consequences (5). After
injury, the neuron has a relatively low capacity to undergo
self-repair and regeneration, the regulation of which requires the
participation of numerous genes, including nerve growth factor and
apoptosis-inhibiting genes (6). The
initiation of these genes requires signal transduction through the
associated pathways (3).
Forkhead box (FOX)O transcription factors are
regulated by a variety of external stimuli, including insulin,
insulin-like growth factor I, nutritional status, cytokines and
stress (7). These external factors
regulate the subcellular localization, DNA binding properties,
protein levels and transcriptional activity of FOXO through the
complex combination of modification and translation of FOXO,
including phosphorylation, acetylation and methylation (8). FOXO has been proved to be involved in
protein degradation and synthesis, which also take part in the
regulation of skeletal muscle growth and development (9).
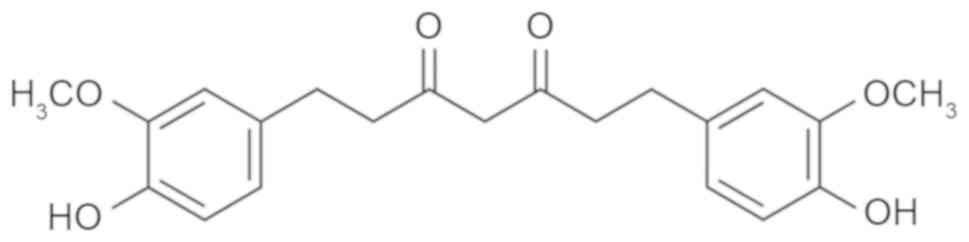
Tetrahydrocurcumin (Fig.
1), the most active and major metabolite of curcumin in
vivo, may be isolated from the cytoplasm of the small intestine
and liver after the administration of curcumin to humans or mice
(10). It inhibits tyrosinase and
the formation of oxygen free radicals, and removes already formed
free radicals, thereby exerting a significant antioxidant effect;
it has therefore been employed as a natural functional whitening
raw material for cosmetic research and development (11). Tetrahydrocurcumin has a
pharmacological effect similar to that of curcumin (11). To date, tetrahydrocurcumin has been
reported to have various pharmacological activities, including
anti-metastasis, anticancer, antioxidant, free radical scavenging,
hypoglycemic and hypolipidemic effects (12,13). The
present study assessed the potential neuroprotective effects of
tetrahydrocurcumin in a rat model of SCI and investigated the
underlying mechanisms.
Materials and methods
Animals and establishment of the SCI
model
Male Sprague Dawley rats (weight, 180–230 g; age,
8–10 weeks) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd (Beijing, China) and kept under standard
housing conditions (temperature, 22–23°C; humidity, 55–65%; 12-h
light/dark cycle). Food and water were provided ad libitum.
The rats were randomly assigned to one of three groups (n=8 in
each): Sham-control group, SCI group and tetrahydrocurcumin
treatment group. In brief, the rats were anesthetized with sodium
pentobarbital (30 mg/kg body weight, i.p.). For the SCI and
tetrahydrocurcumin treatment groups, a dorsal laminectomy was
performed at the 8th thoracic vertebra level to expose the spinal
cord. Subsequently, T6 and T10 were clamped to secure the vertebral
column and a hammer was dropped to produce a moderate SCI model.
Following aneasthetisation, rats in the sham-control group were
treated with 100 µl normal saline. Following SCI and wound
suturing, the injured rats were treated with 100 µl normal saline
or tetrahydrocurcumin (80 mg/kg/day, 2 weeks, i.p.) in the SCI and
tetrahydrocurcumin treatment groups, respectively. All animal
experiments were approved by the Ethics Committee of The 309th
Hospital of The People's Liberation Army (Beijing, China).
Analysis of rat behavior and water
accumulation
After treatment with tetrahydrocurcumin, the motor
function of the rats was evaluated according to the Basso, Beattie
and Bresnahan (BBB) scale (0–21 points) at week 1 and 2. Rats were
allowed to freely walk around for 4 min in an open field, and the
movements of the hindlimbs were observed and scored (14). Then, the spinal cord tissue was
collected and weighed; this was termed the wet weight. Spinal cord
tissue was dried at 80°C for 48 h and weighed again; this was term
the dry weight. Water accumulation was calculated as (dry
weight/wet weight) ×100%.
Analysis of inflammation and oxidative
stress
Following tetrahydrocurcumin treatment whole blood
was collected and centrifugation at 2,000 × g at 4°C for 10 min to
obtain serum, the serum was then stored at −80°C for analysis.
(NF)-κB p65 (cat. no. H202), tumor necrosis factor (TNF)-α (cat.
no. H052), interleukin (IL)-1β (cat. no. H002), IL-6 (cat. no.
H007) and malondialdehyde (MDA; cat. no. A003-1) levels, as well as
superoxide dismutase (SOD; cat. no. A001-1-1), glutathione (GSH;
cat. no. A006-2) and GSH peroxidase (GSH-PX; cat. no. A005)
activity were measured using ELISA kits (Nanjing Jiancheng Biology
Engineering Institute, Nanjing, China).
Western blot analysis
Spinal cord tissue samples were placed in ice-cold
saline and homogenized with radioimmunoprecipitation assay buffer
for 30 min. The supernatant was collected after centrifugation at
15,000 × g for 10 min at 4°C and the protein concentration was
determined using a BSA assay (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Protein (50 µg/lane) was loaded onto a 10% SDS-PAGE gel
for electrophoresis, and then transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 5% non-fat powdered milk in Tris-buffered
saline containing Tween-20 (TBST) for 1 h at 37°C and incubated
with antibodies to B-cell lymphoma 2 (Bcl-2)-associated X protein
(Bax; cat. no. sc-6236; 1:1,000), matrix metalloproteinase (MMP)-3
(cat. no. 14351; 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), MMP-13 (cat. no. sc-30073; 1:1,000), cyclooxygenase
(COX)-2 (cat. no. sc-7951; 1:1,000), phosphorylated (p)-Akt (cat.
no. sc-7985-R; 1:2,000), FOXO4 (cat. no. sc-373877; 1:1,000) and
GAPDH (cat. no. sc-25778; 1:5,000; all Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) overnight at 4°C. The membrane was washed
with TBST and then incubated with horseradish peroxidase-conjugated
goat anti-mouse or anti-rabbit immunoglobulin G (cat. nos. sc-2005
and sc-2004, respectively; 1:5,000; Santa Cruz Biotechnology, Inc.)
as the secondary antibody at room temperature for 1 h. Protein
bands was observed using BeyoECL Moon (Beyotime Institute of
Biotechnology, Haimen, China) and analyzed using Image Lab™
software (version 3.0; Bio-Rad Laboratories, Inc.).
Caspase-3 activity
Spinal cord tissue samples were placed in ice-cold
saline and homogenized with radioimmunoprecipitation assay buffer
for 30 min. The supernatant was collected after centrifugation at
15,000 × g for 10 min at 4°C and the protein concentration was
determined using a Bio-Rad kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Protein (10 µg) was incubated with a caspase-3
activity kit (cat. no. C1116; Beyotime Institute of Biotechnology).
Caspase-3 activity was measured in a spectrophotometer at 405
nm.
Statistical analysis
Values are expressed as the mean ± standard
deviation using SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA). Data were analyzed using one-way analysis of variance
followed by Dunnett's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
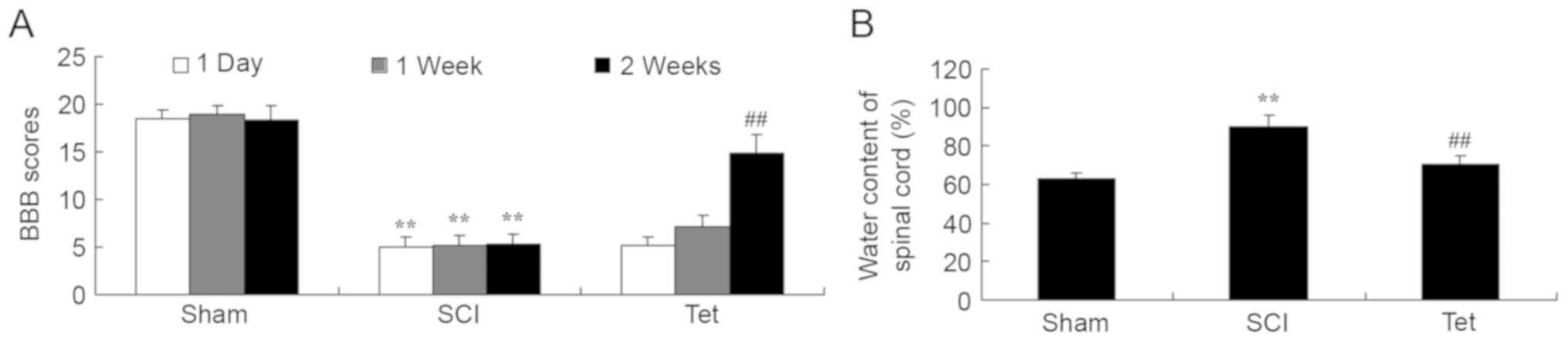
Tetrahydrocurcumin enhances BBB scores
and reduces the water content in the spinal cord of SCI rats
Compared with the control group, a significant
reduction in BBB scores at all time points and an increase in the
water content in the spinal cord was observed in SCI rats (Fig. 2). Administration of
tetrahydrocurcumin to SCI rats resulted in a significant increase
of BBB scores at week 2 and inhibition of water accumulation in the
spinal cord compared with that in the SCI model group (Fig. 2).
Tetrahydrocurcumin inhibits
inflammation in SCI rats
ELISAs indicated that the serum levels of p65 of
NF-κB, TNF-α, IL-1β and IL-6 levels were notably enhanced in the
SCI model group compared with those in the control group (Fig. 3). Treatment of SCI rats with
tetrahydrocurcumin significantly inhibited p65 of NF-κB, TNF-α,
IL-1β and IL-6 levels (Fig. 3).
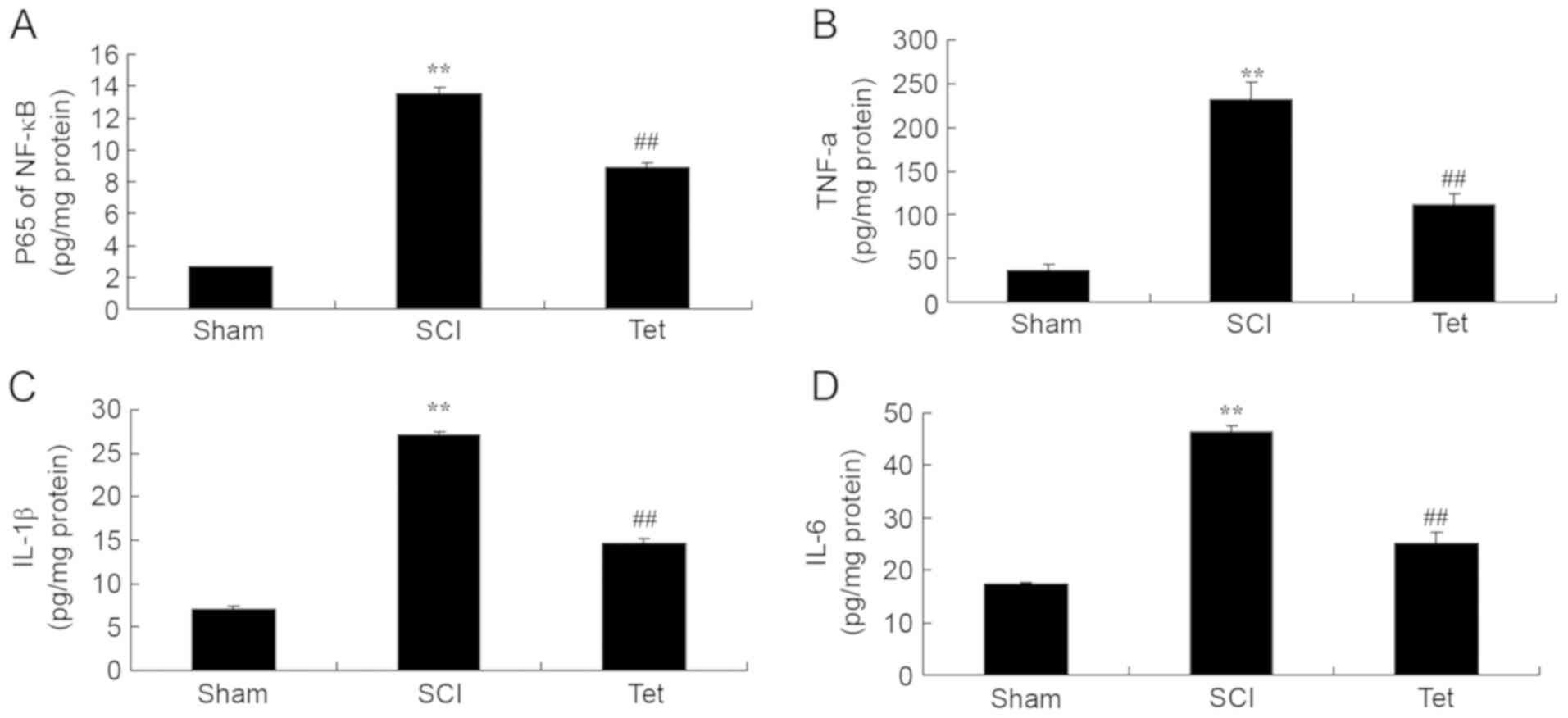 | Figure 3.Tetrahydrocurcumin inhibits
inflammation in SCI rats. Tetrahydrocurcumin inhibits (A) NF-κB
p65, (B) TNF-α, (C) IL-1β and (D) IL-6 in SCI rats. **P<0.01
compared with sham control group, ##P<0.01 compared
with SCI model group. Groups: Sham, sham control group; SCI, SCI
model group; Tet, tetrahydrocurcumin treatment group; SCI, spinal
cord injury; NF, nuclear factor; TNF, tumor necrosis factor; IL,
interleukin. |
Tetrahydrocurcumin inhibits oxidative
stress in SCI rats
As presented in Fig.
4, overproduction of MDA, as well as inhibition of SOD, GSH and
GSH-PX activity were observed in SCI rats compared with the control
group (Fig. 4). Administration of
tetrahydrocurcumin to SCI rats significantly decreased MDA levels,
and promoted the activity of SOD, GSH and GSH-PX (Fig. 4).
 | Figure 4.Tetrahydrocurcumin inhibits oxidative
stress in SCI rats. Tetrahydrocurcumin inhibits (A) MDA, (B) SOD,
(C) GSH and (D) GSH-PX in SCI rats. **P<0.01 compared with sham
control group, ##P<0.01 compared with SCI model
group. Groups: Sham, sham control group; SCI, SCI model group; Tet,
tetrahydrocurcumin treatment group; SCI, spinal cord injury; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH, glutathione;
GSH-PX, GSH peroxidase. |
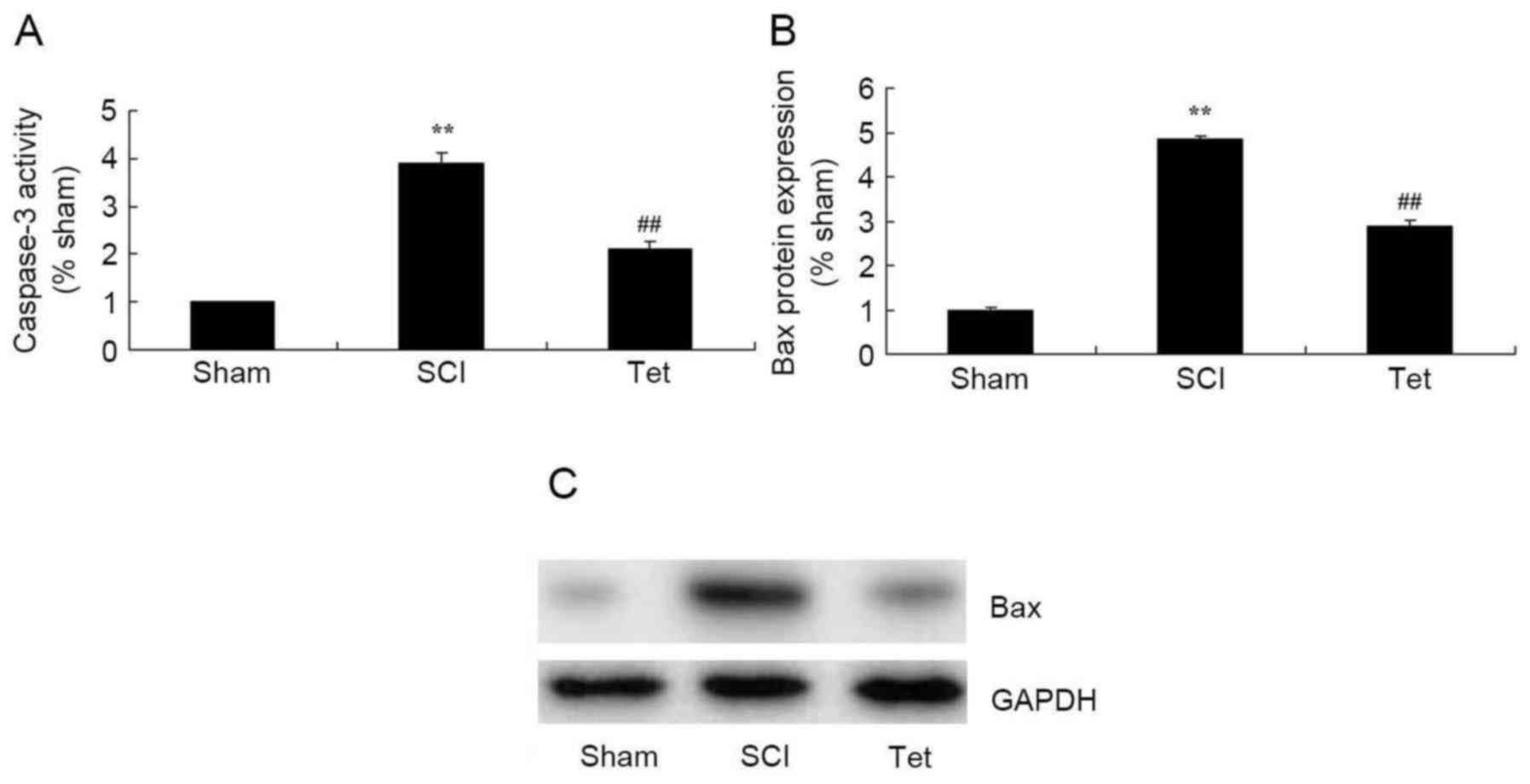
Tetrahydrocurcumin inhibits apoptosis
in SCI rats
As indicated in Fig.
5, caspase-3 activity and Bax protein expression were
significantly enhanced in the SCI model group compared with those
in the control group. However, treatment of SCI rats with
tetrahydrocurcumin significantly suppressed caspase-3 activity and
Bax protein expression (Fig. 5).
Tetrahydrocurcumin inhibits the
protein expression of MMP-3, MMP-13 and COX-2 in SCI rats
To evaluate the mechanisms by which
tetrahydrocurcumin attenuates SCI, MMP-3, MMP-13 and COX-2
expression were measured in the rats of the different experimental
groups. As presented in Fig. 6,
MMP-3, MMP-13 and COX-2 expression in SCI rats were higher than
those in the control group. Treatment with tetrahydrocurcumin
suppressed the protein expression of MMP-3, MMP-13 and COX-2 in SCI
rats.
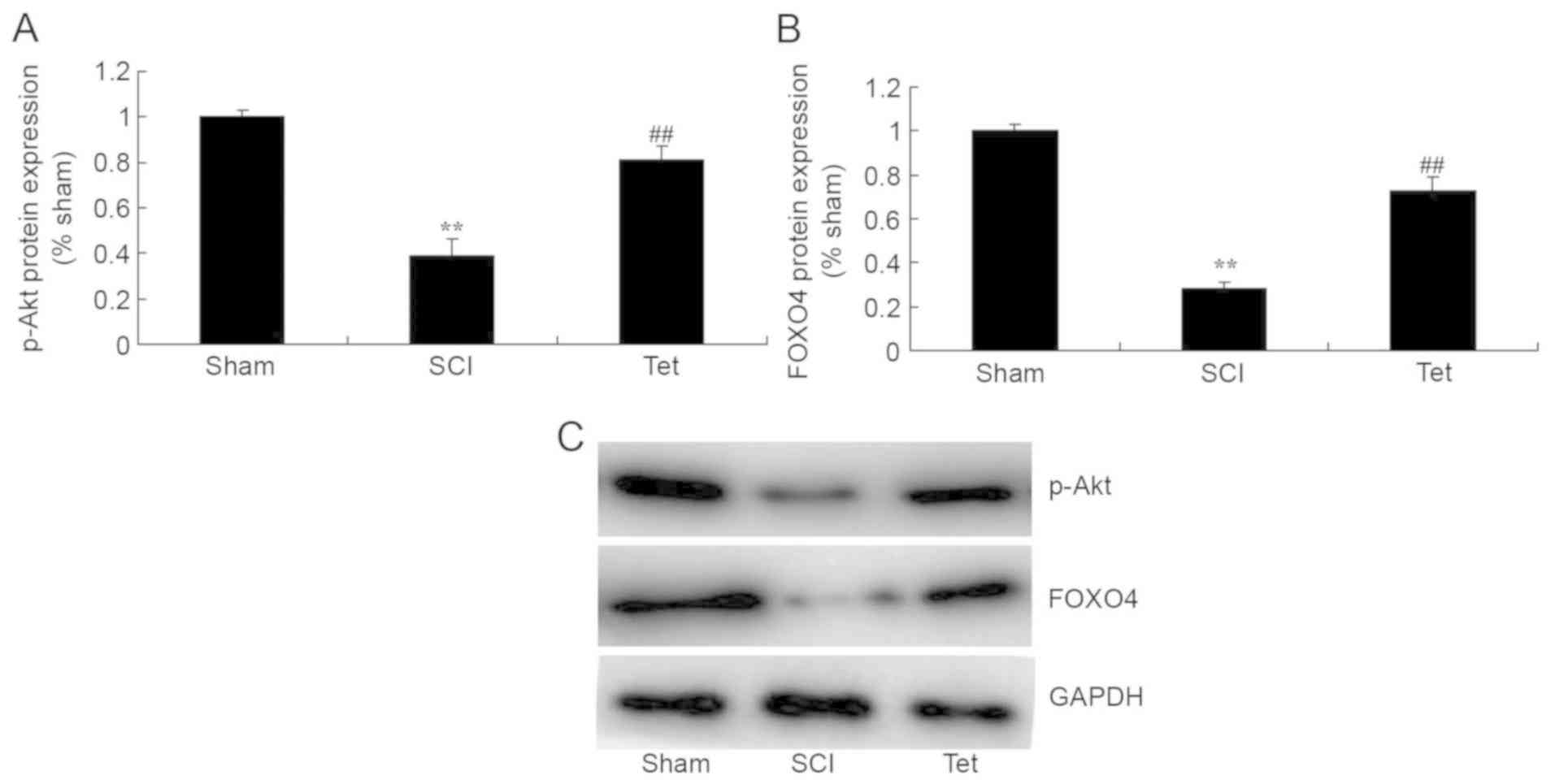
Tetrahydrocurcumin enhances the
protein levels of p-Akt and FOXO4 in SCI rats
To further evaluate the mechanism by which
tetrahydrocurcumin attenuates SCI, p-Akt levels and FOXO4
expression were measured in the rats of the different experimental
groups. p-Akt levels and FOXO4 expression in SCI rats were markedly
lower than those in the control group (Fig. 7). However, tetrahydrocurcumin induced
the production of p-Akt and the protein expression of FOXO4 in SCI
rats (Fig. 7).
Discussion
SCI is a serious injury of the central nervous
system and severely impairs the quality of life of affected
patients (15). Although the
survival rate and survival time of SCI patients has significantly
increased with the development of modern medicine, most patients
affected are disabled for life due to the difficulty of neuronal
regeneration (16). SCI represents a
global medical challenge, and a vast amount of scientific and
clinical research has achieved significant progress in the field
(17). In particular, a large number
of clinical studies and animal experiments have demonstrated that a
series of changes in molecular signaling and pathological processes
occur internally after SCI, including neuronal apoptosis,
inflammatory response and axonal demyelination (18). The results of the present study
preliminarily confirm that tetrahydrocurcumin enhances BBB scores,
inhibits water accumulation in the spinal cord, and decreases
inflammatory factors, oxidative stress and apoptosis in SCI rats.
Sangartit et al (10)
reported that tetrahydrocurcumin protects against cadmium-induced
hypertension in mice through exerting antioxidative and
anti-inflammatory effects.
MMPs have an important role in regulating the
development of the central nervous system (19,20).
However, in the presence of neurological disorders, MMP members
exhibit an abnormally increased expression. In recent years, a
large number of basic studies indicated that MMPs are involved in
various pathological processes after SCI (21). The extracellular matrix (ECM) is
composed of a variety of proteins and non-proteins, which make up
the microenvironment for cells and exert supporting, connecting,
nutritional and defense functions (22). The basement membrane of blood vessels
and the ECM are important components maintaining blood-spinal cord
barrier integrity (23). MMPs may
cause the damage to the blood-spinal cord barrier by degrading ECM
components, leading to increased permeability and extravasation of
capillary water and plasma protein, resulting in the increases in
the water content in the intracellular clearance and the formation
of spinal cord edema (24). It was
also demonstrated that MMPs have an important role in the
inflammatory response and apoptosis after SCI (20). The results of the present study
indicate that treatment with tetrahydrocurcumin suppressed the
protein expression of MMP-3, MMP-13 and COX-2 in SCI rats.
Yodkeeree et al (25)
revealed that tetrahydrocurcumin inhibits the migration and
invasion of HT1080 cells through MMPs and urokinase-type
plasminogen activator.
FOXOs exert their effects as transcription factors
through direct binding with target genes and interactions with
other transcriptional regulators, and their presence in cells
affects their function, including cell cycle regulation, apoptosis
and cellular metabolism (7,26). It has been demonstrated that FOXO is
involved in the degradation and synthesis of protein in skeletal
muscle, which maintains the protein content of skeletal muscle via
interaction with phosphoinositide-3 kinase (PI3K), mammalian target
of rapamycin (mTOR) complex 1 (mTORC1) and NF-κB in the face of
external stimuli (27). Study of the
molecular mechanisms of FOXO and its associated signaling pathways
in the regulation of muscle degradation and synthesis may provide
novel approaches for maintaining the normal development of skeletal
muscle (28). The present study
confirmed that tetrahydrocurcumin induces FOXO4 expression in SCI
rats, which was decreased after SCI. Xiang et al (13) reported that tetrahydrocurcumin
inhibits the oxidative stress response through FOXO forkhead
transcription factor.
The PI3K/Akt/mTOR signal transduction pathway is an
important pathway for receptor signal transduction to the cell,
which may regulate cell differentiation and proliferation, as well
as inhibit apoptosis (19). Under
normal physiological conditions, intracellular expression of PI3K
tends to be low (19). When the
cells are damaged its expression rapidly increases, leading to the
phosphorylation of phosphatidylinositol to generate
phosphatidylinositol 3,4-bisphosphate, the latter of which may
phosphorylate Akt to generate p-Akt, which in turn phosphorylates
mTOR at the Ser2448 site to thereby activate it (28). The PI3K/Akt signaling pathway is an
important signal transduction pathway inside cells, which is
closely associated with numerous vital cellular activities
(29). Phosphorylation of Akt may
promote cell survival by inhibiting glycogen synthase kinase, tumor
suppressors and caspase-3 phosphorylation; following
phosphorylation, Akt then dissociates from the Bcl-2 and 14-3-3
receptor protein complex, resulting in an anti-apoptotic effect
(30). The present study
demonstrated that tetrahydrocurcumin reduces the SCI-associated
inhibition of p-Akt levels and FOXO4 expression in rats. Wu et
al (12) demonstrated that
tetrahydrocurcumin induces autophagic cell death of human leukemia
HL-60 cells through coordinative modulation of PI3K/Akt/mTOR
signaling pathways.
In conclusion, the present study indicated that
tetrahydrocurcumin improves BBB scores and inhibits the oxidative
stress response by regulating the FOXO4 in SCI model rats.
Therefore, tetrahydrocurcumin may be applied as one of the clinical
adjunctive therapies for SCI. The present study should be followed
by further clinical studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JX designed the experiment and wrote the manuscript.
XL, YW, JL, LG, GW and QL performed the experiments. JX and XL
analyzed the data.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of The 309th Hospital of The People's Liberation
Army.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang W, Huang Y, He F, Liu J, Li M, Sun
T, Ren W, Hou J and Zhu L: Dopamine D1 receptor agonist A-68930
inhibits NLRP3 inflammasome activation, controls inflammation, and
alleviates histopathology in a rat model of spinal cord injury.
Spine (Phila Pa 1976). 41:E330–E334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hossain MS, Harvey LA, Rahman MA, Muldoon
S, Bowden JL, Islam MS, Jan S, Taylor V, Cameron ID, Chhabra HS, et
al: Community-based InterVentions to prevent serIous Complications
(CIVIC) following spinal cord injury in Bangladesh: Protocol of a
randomised controlled trial. BMJ Open. 6:e0103502016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jan YK and Crane BA: Wheelchair
tilt-in-space and recline does not reduce sacral skin perfusion as
changing from the upright to the tilted and reclined position in
people with spinal cord injury. Arch Phys Med Rehabil.
94:1207–1210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triolo RJ, Bailey SN, Miller ME, Rohde LM,
Anderson JS, Davis JA Jr, Abbas JJ, DiPonio LA, Forrest GP, Gater
DR Jr and Yang LJ: Longitudinal performance of a surgically
implanted neuroprosthesis for lower-extremity exercise, standing,
and transfers after spinal cord injury. Arch Phys Med Rehabil.
93:896–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kressler J, Nash MS, Burns PA and
Field-Fote EC: Metabolic responses to 4 different body
weight-supported locomotor training approaches in persons with
incomplete spinal cord injury. Arch Phys Med Rehabil. 94:1436–1442.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Lu J, Li YA, Zhou H, Ni WF, Zhang
XL, Zhu SP, Chen BB, Xu H, Wang XY, et al: Autologous olfactory
lamina propria transplantation for chronic spinal cord injury:
Three-year follow-up outcomes from a prospective double-blinded
clinical trial. Cell Transplant. 25:141–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia M and Zhu Y: FOXO3a involvement in the
release of TNF-α stimulated by ATP in spinal cord astrocytes. J Mol
Neurosci. 51:792–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Huan W, Wei H, Shi J, Fan J, Zhao
J, Shen A and Teng H: FOXO3a/p27kip1 expression and essential role
after acute spinal cord injury in adult rat. J Cell Biochem.
114:354–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Léger B, Senese R, Al-Khodairy AW, Dériaz
O, Gobelet C, Giacobino JP and Russell AP: Atrogin-1, MuRF1, and
FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in
skeletal muscle of chronic spinal cord-injured patients. Muscle
Nerve. 40:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sangartit W, Kukongviriyapan U, Donpunha
W, Pakdeechote P, Kukongviriyapan V, Surawattanawan P and Greenwald
SE: Tetrahydrocurcumin protects against cadmium-induced
hypertension, raised arterial stiffness and vascular remodeling in
mice. PLoS One. 9:e1149082014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park S, Lee LR, Seo JH and Kang S:
Curcumin and tetrahydrocurcumin both prevent osteoarthritis
symptoms and decrease the expressions of pro-inflammatory cytokines
in estrogen-deficient rats. Genes Nutr. 11:22016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu JC, Lai CS, Badmaev V, Nagabhushanam K,
Ho CT and Pan MH: Tetrahydrocurcumin, a major metabolite of
curcumin, induced autophagic cell death through coordinative
modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human
leukemia HL-60 cells. Mol Nutr Food Res. 55:1646–1654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang L, Nakamura Y, Lim YM, Yamasaki Y,
Kurokawa-Nose Y, Maruyama W, Osawa T, Matsuura A, Motoyama N and
Tsuda L: Tetrahydrocurcumin extends life span and inhibits the
oxidative stress response by regulating the FOXO forkhead
transcription factor. Aging (Albany NY). 3:1098–1109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukhamedshina YO, Akhmetzyanova ER,
Kostennikov AA, Zakirova EY, Galieva LR, Garanina EE, Rogozin AA,
Kiassov AP and Rizvanov AA: Adipose-derived mesenchymal stem cell
application combined with fibrin matrix promotes structural and
functional recovery following spinal cord injury in rats. Front
Pharmacol. 9:3432018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharp KG, Gramer R, Butler L, Cramer SC,
Hade E and Page SJ: Effect of overground training augmented by
mental practice on gait velocity in chronic, incomplete spinal cord
injury. Arch Phys Med Rehabil. 95:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Scheer JW, de Groot S, Tepper M,
Faber W; ALLRISC group, ; Veeger DH and van der Woude LH:
Low-intensity wheelchair training in inactive people with long-term
spinal cord injury: A randomized controlled trial on fitness,
wheelchair skill performance and physical activity levels. J
Rehabil Med. 48:33–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee
H, Jung K, Hwang K, Kim M, Lee IS, et al: Clinical trial of human
fetal brain-derived neural stem/progenitor cell transplantation in
patients with traumatic cervical spinal cord injury. Neural Plast.
2015:6309322015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murai T, Murata R, Manabe Y, Sugie C,
Tamura T, Ito H, Miyoshi Y and Shibamoto Y: Intensity modulated
stereotactic body radiation therapy for single or multiple
vertebral metastases with spinal cord compression. Pract Radiat
Oncol. 4:e231–e237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng B, Ye L, Zhou Y, Zhu S, Wang Q, Shi
H, Chen D, Wei X, Wang Z, Li X, et al: Epidermal growth factor
attenuates blood-spinal cord barrier disruption via PI3K/Akt/Rac1
pathway after acute spinal cord injury. J Cell Mol Med.
20:1062–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miranpuri GS, Schomberg DT, Alrfaei B,
King KC, Rynearson B, Wesley VS, Khan N, Obiakor K, Wesley UV and
Resnick DK: Role of matrix metalloproteinases 2 in spinal cord
injury-induced neuropathic pain. Ann Neurosci. 23:25–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schreiber R, Paim LR, de Rossi G,
Matos-Souza JR, Costa E Silva Ade A, Souza CM, Borges M, Azevedo
ER, Alonso KC, Gorla JI, et al: Matrix metalloproteinases and left
ventricular function and structure in spinal cord injured subjects.
Clin Chim Acta. 437:136–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Chu G, Pan C, Hu J, Guo C, Liu J,
Wang Y and Wu J: A nutrient mixture reduces the expression of
matrix metalloproteinases in an animal model of spinal cord injury
by modulating matrix metalloproteinase-2 and matrix
metalloproteinase-9 promoter activities. Exp Ther Med. 8:1835–1840.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JY, Kim HS, Oh TH and Yune TY: Ethanol
extract of Bupleurum falcatum improves functional recovery by
inhibiting matrix metalloproteinases-2 and −9 activation and
inflammation after spinal cord injury. Exp Neurobiol. 19:146–154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cirillo G, Colangelo AM, De Luca C,
Savarese L, Barillari MR, Alberghina L and Papa M: Modulation of
matrix metalloproteinases activity in the ventral horn of the
spinal cord re-stores neuroglial synaptic homeostasis and
neurotrophic support following peripheral nerve injury. PLoS One.
11:e01527502016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yodkeeree S, Garbisa S and Limtrakul P:
Tetrahydrocurcumin inhibits HT1080 cell migration and invasion via
downregulation of MMPs and uPA. Acta Pharmacol Sin. 29:853–860.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Z, Yan B, Yu WY, Yao X, Ma X, Sheng G
and Ma Q: Vitexin attenuates acute doxorubicin cardiotoxicity in
rats via the suppression of oxidative stress, inflammation and
apoptosis and the activation of FOXO3a. Exp Ther Med. 12:1879–1884.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu
J, Li X and Qin ZH: Chronic resistance training activates autophagy
and reduces apoptosis of muscle cells by modulating IGF-1 and its
receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp
Gerontol. 48:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshihara T, Kobayashi H, Kakigi R,
Sugiura T and Naito H: Heat stress-induced phosphorylation of
FoxO3a signalling in rat skeletal muscle. Acta Physiol (Oxf).
218:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luan Y, Chen M and Zhou L: MiR-17 targets
PTEN and facilitates glial scar formation after spinal cord
injuries via the PI3K/Akt/mTOR pathway. Brain Res Bull. 128:68–75.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CH, Sung CS, Huang SY, Feng CW, Hung
HC, Yang SN, Chen NF, Tai MH, Wen ZH and Chen WF: The role of the
PI3K/Akt/mTOR pathway in glial scar formation following spinal cord
injury. Exp Neurol. 278:27–41. 2016. View Article : Google Scholar : PubMed/NCBI
|