Introduction
Systemic lupus erythematosus (SLE) is a chronic
inflammatory autoimmune disease (1)
that may be influenced by genetic variation (2), environmental stimuli or estrogen
(3,4). Pathological hallmark features include
the overproduction of auto-antibodies secreted by overactive B
lymphocytes, accompanied by aberrant T-cell function, accumulation
of immune complexes and excessive production of pro-inflammatory
cytokines (2). It has been
demonstrated that SLE may be activated by a variety of
environmental stimuli, including strong ultraviolet (UV) light
(5), ambient fine particulate and
infections (6,7). Of note, high UV irradiation is a
feature associated with high altitude conditions of the Yunnan
plateau (YP), China (8). However, to
date, only few studies (9,10) have focused on the pathogenesis of SLE
in YP, and studies into the relationship between SLE and high
altitude have not been previously reported. Therefore, the
mechanism of the pathological characteristics associated with SLE
in populations exposed to high UV remains to be fully
elucidated.
Accumulated evidence indicates that the type-I
interferon (IFN) system is involved in cutaneous autoimmune
diseases. IFNs induce the expression of pro-inflammatory cytokines
and chemokines, which support the cellular immune response
(11). Elevated IFN levels in the
serum and increased IFN expression in peripheral blood mononuclear
cells (PBMCs) have been indicated to serve a key role in the
pathogenesis of SLE (12). In the
present study, dozens of differentially expressed genes (DEGs) in
SLE patients vs. normal controls were identified by using
high-throughput sequencing. Further computational biological
analysis revealed central and interactional targets. Among these
potential key genes, chemokine C-X-C motif ligand 10 (CXCL10), as a
chemokine, also known as IFN-inducible protein (IP-10) has drawn
our attention; CXCL10 has a significant role in inflammation and
immunity by binding to chemokine C-X-C motif receptor 3 (13).
CXCL10 is highly expressed in a wide range of human
diseases. It is involved in the pathological processes of three
major human disorders, namely infectious, inflammatory and
autoimmune diseases, including Graves' disease, psoriasis, type I
diabetes and SLE (13–15). In SLE patients, the serum levels of
CXCL10 are highly elevated and correlate with the level of disease
activity (16,17). However, to the best of our knowledge,
the mRNA expression of CXCL10 in SLE patients particularly in
crucial lymphocytes, including T-helper (Th) cells and B
lymphocytes, has remained elusive and its molecular mechanisms in
SLE has not been previously explored.
In the present study, DEG profiles in PBMCs from SLE
patients and healthy controls (Ctrl) were first analyzed.
Subsequently, the expression levels of CXCL10 in PBMCs,
CD4+ Th cells and CD19+ B lymphocytes were
examined using reverse transcription-quantitative PCR (RT-qPCR).
Furthermore, a tentative experiment was performed to screen for
polymorphisms in the 3′-untranslated region (UTR) of CXCL10 to
explore any possible association with susceptibility to SLE.
Materials and methods
Participants
A total of 3 patients meeting the American College
of Rheumatology (ACR) classification criteria (18) for SLE [SLE disease activity index
(SLEDAI) score ≥4] and three gender-matched healthy individuals
were enrolled in the high-throughput screening experiment (all
female subjects, with an average age of 39.08±7.23 and 35.71±6.83
years, respectively). A total of 66 patients meeting the ACR
criteria (SLEDAI score ≥4) and 42 unrelated, unaffected controls
were then recruited for mRNA detection. The characteristics and
laboratory data of the cohort are listed in Table I. All cases of inpatients were
recruited from the Department of Dermatology in the Second
Affiliated Hospital of Kunming Medical University (Kunming, China),
and the health controls were recruited from the check-up department
of the same hospital at random during the same period (January to
October 2018). Informed consent for the anonymized analysis of
their blood samples, in addition to personal data regarding all the
parameters listed in Table I were
obtained from all subjects. The present study was approved by the
Ethics Committee of the Second Affiliated Hospital of Kunming
Medical University (Kunming, China).
 | Table I.Demographic data of the two groups
and SNPs in the 3′-untranslated region of chemokine C-X-C motif
ligand 10. |
Table I.
Demographic data of the two groups
and SNPs in the 3′-untranslated region of chemokine C-X-C motif
ligand 10.
|
Characteristics | SLE (n=66) | Ctrl (n=42) | P-value |
|---|
| Age (years; mean ±
SD, M (Q3-Q1)) | 39±7.23 39
(45.25–34) | 36±6.83 36
(42–28.75) | 0.826 |
| BMI
(kg/m2; mean ± SD) | 22.44±2.35 | 21.10±2.75 | 0.293 |
| Gender [n (%)] |
|
| 0.958 |
|
Female | 63 (95.45) | 40 (95.24) |
|
|
Male | 3 (4.55) | 2 (4.76) |
|
| Ethnicity [n
(%)] |
|
| 0.793 |
|
Han | 57 (86.36) | 37 (88.10) |
|
|
Others | 9 (13.64) | 5 (11.90) |
|
| Disease duration
[months; M (Q3-Q1)] | 78
(156.0–21.5) | NA | NA |
| SLEDAI [score;
M(Q3-Q1)] | 9 (15–7) | NA | NA |
| 24-h urinary
protein [g; M (Q3-Q1] | 0.29
(1.39–0.06) | ND | NA |
| Low C3 [<0.79
g/l, n (%)] | 54 (81.82) | ND | NA |
| Low C4 [<0.10
g/l, n (%)] | 47 (71.21) | ND | NA |
| Anti-dsDNA [+, n
(%)] | 14 (21.21) | ND | NA |
| Anti-ENA [+, n
(%)] | 19 (28.79) | ND | NA |
| Anti-Sm [+, n
(%)] | 16 (24.24) | ND | NA |
| SSA (anti-Ro) [+, n
(%)] | 40 (60.71) | ND | NA |
| SSB (anti-La) [+, n
(%)] | 2 (3.03) | ND | NA |
| SNPs |
|
rs35795399 (C/G/T) | 0/0/66 | 0/0/42 | NA |
|
rs58658570 (A/T) | 66/0 | 42/0 | NA |
|
rs34836828 (−/G) | 66/0 | 42/0 | NA |
|
rs148141229 (A/G) | 66/0 | 42/0 | NA |
High-throughput sequencing and
analysis
PBMCs were purified from heparinized venous blood by
density-gradient centrifugation over Lymphoprep™ (Axis-Shield),
performed by fractionation on Lymphoprep™ and separation by
density-gradient centrifugation (400 × g, 20 min, 18–20°C). To
isolate mononuclear cells, the Lymphoprep™ interface was aspirated
without disturbing the erythrocyte/granulocyte pellet and then
washed once with Phosphate-buffered saline (PBS). The cells were
then counted and re-suspended in PBS at a concentration of
2×106/ml. Furthermore, the leukocytes deposited at the
bottom were stored at −80°C.
RNA-sequencing (RNA-seq) was performed by Vazyme
Biotech Co., Ltd. After mRNA was extracted, concentrated and
sheared into fragments, complementary DNA (cDNA) was synthesized,
followed by purification of fragments, terminal repair,
polyA-tailing and ligation of Illumina sequencing adapters.
Finally, raw data from a chain-specific library were obtained by
amplification using qPCR. The Q-value was strictly >30 according
to the quality-control assessment criteria. As a procedure for the
final report, genes were annotated according to the National Center
for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/refseq/) (19), University of California Santa Cruz
Genome Browser database (https://genome.ucsc.edu) (20) and Ensembl genome databases
(http://www.ensembl.org/Homo_sapiens/Info/Index)
(21) after data were cleaned
according to alignment, assembly and qualification. mRNA abundance
was estimated by the expected number of fragments per kilobase of
transcript sequence per million base pairs sequenced. DEGs,
identified by comparison of SLE patients and controls with a cutoff
of |log2 fold change| ≥1 and P≤0.05, were included in
the final report.
Cell isolation
Isolation of PBMCs was performed as previously
described within 2 h after the blood specimen was obtained.
CD4+ cells were enriched by positive selection with CD4
magnetic microbeads (Miltenyi Biotec) according to the
manufacturer's protocol. To obtain highly purified CD4 cells, PBMCs
were incubated with the microbeads (20 µl/107 cells) at
4°C for 15 min prior to being passed through LS columns using a
quadroMACS separator (Miltenyi Biotec). During the process, degas
buffer containing PBS (pH 7.2), bovine serum albumin (0.5% BSA,
Sigma-Aldrich; Merck KGaA) and EDTA (2 mM) was used for washing the
columns. A purity of >90% was confirmed by fluorescence staining
with CD4-FITC (OKT-4; cat. no. 11-0048-42; eBioscience; Thermo
Fisher Scientific, Inc.) and analysis using an LSR Fortessa
fluorescence-activated cell sorting instrument (BD Biosciences).
The same procedure was used to isolate and identify
CD19+ lymphocytes by using B Cell Isolation Kit II (cat.
no. 130-091-151; Miltenyi Biotec GmbH) from the remaining cells
according to the manufacturer's instructions.
RT-qPCR
RNA was extracted from PBMCs, Th cells and B
lymphocytes using the RNAclean Kit (Tiangen Biotech Co., Ltd.).
cDNA was synthesized from total RNA with a cDNA synthesis kit
(Takara Biotechnology Co., Ltd.). qPCR for cDNA sample
amplification was performed using the Roche 480 real-time PCR
system with SYBR Green Master Mix (KAPA Biosystems; Roche
Diagnostics). The sequences of the primers are listed in Table II. The relative expression levels of
genes were normalized to those of human GAPDH, calculated using the
2−∆∆Cq method and were log-transformed (22).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene name | Primer sequence
(5′-3′) |
|---|
| IFI27 | F:
CGTCCTCCATAGCAGCCAAGAT |
|
| R:
ACCCAATGGAGCCCAGGATGAA |
| OLFM4 | F:
GACCAAGCTGAAAGAGTGTGAGG |
|
| R:
CCTCTCCAGTTGAGCTGAACCA |
| IFI44 | F:
GTGAGGTCTGTTTTCCAAGGGC |
|
| R:
CGGCAGGTATTTGCCATCTTTCC |
| SLC12A1 | F:
AGGCTCTTTCCTACGTGAGTGC |
|
| R:
GCCACTGTTCTTGGTAAAGGCG |
| KRT1 | F:
CAGCATCATTGCTGAGGTCAAGG |
|
| R:
CATGTCTGCCAGCAGTGATCTG |
| TUBB2A | F:
TTGGGAGGTCATCAGCGATGAG |
|
| R:
AGGCTCCAGATCCACCAGGATG |
Selection and detection of
single-nucleotide polymorphisms (SNPs)
A total of four candidate SNPs were investigated in
the 3′UTR of CXCL10: rs35795399, rs58658570, rs34836828 and
rs148141229. These had been identified from Genome-Wide Association
Studies from the NCBI (https://www.ncbi.nlm.nih.gov/snp/?term=CXCL10).
Genomic DNA was extracted from the remaining leucocytes using the
TIANamp Blood DNA Kit (Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocol. The gene polymorphisms were analyzed using
the SNaPshot assay (Sangon Biotech Co., Ltd.). To confirm the
genotyping results, PCR-amplified DNA samples were examined by DNA
sequencing and the results were 100% concordant.
Bioinformatics analysis
Networks of DEGs were algorithmically generated
based on the potential connectivity of their products using the
Database for Annotation Visualization and Integrated Discovery
online database (DAVID, version 6.8; http://david.ncifcrf.gov) (23), the Search Tool for the Retrieval of
Interacting Genes and proteins (STRING) database (version 10.5;
http://string-db.org) (24) and Cytoscape (version 3.6.1) (25), which is an open-source Bioinformatics
software platform for visualizing molecular interaction networks.
Enrichment analysis of DEGs, including Gene Ontology (GO)
functional analysis and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analysis, was performed using DAVID. STRING was used
to analyze the functional interactions between proteins and
construct protein-protein interaction (PPI) networks. In the
present study, the threshold of protein interaction was set to
medium confidence, which is the default setting of the website.
Furthermore, the plug-in MCODE (version 1.4.2) of
Cytoscape that is able to visualize PPI networks was used to
cluster a given network based on topology to identify densely
connected regions. Finally, hub genes were selected with degrees of
connectivity >10.
Statistical analysis. Statistically significant
differences between two groups were determined using an unpaired
Student's t-tests for data conforming to a normal distribution, or
a Mann-Whitney U-test for those with an abnormal distribution.
Kolmogorov-Smirnov test was used to determine whether the data
conformed to normal distribution. Pearson's χ2 test was
used to investigate any association with gender and ethnicity. A
two-tailed P<0.05 was considered to indicate statistical
significance. All statistical tests were performed using GraphPad
Prism 7.0 (GraphPad, Inc.).
Results
Bioinformatics analysis of DEGs
To identify the most significant genes, DEGs were
first sorted by their ascending P-value and then listed by the
absolute values of logarithmic conversion
(|log2SLE/Ctrl|) in descending order. A positive value
indicated higher expression in SLE patients relative to controls;
correspondingly, a negative value indicated a lower relative
expression. The top 20 significantly up- or downregulated DEGs are
listed in Table III. Of note, the
expression of IFN-α-inducible protein 27 (IFI27) in the SLE group
was 102.62 times that of the value in the control group. Of all of
the DEGs, 76.97% (117/152) were upregulated and 23.03% were
downregulated.
 | Table III.Details of the top 20
up-/downregulated differentially expressed genes. |
Table III.
Details of the top 20
up-/downregulated differentially expressed genes.
|
| Upregulation | Downregulation |
|---|
|
|
|
|
|---|
| Rank | Gene name |
log2(SLE/Ctrl) | P-value | Gene name |
log2(SLE/Ctrl) | P-value |
|---|
| 1 | IFI27 | 6.68123 |
5.00×10−5 | SLC12A1 | −4.01723 |
5.00×10−5 |
| 2 | FBRSL1 | 4.38689 |
5.00×10−5 | INTS1 | −3.73539 |
5.00×10−5 |
| 3 | OLFM4 | 3.91972 |
5.00×10−5 | PRR12 | −3.20664 |
5.00×10−5 |
| 4 | SPTSSB | 3.90728 |
5.00×10−5 | TUBB2A | −2.46642 |
5.00×10−5 |
| 5 | MMP8 | 3.65456 |
5.00×10−5 | FAM153B | −2.38627 |
5.00×10−5 |
| 6 | IFI44L | 3.57125 |
5.00×10−5 | ETS1 | −2.145136 |
5.00×10−5 |
| 7 | SIGLEC1 | 3.258 |
5.00×10−5 | SLC38A7 | −2.14331 |
5.00×10−5 |
| 8 | RSAD2 | 3.24366 |
5.00×10−5 | HECW2 | −2.09099 |
5.00×10−5 |
| 9 | USP18 | 3.2127 |
5.00×10−5 | PFDN4 | −1.8775 |
5.00×10−5 |
| 10 | DLGAP1 | 3.15073 |
5.00×10−5 | KRT1 | −1.73932 |
5.00×10−5 |
| 11 | EPSTI1 | 3.03301 |
5.00×10−5 | DDIT4 | −1.56707 |
5.00×10−5 |
| 12 | MPO | 3.00225 |
5.00×10−5 | FOS | −1.4891 |
5.00×10−5 |
| 13 | G0S2 | 2.99775 |
5.00×10−5 | RNF182 | −1.34662 |
5.00×10−5 |
| 14 | IFI44 | 2.78607 |
5.00×10−5 | MPZL1 | −1.32971 |
5.00×10−5 |
| 15 | LTF | 2.77532 |
5.00×10−5 | FCRL2 | −1.26173 |
5.00×10−5 |
| 16 | AGBL2 | 2.77292 |
5.00×10−5 | FCER1A | −1.2243 |
5.00×10−5 |
| 17 | LY6E | 2.70831 |
5.00×10−5 | BCAP29 | −1.21236 |
5.00×10−5 |
| 18 | ISG15 | 2.67862 |
5.00×10−5 | KLRF1 | −1.07264 |
5.00×10−5 |
| 19 | CXCL10 | 2.64825 |
5.00×10−5 | GNLY | −1.06844 |
5.00×10−5 |
| 20 | SPATS2L | 2.62536 |
5.00×10−5 | ADGRG1 | −1.06523 |
5.00×10−5 |
To cluster DEGs into ontology subsets, they were
further analyzed using DAVID. GO analysis indicated that in the
category Biological Process (BP), the DEGs were significantly
enriched in the following terms: ‘Defense response to virus’
(GO:0051607), ‘type I interferon signaling pathway’ (GO:0060337),
‘response to virus’ (GO:0009615), ‘negative regulation of viral
genome replication’ (GO:0045071), ‘innate immune response’
(GO:0045087), ‘complement activation’ (GO:0006956, GO:0006958),
‘phagocytosis’ (GO:0006911, GO:0006910) and ‘positive regulation of
B-cell activation’ (GO:0050871) (Fig.
1A). In the category Cellular Component (CC; Fig. 1B), the significantly enriched genes
were concentrated in the following terms: ‘Blood microparticle’
(GO:0072562), ‘circulating immunoglobulin complex’ (GO:0042571),
‘external side of plasma membrane’ (GO:0009897) and ‘nucleosome’
(GO:0000786). Furthermore, in the category Molecular Function (MF),
the DEGs were mainly enriched in ‘immunoglobulin receptor binding’
(GO:0034987), ‘antigen binding’ (GO:0003823), ‘serine-type
endopeptidase activity’ (GO:0004252), ‘double-stranded RNA binding’
(GO:0003725) and ‘2′-5′-oligoadenylate synthetase activity’
(GO:0001730) (Fig. 1C). All of the
aforementioned terms were significantly enriched by the DEGs
(P<0.05). The most significantly different and the most abundant
DEGs identified from the sequencing results were clustered in the
term ‘innate immune response’, followed by enrichment of virus- and
type 1 IFN-associated BP terms, and immune-associated GO terms in
the categories MF, CC and BP.
KEGG pathway analysis revealed that the DEGs were
significantly enriched in ‘systemic lupus erythematosus’ (hsa05322;
12 DEGs), followed by ‘influenza A’ (hsa05164) and ‘herpes simplex
infection’ (hsa05168), as presented in Fig. 1D.
STRING analysis was employed to determine the roles
of various DEGs in the pathogenesis of SLE. The PPI network was
obtained and is displayed in Fig.
1E. The network statistics were as follows: Number of edges,
678; number of nodes, 139; average node degree, 9.76; average local
clustering coefficient, 0.486; PPI enrichment P-value
<1.0×10−16. This level of enrichment indicated that
the proteins of DEGs at least partially biologically interact as a
group during the pathogenesis of SLE. In addition, the most
significant module was obtained from the aforementioned PPI network
(Fig. 1F). A total of 30 upregulated
molecules were identified as hub genes with degrees of ≥10 and a
cluster score of 28.414, as determined by MCODE of Cytoscape.
RT-qPCR validation
To verify the reliability of the high-throughput
sequencing data, several candidate mRNAs of interest were initially
identified for further analysis, including IFI27, olfactomedin 4
(OLFM4), interferon-induced protein 44 (IFI44), solute carrier
family 12 member 1 (SLC12A1), keratin 1 (KRT1) and tubulin β 2A
class IIa (TUBB2A). The expression levels of these mRNAs were
confirmed by RT-qPCR. The results demonstrated that IFI27, OLFM4
and IFI44 were upregulated and that SLC12A1, KRT and TUBB2A were
downregulated in SLE patients relative to those in the healthy
group (all P<0.05; Fig. 2). These
results demonstrated that the expression patterns of IFI27, OLFM4,
IFI44, SLC12A1, KRT1 and TUBB2A were consistent with those obtained
by high-throughput sequencing analysis.
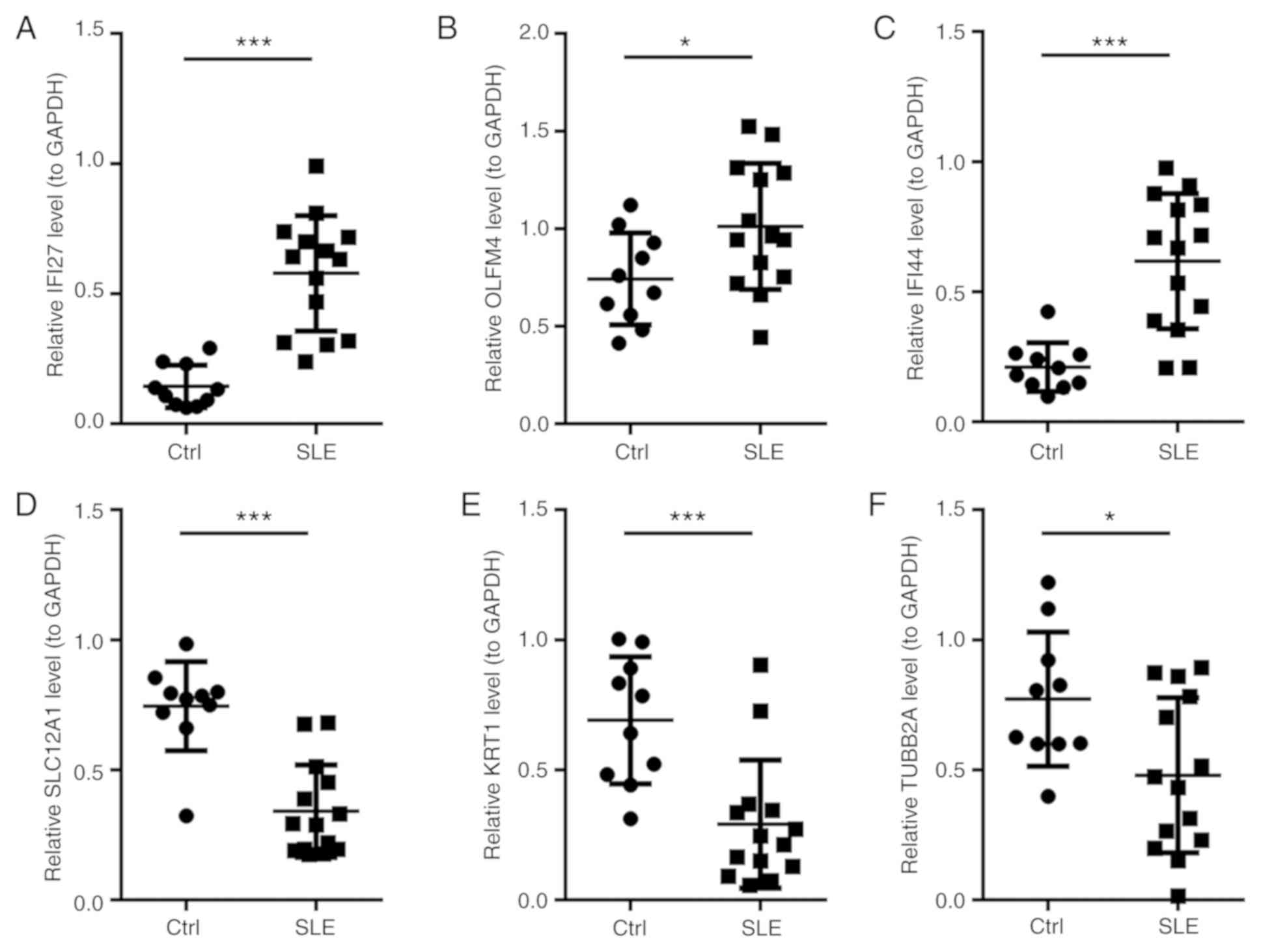 | Figure 2.Relative expression levels of genes
in SLE patients and healthy controls determined by reverse
transcription-quantitative PCR. (A) IFI27, (B) OLFM4 and (C) IFI44
were significantly upregulated in SLE patients, whilst (D) SLC12A1,
(E) KRT1 and (F) TUBB2A were significantly downregulated in SLE
patients compared with those in the healthy group. The expression
levels of mRNA were normalized against GAPDH, which served as an
internal control. *P<0.05, ***P<0.001. Ctrl, healthy
controls; SLE, systemic lupus erythematosus patients; IFI27,
interferon-α-inducible protein 27; OLFM4, olfactomedin 4; IFI44,
interferon-induced protein 44; SLC12A1, solute carrier family 12
member 1; KRT1, keratin 1; TUBB2A, tubulin β 2A class IIa. |
Expression of CXCL10 mRNA in
monocytes
Through Bioinformatics analysis of the
high-throughput RNA-seq profiling data, it was determined that
CXCL10 was upregulated by 6.27-fold in PBMCs from SLE patients
compared with those in controls (P<0.01). In the mRNA PPI
network, CXCL10 was one of the hub genes and significantly
interacted with interferon induced with helicase C domain 1,
2′-5′-oligoadenylate synthetase (OAS)-like, OAS3, IFI27, receptor
transporter protein 4, HECT and RLD domain containing E3 ubiquitin
protein ligase family member 6 (HERC6), ubiquitin specific
peptidase 18 (USP18) and HERC5. Since CXCL10 appeared to contribute
to the pathogenesis of numerous autoimmune diseases, the expression
of CXCL10 was further assessed in a total of 66 patients with SLE
and 42 controls, with demographic data and SLEDAI scores listed in
Table I.
It is well known that in the PBMC population, Th
cells and B lymphocytes have pivotal roles in the initiation and
progression of SLE (26). Therefore,
CXCL10 mRNA was measured not only in PBMCs but also in Th cells and
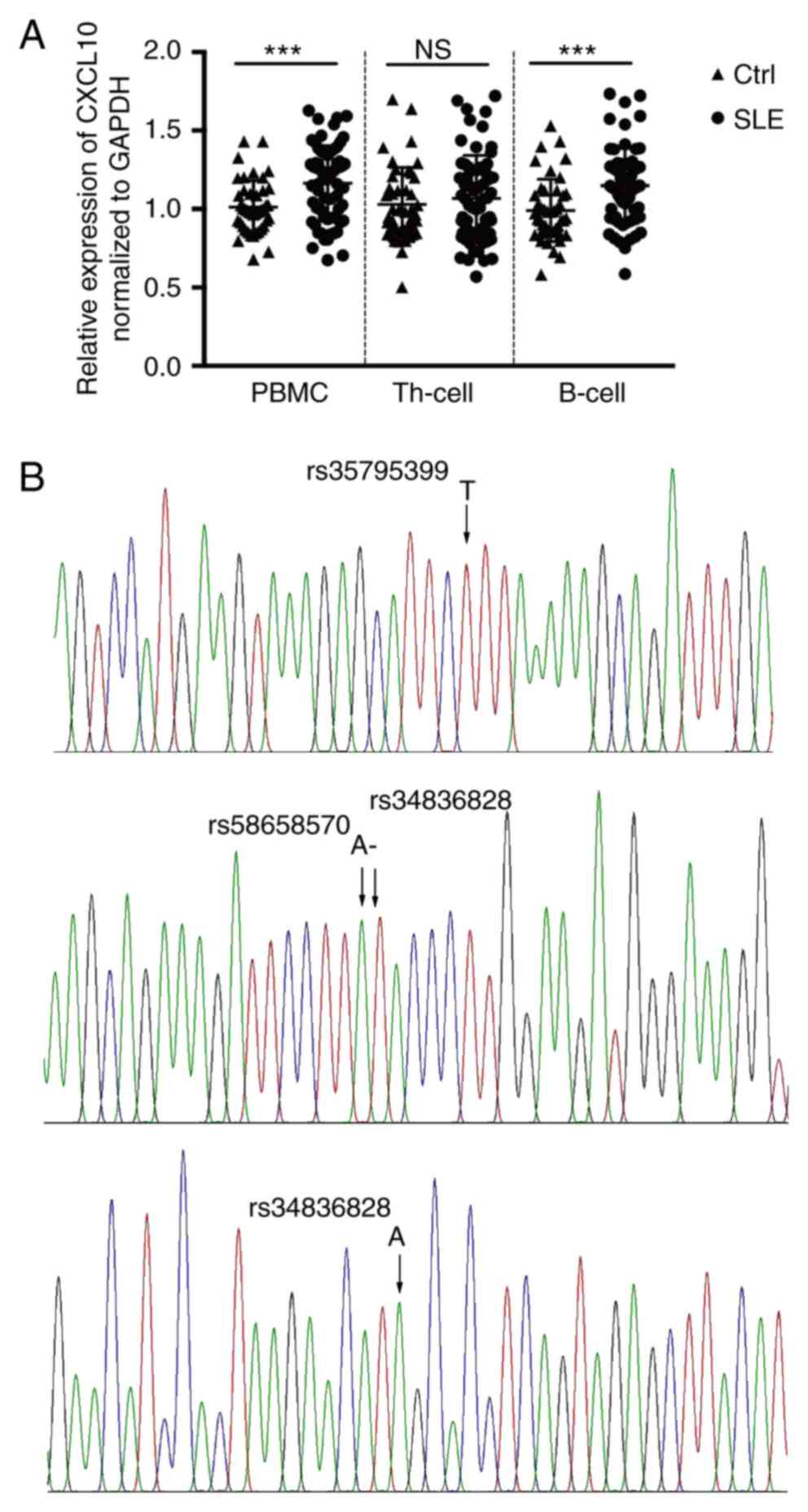
B lymphocytes. The results, presented in Fig. 3A, demonstrated that the expression of
CXCL10 was significantly increased in PBMCs (P<0.001) and was
similar in B lymphocytes (P<0.001), yet unchanged (P=0.881) in
Th cells when comparing SLE patients with normal controls. In the
present study, CXCL10 was identified as a DEG at the
transcriptional level in the screen as well as in the verification
experiments using RT-qPCR analysis. The trend of increased
expression present in PBMCs from SLE patients was consistent with
the results of the RNA-seq. Of note, the expression of CXCL10 in
the Th-cell subpopulation was unchanged.
Allele-specific expression of
CXCL10
SNPs are considered to have a vital role in the
function of CXCL10, which contributes to the pathogenesis of
several diseases, including Graves' disease (27). Therefore, all of the DNA specimens of
the present study were used to detect polymorphisms at selected SNP
loci. The results indicated that when assessing the samples for
rs35795399 (C/G/T), rs58658570 (A>T), rs34836828 (−>G) or
rs148141229 (A>G), no mutation was identified in any of them, as
demonstrated in Table I and Fig. 3B.
Discussion
Epidemiological surveys have indicated a five-year
survival rate for SLE of 93.8% in China (28,30), and
the prevalence in Asian patients is 2–3-fold higher than that in
Caucasians; these rates in Asian patients are accompanied by
serious clinical manifestations and higher fatality rates (1,28,30).
Strong UV light unique to the plateau in Yunnan province may have
an effect on the incidence and mortality associated with SLE
(6,29,30).
Therefore, the high-throughput mRNA-seq profiles of
PBMC from SLE patients from Yunnan province were first compared
with those of health controls. As expected, the results indicated
that the DEGs were enriched in autoimmune-associated pathways,
including SLE, Rheumatoid arthritis and RIG-I-like receptor
signaling pathway, as shown in Fig.
1D. In addition, GO and KEGG analysis indicated that the DEGs
were enriched in viral infection, including defense response to
virus and influenza A, as well as herpes simplex infection.
Previous studies reported the roles of viruses in the pathogenesis
of autoimmune diseases as epitope spreading, molecular mimicry,
cryptic antigens and bystander activation (30). Viral infection has been frequently
associated with a high level of IFN in most lupus patients
(31). This was consistent with
differentially expressed IFN-associated genes (IFI27, IFI44,
CXCL10, IFI44L and sialic acid-binding Ig-like lectin 1) that were
identified in the present study.
In past decades, RNA-seq, an innovative technology
for comprehensive transcriptome profiling on a genomic scale,
performed in a high-throughput and quantitative manner, has
provided clues for identifying aberrantly expressed RNAs. First,
the present analysis regarding the pathogenesis of SLE indicated
that the enriched DEGs had an important role in the IFN signaling
which is consistent with the results of Becker et al
(32). This result has also been
confirmed by a large number of studies for numerous years (33). Furthermore, IFN-α is an essential
factor that acts against viral infection in innate immunity
(34,35). Consequently, it is to be expected
that the enriched molecules and pathways are linked to viral
infection and initiation of an inherent anti-viral immune response.
Finally, a variety of autoimmune cellular components and
immune-associated pathways have also been identified in present
study, which are identical to previous findings from patients with
SLE (13,31,32). In
summary, the conclusions of the present Bioinformatics analysis
were highly consistent with the results of previous studies,
including relatively high expression of IFI44, IFI44L, USP18,
IFN-induced protein with tetratricopeptide repeats 3 and OAS3
(32,36). However, discrepancies with previous
results were also present, including the upregulation of CXCL10 and
downregulation of proline-rich 12 in the present study (32,36).
Elevated CXCL10 levels in serum has been considered
to be an important potential biomarker in predicting SLE flares and
making overall clinical decisions (30,37–39),
with a particular role in the diagnosis of arthritis (30). However, to the best of our knowledge,
few studies have investigated its involvement in the molecular
mechanisms of SLE. Based on the dysregulation identified from the
RNA-seq results of the present study, RT-qPCR was used and
confirmed increased CXCL10 in PBMCs from SLE patients compared to
controls. Recently, Lee et al (40) reported that IP-10/CXCL10 expression
was 1.5-fold higher in the plasma of SLE patients relative to that
in a healthy group. The fold change was even greater in the present
study, demonstrating an up to 6.28-fold upregulation in SLE vs.
Ctrl. It may be speculated that this is associated with the strong
UV light in Yunnan. UV light, as an important initiation factor
triggering the mechanisms of SLE, may activate an innate and
subsequent adaptive immune response through Toll-like receptor or
Th lymphocytes (6,29,30). In
addition, CXCL10 in serum and urine increases in patients with SLE,
depending on the level of disease activity and of anti-double
stranded DNA antibody (41). In the
present study, a correlation analysis of CXCL10 mRNA and disease
activity was performed. The results indicated no difference in
CXCL10 mRNA levels between patients with active and inactive SLE
(data not shown). Furthermore, CXCL10 mRNA levels were not
associated with any clinical indicators (data not shown).
Therefore, it is likely that CXCL10 may be involved in the
pathogenesis of SLE through other mechanisms at the transcriptional
level, including cAMP-dependent protein kinase A signaling
pathways, mitogen-activated protein kinase/PI3K and JNK/STAT
(42). It has been reported that
CXCL10 was able to polarize effector type I Th (Th1) and Th17
cells, whose imbalance contributes to the symptoms of SLE (30,43).
In the present study, CXCL10 mRNA expression in
CD4+ Th cells and CD19+ B cells was compared
between SLE patients and health controls for the first time, to the
best of our knowledge. The absence of a significant difference of
CXCL10 in Th cells between the two groups was not expected, but it
may be explained by multiple antagonistic CD4+
lymphocyte subpopulations. It is well-known that the balance of
Th1/Th2 and Th17/T-regulatory (Treg) cells as a large regulatory
network is important in biological processes, and opposite
alternations of CXCL10 in these cells may lead to unchanged total
expression. Further research by our group will investigate possible
causes. Furthermore, CXCL10 expression in the B lymphocytes of SLE
patients was increased compared with that in controls. This result
was similar to previous results in immature dendritic cells (DCs)
(30,44). The expected result may be due to the
UV light, as previously indicated (5,45),
particularly when directly considering DCs (46). As a result, enhanced expression of
CXCL10 may contribute to the interaction of immune and inflammatory
responses and may have a vital role in the activity of SLE.
Finally, potential factors that may facilitate the
expression of CXCL10 were explored in the present study. A previous
study from Germany reported that polymorphisms in the CXCL10 gene
may change the chemotaxis of activated T cells to targets and
directly influence the severity of Grave's disease (27). Furthermore, polymorphisms in CXCL10
determine the secretion of the chemokine by monocyte-derived DCs
upon exposure to Aspergillus fumigatus, and are closely
associated with the risk of invasive aspergillosis after allogeneic
stem-cell transplantation (44).
Numerous studies on the function of genes have focused on the
binding of micro (mi)RNA with SNPs of their target gene (47,48). In
addition, it is well known that miRNAs inhibit the translation of
their corresponding mRNA by directly binding to their 3′UTR, so it
may be hypothesized that SNPs at the 3′UTR of CXCL10 are associated
with its expression. Thus, four potential SNPs within the 3′UTR of
CXCL10 we examined in the present study, but no mutation was
identified in these polymorphic sites. However, correlations of
CXCL10 gene promoter polymorphisms with the protein in plasma and
disease susceptibility have been reported in infectious diseases,
including malaria (49) and
hepatitis B virus (50). Similar
research on CXCL10 will be performed by our group in the
future.
In conclusion, the present study indicated that
CXCL10, a pivotal node in transcriptional gene expression networks,
synergistically functions with other genes in the pathogenesis of
SLE. It was revealed that CXCL10 mRNA is highly and significantly
expressed in the PBMCs and B lymphocytes of SLE patients compared
with that in controls, while its expression is unaltered in Th
cells. However, no mutation was identified within four SNPs in the
3′UTR of CXCL10. Future studies on the expression of CXCL10 in
other subgroups of Th cells, including Th1, Th2, Th17 and Treg
cells, are required in order to better understand the role of
CXCL10 in the mechanisms of SLE, or of SNPs in the promoter region
of CXCL10.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Applied Basic
Research Foundation of Yunnan Province (Grant no. 2017FE468 (−004))
and the National Natural Science Foundation of China (Grant no.
81360457).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XLL participated in the study design. RXZ and YaLi
performed the PCR experiments. YiLi, BPP and AML performed data
analysis and interpreted the patients' clinical data. RXZ was a
major contributor in writing the manuscript. YaLi and AML revised
the manuscript. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Kunming Medical University
(Shen-PJ-2018-40). All subjects provided written informed
consent.
Patient consent for publication
Patient consent for publication was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stojan G and Petri M: Epidemiology of
systemic lupus erythematosus: An update. Curr Opin Rheumatol.
30:144–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghodke-Puranik Y and Niewold TB:
Immunogenetics of systemic lupus erythematosus: A comprehensive
review. J Autoimmun. 64:125–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quintero OL, Amador-Patarroyo MJ,
Montoya-Ortiz G, Rojas-Villarraga A and Anaya JM: Autoimmune
disease and gender: Plausible mechanisms for the female
predominance of autoimmunity. J Autoimmun. 38:J109–J119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barbhaiya M and Costenbader KH:
Environmental exposures and the development of systemic lupus
erythematosus. Curr Opin Rheumatol. 28:497–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbhaiya M and Costenbader KH:
Ultraviolet radiation and systemic lupus erythematosus. Lupus.
23:588–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernatsky S, Fournier M, Pineau CA, Clarke
AE, Vinet E and Smargiassi A: Associations between ambient fine
particulate levels and disease activity in patients with systemic
lupus erythematosus (SLE). Environ Health Perspect. 119:45–49.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zandman-Goddard G and Shoenfeld Y:
Infections and SLE. Autoimmunity. 38:473–485. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Yin Y, Zhang E, Zhu G, Liu M,
Feng L, Qin B and Liu X: Spectral attenuation of ultraviolet and
visible radiation in lakes in the Yunnan Plateau, and the middle
and lower reaches of the Yangtze River, China. Photochem Photobiol
Sci. 10:469–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong H, Li XL, Li M, Hao LX, Chen RW,
Xiang K, Qi XB, Ma RZ and Su B: Replicated associations of TNFAIP3,
TNIP1 and ETS1 with systemic lupus erythematosus in a southwestern
Chinese population. Arthritis Res Ther. 13:R1862011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian G, Ran X, Zhou CX, Deng DQ, Zhang PL,
Guo Y, Luo JH, Zhou XH, Xie H and Cai M: Systemic lupus
erythematosus patients in the low-latitude plateau of China:
Altitudinal influences. Lupus. 23:1537–1545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dominguez-Gutierrez PR, Ceribelli A, Satoh
M, Sobel ES, Reeves WH and Chan EK: Reduced levels of CCL2 and
CXCL10 in systemic lupus erythematosus patients under treatment
with prednisone, mycophenolate mofetil, or hydroxychloroquine,
except in a high STAT1 subset. Arthritis Res Ther. 16:R232014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crow MK: Type I interferon in the
pathogenesis of lupus. J Immunol. 192:5459–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antonelli A, Ferrari SM, Giuggioli D,
Ferrannini E, Ferri C and Fallahi P: Chemokine (C-X-C motif) ligand
(CXCL)10 in autoimmune diseases. Autoimmun Rev. 13:272–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruck P, Bartsch W, Penna-Martinez M,
Kahles H, Seidl C, Böhme A, Badenhoop K and Ramos-Lopez E:
Polymorphisms of CXCR3-binding chemokines in type 1 diabetes. Hum
Immunol. 70:552–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruffilli I, Ferrari SM, Colaci M, Ferri C,
Fallahi P and Antonelli A: IP-10 in autoimmune thyroiditis. Horm
Metab Res. 46:597–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauer JW, Petri M, Batliwalla FM, Koeuth
T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK,
Behrens TW and Baechler EC: Interferon-regulated chemokines as
biomarkers of systemic lupus erythematosus disease activity: A
validation study. Arthritis Rheum. 60:3098–3107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reynolds JA, McCarthy EM, Haque S,
Ngamjanyaporn P, Sergeant JC, Lee E, Lee E, Kilfeather SA, Parker B
and Bruce IN: Cytokine profiling in active and quiescent SLE
reveals distinct patient subpopulations. Arthritis Res Ther.
20:1732018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Leary NA, Wright MW, Brister JR, Ciufo
S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B,
Ako-Adjei D, et al: Reference sequence (RefSeq) database at NCBI:
Current status, taxonomic expansion, and functional annotation.
Nucleic Acids Res. 44:D733–D745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hinrichs AS, Raney BJ, Speir ML, Rhead B,
Casper J, Karolchik D, Kuhn RM, Rosenbloom KR, Zweig AS, Haussler D
and Kent WJ: UCSC data integrator and variant annotation
integrator. Bioinformatics. 32:1430–1432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aken BL, Ayling S, Barrell D, Clarke L,
Curwen V, Fairley S, Fernandez Banet J, Billis K, García Girón C,
Hourlier T, et al: The Ensembl gene annotation system. Database
(Oxford). 2016(pii): baw0932016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Díaz-Montaña JJ, Gómez-Vela F and
Díaz-Díaz N: GNC-app: A new Cytoscape app to rate gene networks
biological coherence using gene-gene indirect relationships.
Biosystems. 166:61–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foster MH: T cells and B cells in lupus
nephritis. Semin Nephrol. 27:47–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruck P, Bartsch W, Sadet D,
Penna-Martinez M, Kurylowicz A, Bednarczuk T, Robbers I, Paunkovic
J, Böhme A, Badenhoop K and Ramos-Lopez E: A CXC motif ligand 10
polymorphism as a marker to predict severity of Graves' disease.
Thyroid. 20:343–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Li M, Wang Y, Xu D, Wang Q, Zhang
S, Zhao J, Su J, Wu Q, Shi Q, et al: Long-term mortality and
morbidity of patients with systemic lupus erythematosus: A
single-center cohort study in China. Lupus. 27:864–869. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kemp MG, Lindsey-Boltz LA and Sancar A: UV
light potentiates STING (Stimulator of Interferon Genes)-dependent
innate immune signaling through deregulation of ULK1 (Unc51-like
Kinase 1). J Biol Chem. 290:12184–12194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olson JK, Croxford JL and Miller SD:
Virus-induced autoimmunity: Potential role of viruses in
initiation, perpetuation and progression of T-cell-mediated
autoimmune disease. Viral immunol. 14:227–250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng D and Barnes BJ: Bioinformatics
analysis of the factors controlling type I IFN gene expression in
autoimmune disease and virus-induced immunity. Front Immunol.
4:2912013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Becker AM, Dao KH, Han BK, Kornu R,
Lakhanpal S, Mobley AB, Li QZ, Lian Y, Wu T, Reimold AM, et al: SLE
peripheral blood B cell, T cell and myeloid cell transcriptomes
display unique profiles and each subset contributes to the
interferon signature. PLoS One. 8:e670032013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ronnblom L and Alm GV: Systemic lupus
erythematosus and the type I interferon system. Arthritis Res Ther.
5:68–75. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garcia-Sastre A and Biron CA: Type 1
interferons and the virus-host relationship: A lesson in detente.
Science. 312:879–882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawai T and Akira S: Innate immune
recognition of viral infection. Nat Immunol. 7:131–137. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Ni J, Li LJ, Leng RX, Pan HF and Ye
DQ: Decreased UBASH3A mRNA expression levels in peripheral blood
mononuclear cells from patients with systemic lupus erythematosus.
Inflammation. 38:1903–1910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bauer JW, Petri M, Batliwalla FM, Koeuth
T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK,
Behrens TW and Baechler EC: Interferon-regulated chemokines as
biomarkers of systemic lupus erythematosus disease activity: A
validation study. Arthritis Rheum. 60:3098–3107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rose T, Grutzkau A, Hirseland H, Huscher
D, Dähnrich C, Dzionek A, Ozimkowski T, Schlumberger W, Enghard P,
Radbruch A, et al: IFNα and its response proteins, IP-10 and
SIGLEC-1, are biomarkers of disease activity in systemic lupus
erythematosus. Ann Rheum Dis. 72:1639–1645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oke V, Brauner S, Larsson A, Gustafsson J,
Zickert A, Gunnarsson I and Svenungsson E: IFN-λ1 with Th17 axis
cytokines and IFN-α define different subsets in systemic lupus
erythematosus (SLE). Arthritis Res Ther. 19:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee HT, Wu TH, Lin CS, Lee CS, Pan SC,
Chang DM, Wei YH and Tsai CY: Oxidative DNA and mitochondrial DNA
change in patients with SLE. Front Biosci (Landmark Ed).
22:493–503. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narumi S, Takeuchi T, Kobayashi Y and
Konishi K: Serum levels of IFN-inducible protein-10 relating to the
activity of systemic lupus erythematosus. Cytokine. 12:1561–1565.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu M, Guo S, Hibbert JM, Jain V, Singh N,
Wilson NO and Stiles JK: CXCL10/IP-10 in infectious diseases
pathogenesis and potential therapeutic implications. Cytokine
Growth Factor Rev. 22:121–130. 2011.PubMed/NCBI
|
|
43
|
Touzot M, Cacoub P, Bodaghi B, Soumelis V
and Saadoun D: IFN-α induces IL-10 production and tilt the balance
between Th1 and Th17 in Behçet disease. Autoimmun Rev. 14:370–375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mezger M, Steffens M, Beyer M, Manger C,
Eberle J, Toliat MR, Wienker TF, Ljungman P, Hebart H, Dornbusch
HJ, et al: Polymorphisms in the chemokine (C-X-C motif) ligand 10
are associated with invasive aspergillosis after allogeneic
stem-cell transplantation and influence CXCL10 expression in
monocyte-derived dendritic cells. Blood. 111:534–536. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng GM and Tsokos GC: Pathogenesis and
targeted treatment of skin injury in SLE. Nat Rev Rheumatol.
11:663–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wahren-Herlenius M and Dörner T:
Immunopathogenic mechanisms of systemic autoimmune disease. Lancet.
382:819–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Holder A, Jones G, Soutter F, Palmer DB,
Aspinall R and Catchpole B: Polymorphisms in the canine IL7R 3′UTR
are associated with thymic output in Labrador retriever dogs and
influence post-transcriptional regulation by microRNA 185. Dev Comp
Immunol. 81:244–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Wang L, Chi H, Wang J, Zheng Q, Li
J, Li Y and Yang D: The SNP Rs915014 in MTHFR regulated by MiRNA
associates with atherosclerosis. Cell Physiol Biochem.
45:1149–1155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wilson N, Driss A, Solomon W,
Dickinson-Copeland C, Salifu H, Jain V, Singh N and Stiles J:
CXCL10 gene promoter polymorphism-1447A>G correlates with plasma
CXCL10 levels and is associated with male susceptibility to
cerebral malaria. PLoS One. 8:e813292013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deng G, Zhou G, Zhang R, Zhai Y, Zhao W,
Yan Z, Deng C, Yuan X, Xu B, Dong X, et al: Regulatory
polymorphisms in the promoter of CXCL10 gene and disease
progression in male hepatitis B virus carriers. Gastroenterology.
134:716–726. 2008. View Article : Google Scholar : PubMed/NCBI
|