Introduction
Long-term alcohol consumption frequently leads to
development and progression of non-ischemic dilated cardiomyopathy
(NIDCM), also known as alcoholic cardiomyopathy (ACM) (1). Alcohol exerts diverse toxic effects on
the heart contributing to heart failure, conduction block, atrial
fibrillation, myocardial remodeling and cardiac anomalies
associated with metabolism and function. In NIDCM patients, who
never stop their alcohol intake, the 4-year mortality rate was as
high as 50% (2,3). However, the mechanism of action of
alcohol in NIDCM has not been elucidated.
Alterations in the metabolism of fatty acid ethyl
esters cause decreased β-oxidation of fatty acids and contribute to
metabolic disturbances in myocardial cells (4–6).
Previous studies suggest alcohol intake as a cause of increased
plasma homocysteine, which is associated with oxidative stress,
mitochondrial dysfunction and inflammation, all of which induce
myocardial fibrosis and cardiac remodeling (7–9).
Tenascin, a major protein of the extracellular matrix is divided
into 6 subtypes, produced by fibroblasts, along with collagen
mediates the process of fibrosis (10). Peroxisome proliferator-activated
receptor α (PPARα) is a key enzyme involved in the regulation of
fatty acid oxidation (11,12). Retinoid × receptor α (RXRα) PPARα and
RXRα are the major nuclear transcription factors involved in the
energy metabolism of fatty acid in myocardial cells and in
remodeling the myocardium (13).
Angiotensin II via activation of angiotensin II type I receptor
increases superoxide anion generated by NADPH, while suppressing
angiotensin II ameliorates oxidative stress and fibrosis (14). Almost all cases of ACM are associated
with cardiac remodeling induced by myocardial fibrosis and
oxidative stress (14).
Nevertheless, the mechanisms of ACM remain unclear.
Several hypotheses have been postulated regarding
the pathogenesis of ACM, including the toxic effects of alcohol on
the heart and enhanced oxidative stress (15). However, only limited studies have
focused on the effect of Ras homolog gene family, member A (RhoA),
Rho-associated protein kinase 2 (ROCK2) and myosin light chain
(MYL) in the pathogenesis of ACM. A previous study has indicated
that ethanol could disrupt the junction between intestinal
epithelial cells through activation of the RhoA-ROCK pathway
(16). The RhoA-ROCK pathway alters
the smooth muscle cell cytoskeleton and causes remodeling of the
respiratory tract in infant mice (17). In nucleus pulposus cells, renin
activates the RhoA-ROCK pathway, thereby inducing the remodeling of
the cytoskeleton (18). The
RhoA/Rho-kinase pathway serves an important role in various
fundamental cellular functions, including production of excessive
reactive oxygen species, leading to the development of
cardiovascular diseases (19).
Rho-kinase also upregulates NAD(P)H oxidases (Nox1, Nox4, gp91phox
and p22phox), and augments AngII-induced ROS production (20,21). The
role of RhoA-ROCK in the pathogenesis of ACM is still not clearly
elucidated. The present study aims to interpret altered expression
of the RhoA-ROCK pathway, MYL and its downstream targets in the
pathogenesis, and treatment of ACM. In addition, the therapeutic
effects of valsartan on ACM were analyzed. Future research aimed at
elucidating the pathogenesis of ACM may contribute to significant
breakthroughs that might prove beneficial for the diagnosis and
treatment of ACM.
Materials and methods
Instruments and reagents
Refrigerators and deep freezers (4°C, −20°C and
−80°C) (Haier, Qingdao, China); light microscopes (Olympus
Corporation, Tokyo, Japan); color Doppler ultrasound diagnostic
system (GE Healthcare, Chicago, IL, USA); pathological image
analysis system (Motic Images Advanced 3.0; Motic Asia, Hong Kong,
China); gel-image analyzer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA); electronic scale (Shanghai Scale, Shanghai, China);
liquid nitrogen biological container (Chengdu Jinfeng Liquid
Nitrogen Container Co., Ltd., Chengdu, China); Langendorff
perfusion system (Etiological Lab of Harbin Medical University,
Harbin, China); microplate reader (Tekon Scientific Corp., Taipei
city, Taiwan); electrophoresis system and electronic transfer
(Beijing Liuyi Biotechnology Co., Ltd., Beijing, China); centrifuge
(Kaidi Machinery Co., Ltd., Jiangsu, China); quantitative PCR
system (Shanghai Zhiyan, China); thermostatic water (Shanghai
Medical Analytic Instrument Factory, Shanghai, China) were used in
the present study.
In addition the following reagents were purchased:
Valsartan capsules (7 tablets, 80 mg/tablet; Novartis International
AG, Basel, Switzerland); 98% ethanol (500 ml), 10% chloral hydrate,
heparin, Ca2+-free Tyrode solution,
Ca2+-contained Tyrode solution and PBS solution (8.0 g
of NaCl, 0.2 g of KCl, 1.26 g of
Na2HPO4•12H2O, and 0.2 g of
KH2PO4 adjust the pH to 7.2 with 1 mol/l HCl
or 1 mol/l NaOH to 1,000 ml, PBS was provided by the Etiological
Lab of Harbin Medical University), collagenase II and albumin
(Zhongtian World, Harbin, China); radioimmunoprecipitation assay
lysis buffer, Benzonase, TEMED, bicinchoninic acid kit, 10% SDS,
30% Acr-Bis (29:1), Tris, SDS buffer, enhanced chemiluminescence
reagent substrate (cat. no. no32106; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), skimmed milk powder (Beyotime
Institute of Biotechnology, Haimen, China); polyvinylidene
difluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA);
mouse anti-RhoA polyclonal antibody (1:1,000; cat. no. ab54835;
Abcam, Cambridge, MA, USA), mouse anti-MYL1 polyclonal antibody
(1:1,000; cat. no. PA5-29635 Invitrogen; Thermo Fisher Scientific,
Inc.), goat anti-ROCK polyclonal antibody (1:1,000; sc-1851; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA); β-actin (1:5,000; cat.
no. ab8227; Abcam) horseradish peroxidase (HRP)-labeled mouse
anti-immunoglobulin (Ig)G antibody (1:5,000; cat. no. sc-2005;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), HRP-labeled goat
anti-IgG antibody (1:5,000; cat. ab6721; Abcam); citrate sodium
buffer, PBST, 30% H2O2, and hematoxylin
(provided by Etiological Lab of Harbin Medical University); RNA
extraction kit Trizol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); Accupower RocketScript RT PreMix (Bioneer
Corporation, Daejeon, Korea); Real MasterMix (SYBR Green, Tiangen,
China); primer synthesis for qPCR (Bioneer Corporation).
Subjects
A total 120, 8–10 weeks (280–300 g) healthy male
Wistar rats were purchased from the Changchun Yisi Experimental
Animal Co., Ltd., (Changchun, China). Animals were maintained in a
controlled environment (12-h light/dark cycle; temperature, 27±2°C;
humidity, 35±5%). The animals were fed a standard pellet diet and
water was freely available. Animals were maintained at the
Experimental Animal Center of the First Affiliated Hospital of
Harbin Medical University. This study was approved by the First
Affiliated Hospital of Harbin Medical University.
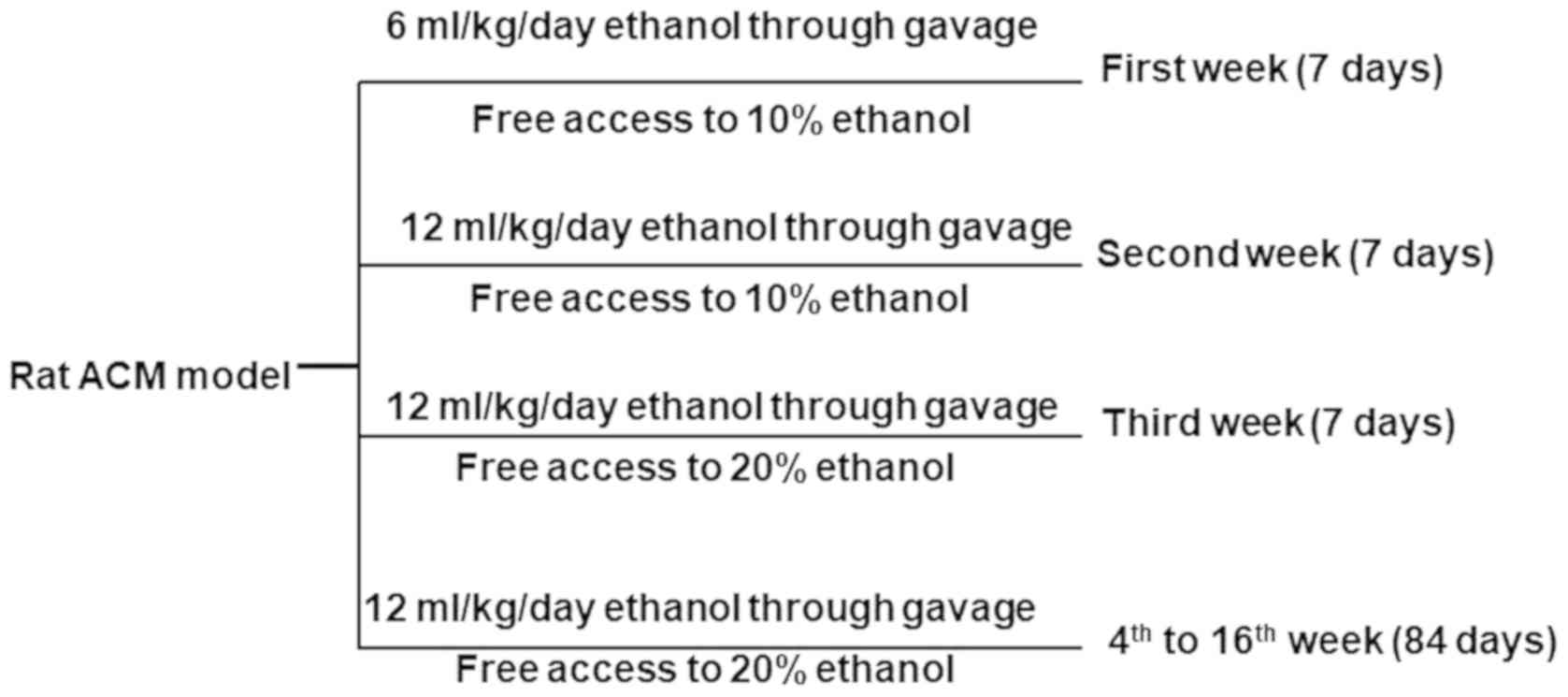
Establishing a rat model of ACM
A total of 120 male Wistar rats were randomly
divided into three groups, n=40 namely, the control group, the
alcohol group and the alcohol + valsartan group (treatment group).
ACM was induced in rats through alcoholic gavage and free access to
alcohol. During the first week, rats received 60% ethanol (6
ml/kg/day) through gavage and had free access to 10% ethanol all
day long. In the second week, they received 12 ml/kg/day ethanol
and had free access to 10% ethanol. Through the third week, rats
were continued to receive 60% ethanol at a dose of 12 ml/kg/day but
had free access to 20% ethanol. From the 4th to 16th week, rats
were received a gavage of 60% ethanol at a dose of 15 ml/kg/day,
which was carried out twice per day, along with free access to 20%
ethanol. For rats in the treatment group, valsartan at a dose of 8
mg/kg/day was additionally administered. Rats in the control group
were fed with regular water and food. The experimental design is
presented in Fig. 1.
Doppler echocardiography
Rats were anesthetized by intraperitoneal (IP)
injection of 10% chloral hydrate (300 mg/kg). Cardiac color
ultrasonic scanner (GE Healthcare) and a probe (10 MHz) was used to
examine the variations in the structure and function by
professional sonographers. The following were examined, namely, the
left ventricular end diastolic diameter (LVDD), ejection fraction
(EF) of the left ventricle, left ventricular fractional shortening
(FS) and the early/atrial ratio in three consecutive cardiac
cycles. The results were averaged.
Collection of specimen
The animals were euthanized under sodium
pentobarbital anesthesia. After sacrifice the rat hearts were
isolated and rinsed with pre-cooled normal saline. Tissue specimens
were collected from the transverse section of the left ventricular
myocardium. Briefly, tissues were cut from the apex of the heart
and isolated from the free wall of the left ventricle parallel to
its longitude axis on ice. Specimens were fixed in 4% formaldehyde
for 4 h at 4°C, were paraffin embedded, serial sectioned and
stained with hematoxylin for 5–10 min at room temperature.
Remaining tissues were preserved at −80°C until further use.
Hematoxylin & eosin (H&E)
staining
Sections were dewaxed twice in xylene (10 min each).
Sections were rehydrated sequentially in descending series of
alcohol for 5 min each in anhydrous, 90, 80 and 70% alcohol.
Sections were then treated with phosphate buffered saline, 0.1%
Tween-20 (PBST) for 2 min. Specimens were stained by immersing in
hematoxylin for 5–10 min at room temperature, treated with 1% acid
alcohol for 3 sec, washed with running water for 10 min, washed
with distilled water for 1 or 2 min, staining with 0.5% eosin for
1–3 min and washed with distilled water for 2 sec. Specimens were
then dehydrated twice in 95% ethanol for 2 min each and cleared by
treating twice with xylene for 5 min each. Sections were then
mounted with neutral balsam and observed under a light microscope.
As anticipated, the nuclei were stained red, while the cytoplasm
was stained pink.
Masson's trichrome staining
Sequentially, specimens were dewaxed, washed with
running water and treated with a mordant for 30 min. Specimens were
then stained with hematoxylin for 20 min at room temperature,
washed with running water, treated with acidic alcohol for 10 to 15
sec, washed again with running water, treated with ammonia for 10
to 15 sec and the reaction was terminated by washing with running
water. Thereafter, specimens were stained in Masson solution for 1
min at room temperature, washed in acetic acid and observed under a
light microscope.
Immunohistochemistry (IHC)
Paraffin sections were dewaxed, incubated with 3%
H2O2 for 5 to 10 min at 25°C to block
endogenous peroxidase activity, rinsed with distilled water and
treated twice with PBS (5 min each). Sections (4–6 µm-thick) were
then blocked at at room temperature in 5 to 10% normal goat serum
diluted in PBS for 10 min and incubated overnight with primary
antibodies at 37°C for 1 to 2 h or 4°C overnight. Sections were
washed thrice in PBS (5 min each), incubated with biotin-labeled
secondary antibodies at 37°C for 10 to 30 min, washed thrice in PBS
(5 min each), incubated with HRP- or alkaline phosphatase-labeled
streptavidin for 10 to 30 min at 37°C and washed thrice with PBS (5
min each). After washing, slides were incubated with
3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 2 h at room temperature and
immediately washed under tap water following color development.
Slides were then counter stained with hematoxylin for 10 min at
room temperature. Slides were mounted with dibutyl phthalate xylene
and observed under a light microscope (Carl Zeiss AG, Oberkochen,
Germany).
Sample extraction
In a water bath set at 39°C, the Langendorff channel
was rinsed twice with deionized water and filled with calcium-free
tyrode solution. Digestive solution was prepared using 8 mg
albumin, 0.5 mg collagenase and 50 ml calcium-free Tyrode solution.
Hank's Balanced Salt Solution stored at −20°C was thawed for later
use.
Extraction of myocardial cells
Rats represented as subjects were IP injected with 2
ml of heparin. Rats were anesthetized 20 min later by IP injection
of 2 ml chloral hydrate (10%). Thoracic surgery was conducted on
the rats to isolate the heart. The heart was isolated by excising
the aorta at the distal end. The heart was immediately transferred
into a calcium-containing Tyrode solution in a culture dish, where
the surrounding pulmonary tissues and vessels were dissected
rapidly. The aorta has three branches at the upper end and is
located beneath the two white thymus glands. At the bifurcation,
the aorta was dissected to expose its outlet into which the
12# needle of the 20 ml injector containing calcium-free
Tyrode solution was inserted, followed by ligation with a suture
for fixation. The injector was slowly pushed and the needle
connected with the heart was inserted in the T-Cock. Thereafter,
perfusion with calcium-free Tyrode solution was carried out. When
the level of the solution decreased beneath the neck of the tube,
digestive solution was placed in the Langendorff device. The above
procedures were repeated and the digestive solution was collected
in a beaker. Digestion was carried out for 30 min with continuous
supplementation of digestive solution. After digestion, the white
and widened heart tissue was placed in a culture dish supplemented
with KB solution. Following excising the atrium and auriculars, the
remaining tissue was dissected and cut into pieces, placed in a
centrifuge tube supplemented with the KB solution, and beaten with
a pipette. A drop of myocardial cell suspension was dripped onto a
glass slide and observed under a light microscope (Carl Zeiss AG)
for the estimation of survival rate. Samples were immediately
centrifuged 2,000 × g for 10 min at 4°C and stored at −80°C until
further use.
Estimation of protein concentration in
samples
Cell lysis was carried out on ice using PMSF
(100:1). Cell lysates were mixed well by beating and vibration.
After 1 min of vibration, lysates were placed for 5 min,
centrifuged at 1,000 × g for 10 min at 4°C and the supernatants
were collected for protein quantification. In a 96-well plate,
samples were diluted in deionized water to a volume of 20 µl. A
total of 200 µl of solution A and B (50:1) was added to each well
and incubated for 30 min. Samples were mixed with the loading
buffer (1:4) and denatured for 10 min. A microplate reader was used
to determine the concentration of protein in the samples.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). RNA
from myocardial tissue was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as previously
described. cDNA synthesis was performed using an RT kit (cat. no.
FSQ-101; Toyobo Life Science, Osaka, Japan), according to the
manufacturer's protocol. qPCR was conducted using the
LightCycler® qPCR apparatus (Roche Molecular Systems,
Inc.). The following thermocycling conditions were used: 40 cycles
of 95°C for 15 sec and 60°C for 60 sec with Fast Start SYBR Green
master mix (Roche Molecular Systems, Inc.). mRNA expression results
were analyzed using the 2−ΔΔCq method (22). The following primer sequences were
used: RhoA forward, 5′-CTCTCTTATCCAGACACCGATGT-3′ and reverse,
5′-TGTGCTCGTCATTCCGAAGG-3′; ROCK forward,
5′-GTTCGTCATAAGGCATCACAGA-3′ and reverse,
5′-TGTTGGCAAAGGCCATAATATCT-3′; MYL forward,
5′-CCCGAAGGGCTTTCACAATCT-3′ and reverse,
5′-CCCACTCTTCCAAACAGCAG-3′.
Western blotting
The composition of the lower gel included, 10 ml
water (H2O), 3.3 ml 30% Arc-Bis (29:1), 3.8 ml 1 M Tris
(pH=8.8), 0.1 ml 10% SDS, 0.1 ml 10% ammonium persulfate and 0.004
ml tetramethylethylenediamine (TEMED). The composition of the upper
gel included, 3.4 ml H2O, 0.85 ml 30% Arc-Bis (29:1),
0.625 ml 1 M Tris (pH=6.8), 0.05 ml 10% SDS, 0.05 ml 10% ammonium
persulfate and 0.005 ml TEMED.
In the plate, the lower gel and upper gel were
sequentially added without air bubbles with the immediate insertion
of the comb in the upper gel. After coagulation, electrophoresis
buffer was added into the electrophoresis apparatus and the comb
was removed. After loading the proteins (25 µg) and marker,
electrophoresis was carried out at 80 V for 40 min and later at 120
V until the proteins and marker reached the bottom of the plate.
For blotting, the following were placed in a sequential order in
the electrophoretic unit: Sponge pad, filter paper, gel, PVDF
membrane, filter paper and sponge pad from the negative electrode
to the positive electrode without air bubbles. In the transfer
apparatus, transfer buffer was added and the transfer was carried
out at a constant current of 200 mA for 3 h at 4°C. Membranes were
blocked in 5% skimmed milk on a shaker for 2 h at room temperature.
Membranes were then incubated with RhoA anti-mouse polyclonal
antibodies (1:200), MYL anti-mouse polyclonal antibody (1:500),
ROCK anti-goat polyclonal antibody (1:200) and β-actin (1:500)
overnight at 4°C, washed thrice with TBST (5 min each), incubated
with the corresponding HRP-conjugated secondary antibodies (cat.
nos. ab7061, ab7125 and ab97085; all Abcam) for 1 h at room
temperature, and washed thrice with TBST. After exposure in the
dark with enhanced chemiluminescence reagent (Beyotime Institute of
Biotechnology; cat. no. P0018M), membranes were scanned in the gel
imaging system (cat. no. 4466613; E-Gel™ Imager System with E-Gel™
Adaptor and Bio-Rad: Universal Hood II) and densitometry was
performed using Image Lab software (version 2.0.1; Bio-Rad
Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
18.0 software (SPSS, Inc., Chicago, IL, USA). Results were
expressed as the mean ± standard deviation. Experiments were
repeated for at least 3 times. The independent sample t-test was
used for comparison between two groups. One-way analysis of
variance followed by Tukey's post-hoc test was applied for
comparison among groups; P<0.05 were considered to indicate a
statistically significant difference.
Results
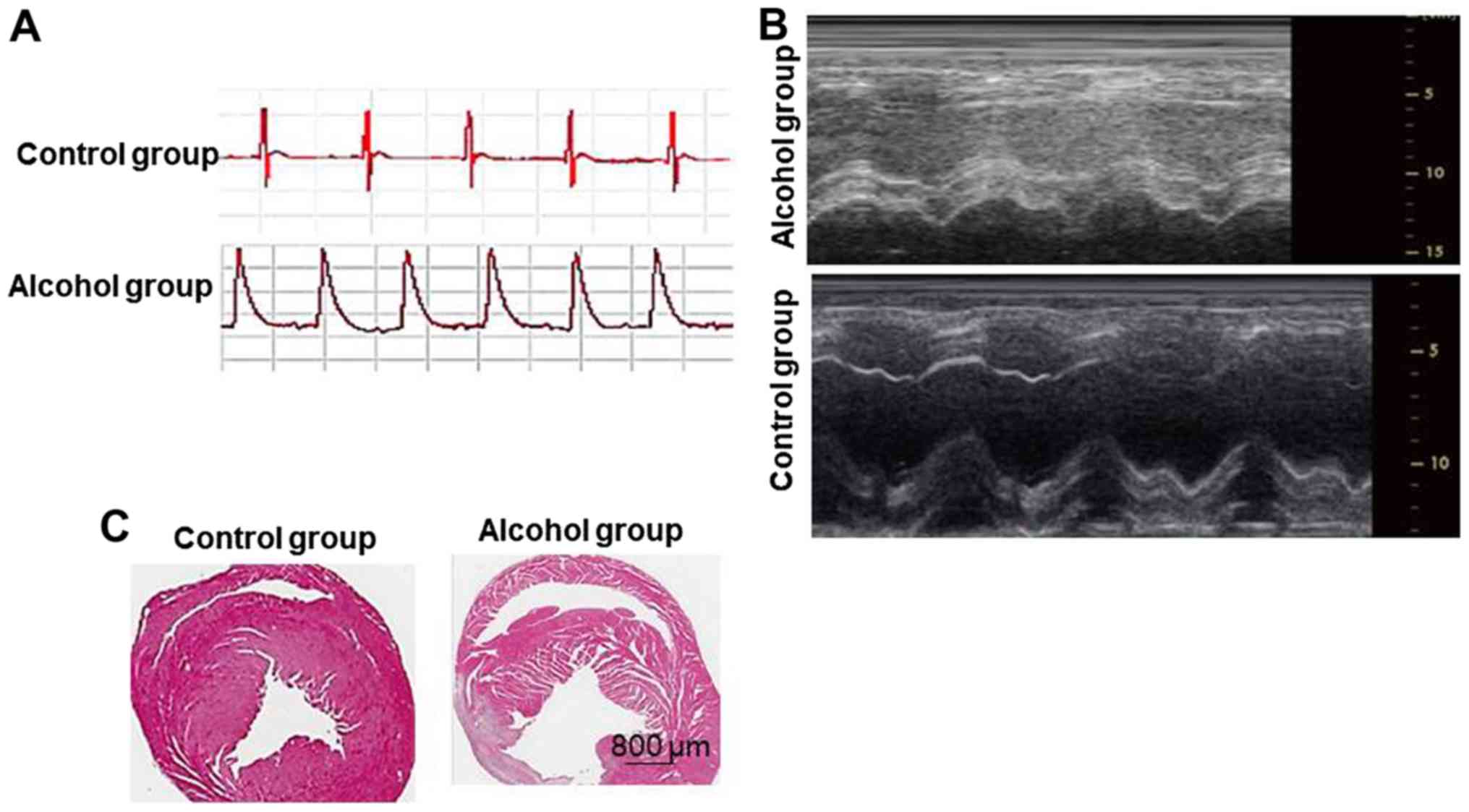
Verification of non-ischemic dilated
cardiomyopathy model
In the alcohol group, ST-segment elevation (>1/2
R waves) in left ventricular coronary arteries following left
coronary artery ligation demonstrated a single-peak curve, which is
a sign of successful rat non-ischemic dilated cardiomyopathy
(Fig. 2A). Ultrasound detection
demonstrated that the thickness of the left ventricular myocardium
in the Control group was uniform, with good activity and no
abnormal beats. In the alcohol group, the activity of the left
ventricular myocardium was weakened (Fig. 2B). HE staining results demonstrated
that the myocardial cells in the alcohol group exhibited
compensatory hypertrophy, no inflammatory cell infiltration; it
also exhibited fibrosis, disordered arrangement of fibers, clear
border between infarct and non-infarct border, a small amount of
inflammatory cell infiltration can be seen (Fig. 2C). The success of model formation
rate was >80%.
Enhanced LVDD with reduced EF and FS
is demonstrated in rat models of ACM-amelioration of cardiac
functions by valsartan
LVDD was increased with a decreased EF and FS in rat
models of ACM (alcohol group) compared with the control group. The
corresponding levels in the treatment group were between the
control group and the alcohol group. The detected differences were
statistically significant. The results of the present study suggest
enlargement of the left ventricle with decreased EF and myocardial
contractility associated with alcohol intake (Table I), which were ameliorated by the use
of valsartan that led to improved cardiac function.
 | Table I.LVDD, EF and FS in every group. |
Table I.
LVDD, EF and FS in every group.
| Groups | LVDD (mm) | EF (%) | FS (%) | LVSD (mm) | E/A ratio |
|---|
| Control (n=15) | 5.23±0.69 | 73.45±8.35 | 47.46±4.36 | 2.89±0.65 | 1.98±0.32 |
| Alcohol (n=15) |
7.76±0.65a |
43.12±5.34a |
30.56±2.45a | 5.78±0.46 | 0.94±0.25 |
| Alcohol + valsartan
(n=15) |
6.39±0.73a,b |
54.34±5.38a,b |
35.74±3.65a,b | 4.15±0.39 | 1.63±0.21 |
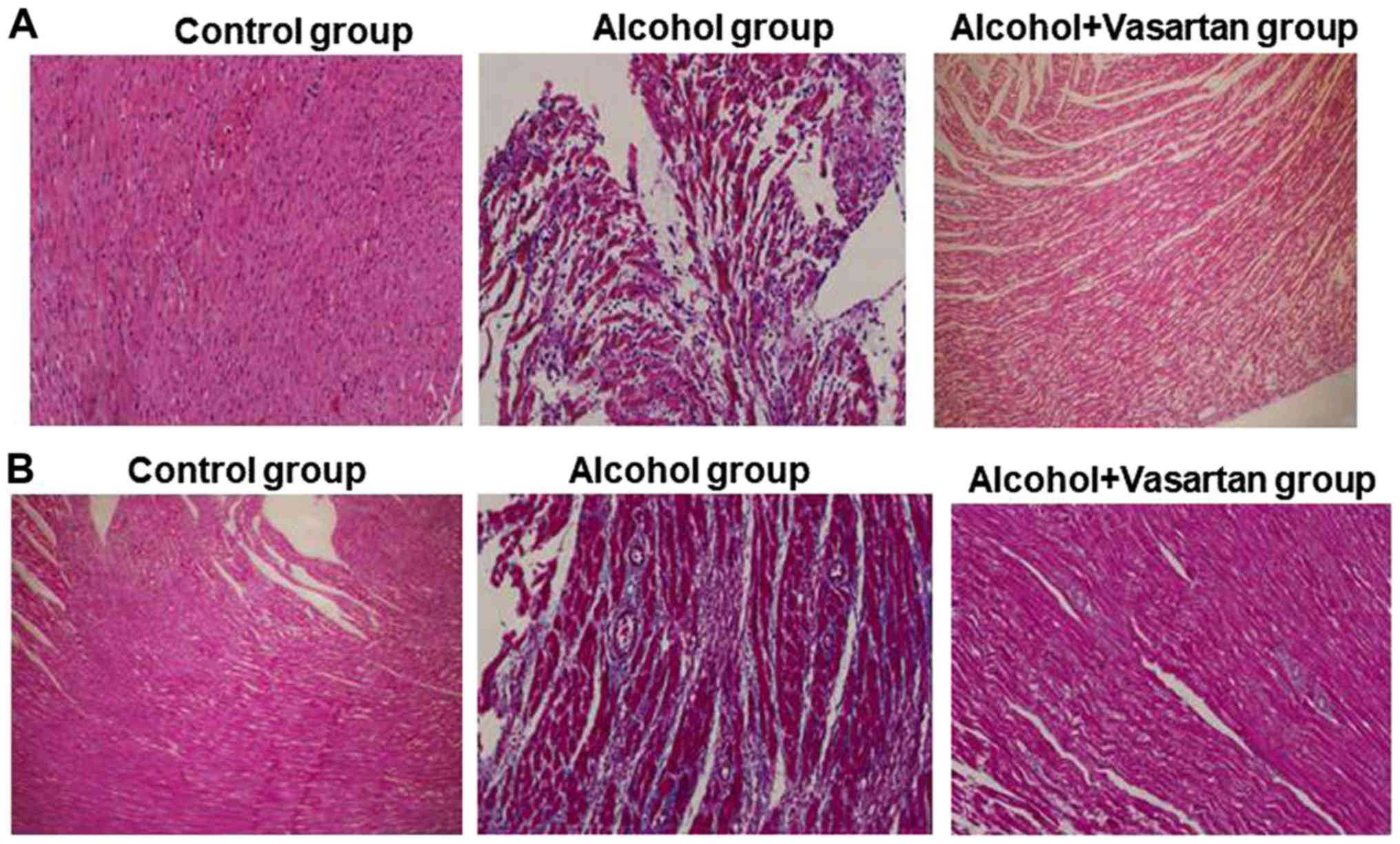
Disorganized arrangement and increased
fibrosis of myocardial filaments in rat model of ACM-rectification
by valsartan
In the alcohol group, HE staining revealed a
disorganized arrangement and rupture of myocardial filaments, an
enlarged intercellular space with edema and massive inflammatory
infiltration indicated that cells were signaled to undergo
apoptosis (Fig. 3A) compared with
the control group, which displayed ordered arrangement of
myocardial filaments, evenly distributed cytoplasm without rupture,
enlargement of intercellular space, effusion edema or inflammatory
infiltration. In the treatment group, cells were in a closely
packed arrangement with reduced infiltration of inflammatory cells
compared with the alcohol group. Masson's trichrome staining
revealed increased fibrosis of myocardial cells in the alcohol
group compared with the control group with no fibrosis (Fig. 3B). The degree of fibrosis in the
treatment group was between the control and alcohol groups. The
results of the present study indicate alleviation of enhanced
fibrosis of myocardial cells in ACM by valsartan.
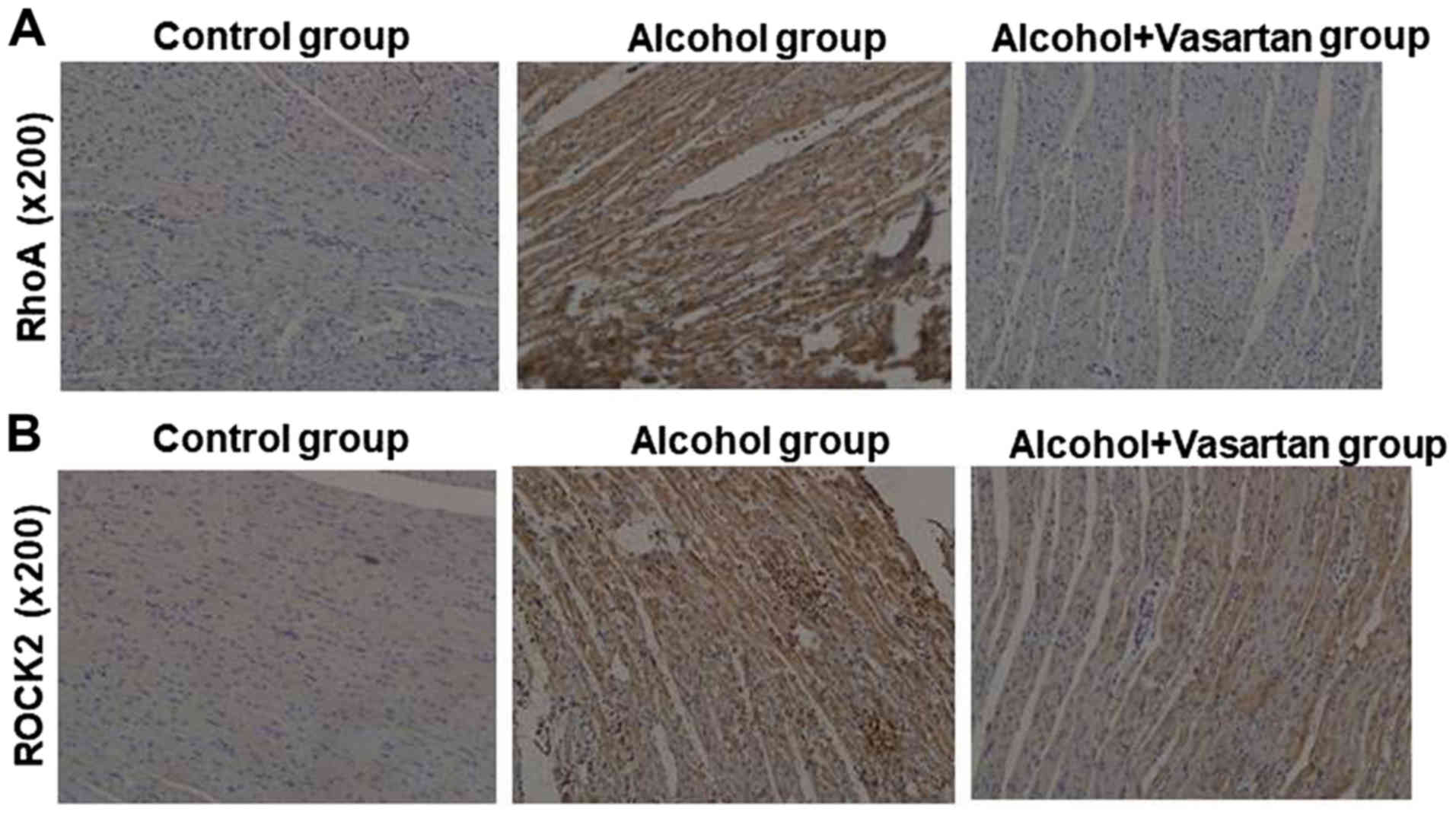
Elevated RhoA and ROCK in myocardial
tissues of rat models of ACM-reversal by valsartan
In the myocardial tissue, IHC results demonstrated
elevated expression of RhoA and ROCK in the alcohol group compared
with the control group (Fig. 4A and
4B). The myocardial expression of
RhoA and ROCK was decreased in the treatment group compared with in
the alcohol group.
Augmented protein and mRNA expressions
of RhoA and ROCK and decreased MYL in myocardial cells-amelioration
by valsartan
In the myocardial tissue, the expression of RhoA and
ROCK were significantly elevated and MYL was significantly
decreased in the alcohol group compared with the control group
(P<0.05; Fig. 5A-D). The
myocardial expression of RhoA and ROCK were significantly
downregulated along with upregulation of MYL in the treatment group
compared with the alcohol group (P<0.05). In the myocardial
tissue, fluorescence quantitative PCR results indicated elevated
mRNA expressions of RhoA and ROCK in the alcohol group compared
with the control group (P<0.05; Fig.
5E and F). The mRNA expressions of RhoA and ROCK were decreased
in the treatment group compared with the alcohol group (P<0.05;
Fig. 5E and 5F). The mRNA expression of MYL was
decreased in the alcohol group compared with the control group. The
mRNA expression of MYL was increased in the treatment group
compared with the alcohol group (P<0.05; Fig. 5G).
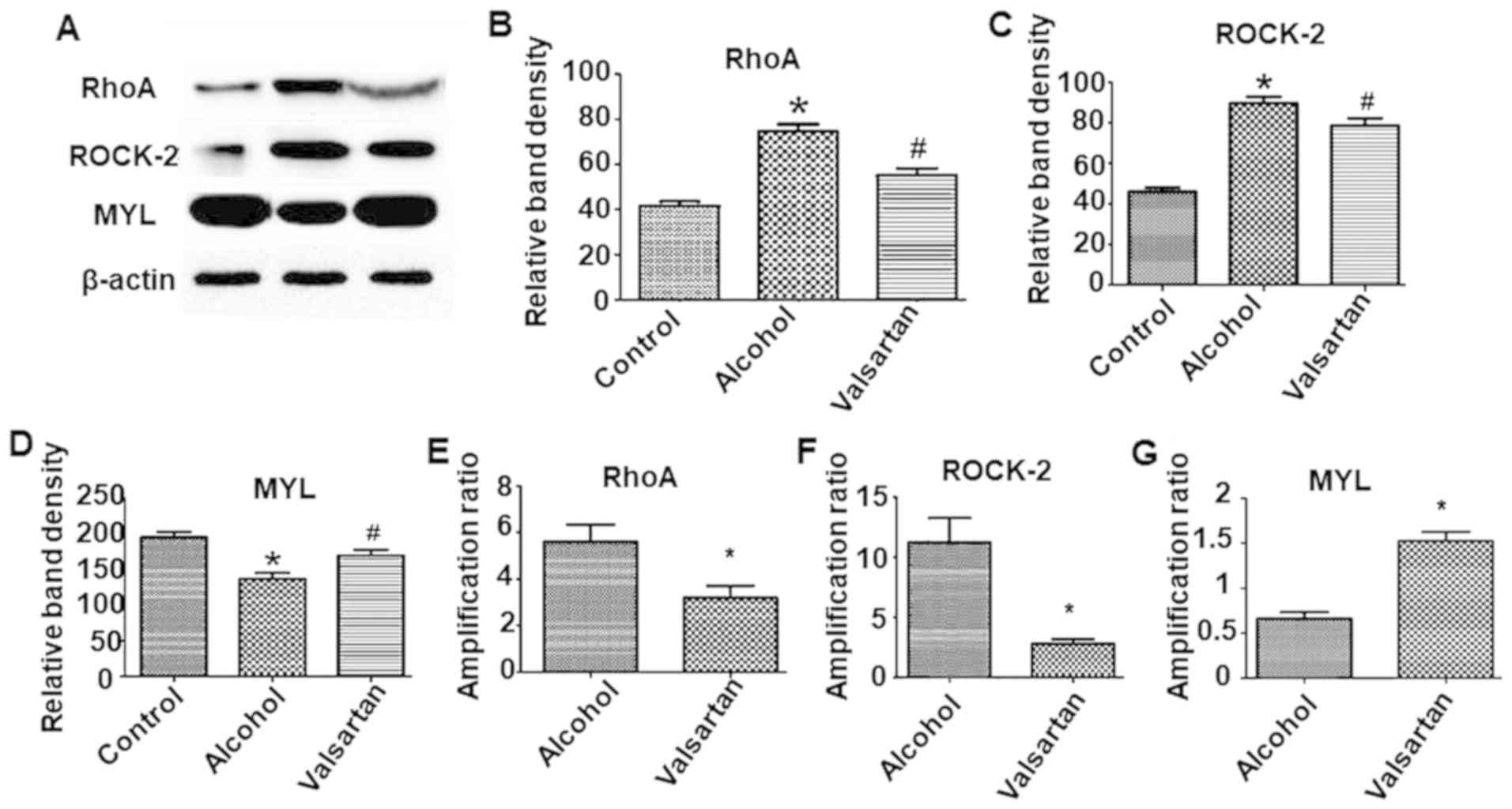 | Figure 5.Effect of valsartan on protein
expression level of RhoA, ROCK-2 and MYL in myocardial
cells-amelioration by valsartan. (A) Western blots results of RhoA,
ROCK-2 and MYL expression in myocardial cells. The experiment was
repeated 3 times with similar results. The figure presents a
representative analysis. Quantification of the western blots (B)
RhoA, (C) ROCK-2 (D) and MYL. Columns are means +/- standard error
of the mean, (n=3). *P<0.05, alcohol group compared with the
control group. #P<0.05, alcohol + valsartan group
compared with the alcohol group. Reverse transcription-quantitative
polymerase chain reaction analysis of the (E) RhoA, (F) ROCK-2 and
(G) MYL mRNA transcription profiles of myocardial cells in the
alcohol group and alcohol + vasartan group (n=3). Expression levels
were normalized to GAPDH levels. *P<0.05, alcohol + valsartan
group compared with the alcohol group. ROCK, Rho-associated protein
kinase; RhoA, Ras homolog gene family, member A; MYL, myosin light
chain. |
Discussion
Alcohol abuse is injurious to health. Long-term or
intermittent addiction to alcohol is accountable for
gastrointestinal diseases, alcoholic liver diseases, myopathy and
encephalopathy. The associated cardiovascular diseases demonstrate
symptoms similar to dilated cardiomyopathy and are referred to as
ACM (23,24). Patients at the end-stage of ACM
suffer from decreased cardiac function leading to heart failure or
arrhythmia. Without any effective measures, including abstinence,
nearly half of the patients would die in a period of 4 years
(25,26). However, the pathogenesis and
treatment methods of ACM remain unclear. In the present study, the
role of the variations in the RhoA-ROCK2-MYL pathway were
investigated in the pathogenesis of ACM and the beneficial effects
of angiotensin-converting enzyme inhibitor I drugs.
Ras, the first-identified low-molecular weight G
protein (27) belongs to a family of
proteins which is divided into three subgroups, namely, RhoA, RhoB
and RhoC. RhoA is a major member involved in multiple intracellular
signal transduction pathways (28–30).
ROCK is a member of the Ser/Thr protein kinase family. It is the
key and characteristic downstream signaling molecule of RhoA
(31–33) consisting of ROCK1 and ROCK2 (34,35).
ROCK1 is mainly expressed in the lung, liver, kidney, spleen and
testicles, while ROCK2 is expressed in the heart and brain
(36,37). MYL is a major downstream protein of
ROCK and together with the myosin heavy chain constitutes myosin
(29,38). MYL is a key substance in tubulin with
major regulatory effects on the contraction of myocardial cells.
Previous studies (39–41) have demonstrated that chronic alcohol
intake activates the renin-angiotensin system and through
Angiotensin II (AngII) facilitates cardiac remodeling. Blocking the
angiotensin type l (AT1) receptor ameliorates cardiac
remodeling.
A massive intake of alcohol activates RAS and
facilitates binding of AngII to AT1 (42). This activates the downstream RhoA
resulting in the induction of the expression of ROCK. Sequentially,
ROCK inhibits its downstream protein MYL, therefore decreasing its
expression. MYL is critical for the contraction of myocardial cells
(43). Reduced expression of MYL is
associated with decreased contraction of myocardial cells and as
time lapses, patients become more susceptible to heart failure.
Through alcohol gavage and free access to alcohol,
rat models of ACM were established. The method surmounted the
limitations of having only free access to alcohol, individual
differences in models and long time needed for model establishment.
Pathological manifestations in the alcohol group included
disorganized arrangement and rupture of myocardial filaments, an
enlarged intercellular space with edema, and massive inflammatory
infiltration which indicated that cells were signaled to undergo
apoptosis the alcohol group. The treatment group demonstrated an
ordered arrangement of myocardial filaments, evenly distributed
cytoplasm without any rupture and absence of enlarged intercellular
space, effusion edema or inflammatory infiltration. Results of
Masson's trichrome staining demonstrated significantly enhanced
fibrosis of myocardial cells in the alcohol group compared with the
control group. Myocardial fibrosis was not observed in the
treatment group suggesting that valsartan alleviated myocardial
fibrosis leading to amelioration of cardiac remodeling in ACM.
Echocardiography revealed decreased EF and increased LVEDD in the
treatment group, suggesting a decline in systolic function. The
treatment group demonstrated decreased LVEDD and increased EF
compared with the alcohol group, with a concomitant increase in the
contractility of myocardial cells. These results suggest that
long-term massive intake of alcohol initiates myocardial injury,
fibrosis and systolic dysfunction, leading to decreased cardiac
function, or heart failure. Valsartan improves cardiac function by
preventing the progression of ACM into heart failure, thereby
benefiting majority of the patients.
IHC was employed to detect the expressions of RhoA
and ROCK in the myocardial cells (44), while western blotting was used to
detect the expressions of RhoA, ROCK and MYL. Results demonstrated
that in comparison with the control group, western blot analysis
detected elevated expression of RhoA and ROCK and decreased
expression of MYL in the alcohol group compared with the control
group. Decreased RhoA and ROCK, and elevated MYL expression was
detected in the treatment group compared with the alcohol group.
PCR analysis revealed increased RhoA and ROCK and downregulated MYL
mRNA expressions in the alcohol group compared with the control
group. Notably, decreased RhoA and ROCK and elevated MYL mRNA
expression was seen in the treatment group compared with in the
alcohol group, which was consistent with the results of western
blotting. These findings suggest the activation of the
RhoA-ROCK2-MYL pathway at the protein or mRNA level by alcohol and
its metabolites causing a reduction in myocardial contractility.
Treatment with valsartan inhibited AT1 and suppressed the
expression of RhoA and ROCK to increase the expression of MYL,
thereby enhancing the contractility of myocardial cells. Valsartan
improves cardiac function and exerts a therapeutic and prophylactic
effect in ACM. Early administration of angiotensin receptor
antagonists ameliorates cardiac function and the prognosis of ACM,
therefore indicating its critical protective effect in the
development and progression of ACM. A rat model of ACM revealed
increased LVEDD, decreased EF and systolic function, and cell
rupture associated with increased fibrosis (45). Administration of valsartan
ameliorated myocardial fibrosis and prevented the progression of
ACM. Alcoholic stimulation activates the RhoA-ROCK2-MYL pathway to
curb the systolic function of myocardial cells (46), while valsartan inhibits this pathway
to enhance myocardial contractility and improves cardiac
function.
Although alcohol gavage + free access to alcohol can
shorten the time required to simulate and establish the development
of ACM more effectively, success rate remains quite low due to
incompetence in gavaging, excessively high concentrations of
alcohol, and treatment with industrial alcohol instead of the wine
made from grain. In addition, due to the lack of RhoA and ROCK
inhibitors, the involvement of the RhoA-ROCK2-MYL pathway was not
confirmed. The results of the present study are inconsistent with
other research results that ROCK2 leads to the activation of myosin
light chain by phosphorylation (47). It was speculated that MYL
phosphorylation is determined by the balance between the activities
of Rho-kinase and myosin phosphatase. Also, apart from ROCK2, an
additional pathway(s) may be required for sustained MYL
phosphorylation.
Experimental design determining the drug
concentration was simple and further evaluation on dose-effect
association was not performed. Because of the lack of abstinence
group or abstinence + valsartan group, the prophylactic effect of
valsartan could only be proved in ACM. Therefore, further studies
are necessary for validation of the therapeutic effects of
valsartan.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
In this study, LL and WL conceived the study and
designed the experiments. LJ and JZ contributed to the data
collection, JL and WY performed the data analysis and interpreted
the results. LL wrote the manuscript; WL and LZ contributed to the
critical revision of article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the First Affiliated
Hospital of Harbin Medical University.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piano MR: Alcoholic cardiomyopathy:
Incidence, clinical characteristics, and pathophysiology. Chest.
121:1638–1650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skotzko CE, Vrinceanu A, Krueger L and
Freudenberger R: Alcohol use and congestive heart failure:
Incidence, importance, and approaches to improved history taking.
Heart Fail Rev. 14:51–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berger J and Moller DE: The mechanisms of
action of PPARs. Annu Rev Med. 53:409–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finck BN, Han X, Courtois M, Aimond F,
Nerbonne JM, Kovacs A, Gross RW and Kelly DP: A critical role for
PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic
cardiomyopathy: Modulation by dietary fat content. Proc Natl Acad
Sci USA. 100:1226–1231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barger PM, Brandt JM, Leone TC, Weinheimer
CJ and Kelly DP: Deactivation of peroxisome proliferator-activated
receptor-alpha during cardiac hypertrophic growth. J Clin Invest.
105:1723–1730. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berger J and Moller DE: The mechanisms of
action of PPARs. Annu Rev Med. 53:409–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mishra PK, Tyagi N, Kundu S and Tyagi SC:
MicroRNAs are involved in homocysteine-induced cardiac remodeling.
Cell Biochem Biophys. 55:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hultberg B, Berglund M, Andersson A and
Frank A: Elevated plasma homocysteine in alcoholics. Alcohol Clin
Exp Res. 17:687–689. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stickel F, Choi SW, Kim YI, Bagley PJ,
Seitz HK, Russell RM, Selhub J and Mason JB: Effect of chronic
alcohol consumption on total plasma homocysteine level in rats.
Alcohol Clin Exp Res. 24:259–264. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dettmeyer R, Reith K and Madea B:
Alcoholic cardiomyopathy versus chronic
myocarditis-immunohistological investigations with LCA, CD3, CD68
and tenascin. Forensic Sci Int. 126:57–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang QF and Li YH: Roles of PPARs on
regulating myocardial energy and lipid homeostasis. J Mol Med
(Berl). 85:697–706. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pellieux C, Montessuit C, Papageorgiou I
and Lerch R: Inactivation of peroxisome proliferator-activated
receptor isoforms alpha, beta/delta, and gamma mediate distinct
facets of hypertrophic transformation of adult cardiac myocytes.
Pflugers Arch. 455:443–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loichot C, Jesel L, Tesse A, Tabernero A,
Schoonjans K, Roul G, Carpusca I, Auwerx J and Andriantsitohaina R:
Deletion of peroxisome proliferator-activated receptor-alpha
induces an alteration of cardiac functions. Am J Physiol Heart Circ
Physiol. 291:H161–H166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melendez J, Welch S, Schaefer E, Moravec
CS, Avraham S, Avraham H and Sussman MA: Activation of pyk2/related
focal adhesion tyrosine kinase and focal adhesion kinase in cardiac
remodeling. J Biol Chem. 277:45203–45210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Preedy VR, Patel VB, Reilly ME, Richardson
PJ, Falkous G and Mantle D: Oxidants, antioxidants and alcohol:
Implications for skeletal and cardiac muscle. Front Biosci.
4:e58–e66. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elamin E, Masclee A, Dekker J and Jonkers
D: Ethanol disrupts intestinal epithelial tight junction integrity
through intracellular calcium-mediated Rho/ROCK activation. Am J
Physiol Gastrointest Liver Physiol. 306:G677–G685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei B, Shang YX, Li M, Jiang J and Zhang
H: Cytoskeleton changes of airway smooth muscle cells in juvenile
rats with airway remodeling in asthma and the RhoA/ROCK signaling
pathway mechanism. Genet Mol Res. 13:559–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Liang J, Wu WK, Yu X, Yu J, Weng X
and Shen J: Leptin activates RhoA/ROCK pathway to induce
cytoskeleton remodeling in nucleus pulposus cells. Int J Mol Sci.
15:1176–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimokawa H and Satoh K: Light and dark of
reactive oxygen species for vascular function: 2014 ASVB (Asian
Society of Vascular Biology). J Cardiovasc Pharmacol. 65:412–418.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Satoh K, Godo S, Saito H, Enkhjargal B and
Shimokawa H: Dual roles of vascular-derived reactive oxygen
species-with a special reference to hydrogen peroxide and
cyclophilin A. J Mol Cell Cardiol. 73:50–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higashi M, Shimokawa H, Hattori T, Hiroki
J, Mukai Y, Morikawa K, Ichiki T, Takahashi S and Takeshita A:
Long-term inhibition of Rho-kinase suppresses angiotensin
II-induced cardiovascular hypertrophy in rats in vivo: Effect on
endothelial NAD(P)H oxidase system. Circ Res. 93:767–775. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fiarresga A, Cacela D, Galrinho A, Ramos
R, de Sousa L, Bernardes L, Patrício L and Cruz Ferreira R: Alcohol
septal ablation in obstructive hypertrophic cardiomyopathy: Four
years of experience at a reference center. Rev Port Cardiol.
33:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Veselka J, Lawrenz T, Stellbrink C,
Zemanek D, Branny M, Januska J, Sitar J, Dimitrow P, Krejci J,
Dabrowski M, et al: Early outcomes of alcohol septal ablation for
hypertrophic obstructive cardiomyopathy: A European multicenter and
multinational study. Catheter Cardiovasc Interv. 84:101–107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pridemore WA, Chamlin MB, Kaylen MT and
Andreev E: The effects of the 2006 Russian alcohol policy on
alcohol-related mortality: An interrupted time series analysis.
Alcohol Clin Exp Res. 38:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kycina P and Murin J: Alcoholic
cardiomyopathy and cardiovascular events-an insight from the Liptov
region. Bratisl Med J. 114:337–341. 2013. View Article : Google Scholar
|
|
27
|
Lessey-Morillon EC, Osborne LD,
Monaghan-Benson E, Guilluy C, O'Brien ET, Superfine R and Burridge
K: The RhoA guanine nucleotide exchange factor, LARG, mediates
ICAM-1-dependent mechanotransduction in endothelial cells to
stimulate transendothelial migration. J Immunol. 192:3390–3398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan X, Liu J, Dai XX, Liu HL, Cui XS, Kim
NH, Wang ZB, Wang Q and Sun SC: Rho-GTPase effector ROCK
phosphorylates cofilin in actin-meditated cytokinesis during mouse
oocyte meiosis. Biol Reprod. 90:372014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gabrielli L, Winter JL, Godoy I, McNab P,
Padilla I, Cordova S, Rigotti P, Novoa U, Mora I, García L,
Ocaranza MP and Jalil JE: Increased rho-kinase activity in
hypertensive patients with left ventricular hypertrophy. Am J
Hypertens. 27:838–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hensel N, Stockbrügger I, Rademacher S,
Broughton N, Brinkmann H, Grothe C and Claus P: Bilateral crosstalk
of rho- and extracellular-signal-regulated-kinase (ERK) pathways is
confined to an unidirectional mode in spinal muscular atrophy
(SMA). Cell Signal. 26:540–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morgan-Fisher M, Couchman JR and Yoneda A:
Phosphorylation and mRNA splicing of collapsin response mediator
protein-2 determine inhibition of rho-associated protein kinase
(ROCK) II function in carcinoma cell migration and invasion. J Biol
Chem. 288:31229–31240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasaki T, Oga T, Nakagaki K, Sakai K,
Sumida K, Hoshino K, Miyawaki I, Saito K, Suto F and Ichinohe N:
Developmental genetic profiles of glutamate receptor system,
neuromodulator system, protector of normal tissue and mitochondria,
and reelin in marmoset cortex: Potential molecular mechanisms of
pruning phase of spines in primate synaptic formation process
during the end of infancy and prepuberty (II). Biochem Biophys Res
Commun. 444:307–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaio V, Nunes B, Fernandes A, Mendonça F,
Horta Correia F, Beleza A, Gil AP, Bourbon M, Vicente A, Dias CM
and Barreto da Silva M: Genetic variation at the CYP2C19 gene
associated with metabolic syndrome susceptibility in a South
Portuguese population: Results from the pilot study of the European
health examination survey in portugal. Diabetol Metab Syndr.
6:232014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu PY and Liao JK: A method for measuring
Rho kinase activity in tissues and cells. Methods Enzymol.
439:181–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mertsch S and Thanos S: Opposing signaling
of ROCK1 and ROCK2 determines the switching of substrate
specificity and the mode of migration of glioblastoma cells. Mol
Neurobiol. 49:900–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimizu T, Fukumoto Y, Tanaka S, Satoh K,
Ikeda S and Shimokawa H: Crucial role of ROCK2 in vascular smooth
muscle cells for hypoxia-induced pulmonary hypertension in mice.
Arterioscler Thromb Vasc Biol. 33:2780–2791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laeno AM, Tamashiro DA and Alarcon VB:
Rho-associated kinase activity is required for proper morphogenesis
of the inner cell mass in the mouse blastocyst. Biol Reprod.
89:1222013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Shboul O: The role of the RhoA/ROCK
pathway in gender-dependent differences in gastric smooth muscle
contraction. J Physiol Sci. 66:85–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nzegwu MA, Okafor OC, Olusina DB, Ike V
and Mbah AU: Dilated alcoholic cardiomyopathy and incidental
lymphoma occuring in a 56 year old man who was being managed for
hypertensive heart disease in Enugu Nigeria-a rare finding. Niger J
Med. 20:494–497. 2011.PubMed/NCBI
|
|
40
|
Jing L, Zhou LJ, Zhang FM, Li WM and Sang
Y: Tenascin-x facilitates myocardial fibrosis and cardiac
remodeling through transforming growth factor-β1 and peroxisome
proliferator-activated receptor γ in alcoholic cardiomyopathy. Chin
Med J (Engl). 124:390–395. 2011.PubMed/NCBI
|
|
41
|
Tan Y, Li XK, Prabhu SD, Brittian KR, Chen
Q, Yin X, McClain CJ, Zhou Z and Cai L: Angiotensin II plays a
critical role in alcohol-induced cardiac nitrative damage, cell
death, remodeling, and cardiomyopathy in a protein kinase
C/nicotinamide adenine dinucleotide phosphate oxidase-dependent
manner. J Am Coll Cardiol. 59:1477–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ferrario CM: Cardiac remodelling and RAS
inhibition. Ther Adv Cardiovasc Dis. 10:162–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McArthur L, Chilton L, Smith GL and
Nicklin SA: Electrical consequences of cardiac myocyte: Fibroblast
coupling. Biochem Soc Trans. 43:513–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Liu S, Xia Y and Wu K: MiR-31
regulates Rho-associated kinase-myosin light chain (ROCK-MLC)
pathway and inhibits gastric cancer invasion: Roles of RhoA. Med
Sci Monit. 22:4679–4691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sabater-Molina M, Pérez-Sánchez I,
Hernández Del Rincón JP and Gimeno JR: Genetics of hypertrophic
cardiomyopathy: A review of current state. Clin Genet. 93:3–14.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lopez NC, Ebensperger G, Herrera EA, Reyes
RV, Calaf G, Cabello G, Moraga FA, Beñaldo FA, Diaz M, Parer JT and
Llanos AJ: Role of the RhoA/ROCK pathway in high-altitude
associated neonatal pulmonary hypertension in lambs. Am J Physiol
Regul Integr Comp Physiol. 310:R1053–R1063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bhadriraju K, Yang M, Alom Ruiz S, Pirone
D, Tan J and Chen CS: Activation of ROCK by RhoA is regulated by
cell adhesion, shape, and cytoskeletal tension. Exp Cell Res.
313:3616–3623. 2007. View Article : Google Scholar : PubMed/NCBI
|