Introduction
Brucellosis is caused by the facultative
intracellular pathogen Brucella, which is a gram-negative
coccobacillus lacking capsules, flagella, endospores and native
plasmids. This zoonotic disease affects the reproductive tract of
livestock, resulting in abortion, infertility and consequently
economic loss (1). Human infection
is mainly due to the consumption of unpasteurized milk or
undercooked meat from infected animals or from direct contact with
the secretions of infected animals (1). Infection is characterized by undulant
fever, physical weakness and the presence of diseases including
endocarditis, arthritis and neurological disorders (2). Currently, a number of antibiotics can
be used clinically for the treatment of human brucellosis, but no
effective drugs have been identified for animal brucellosis
(3). Live attenuated Brucella
strains, including B. suis strain 2 (S2), are produced as
vaccines to induce immunization against Brucella infection
in domestic animals (4). The S2
vaccine is widely used in China as an effective method to prevent
brucellosis in goats, sheep, cattle and swine, but it is not
administered worldwide since its capacity to protect against
heterologous virulent Brucella species is still debatable
(5). Additionally, live attenuated
vaccines are potential pathogens for humans and may induce abortion
in pregnant animals (6). No safe or
effective vaccines are currently available for the treatment of
human brucellosis. Therefore, the identification of novel compounds
for the prevention or treatment of brucellosis is urgently
required.
Brucella species are relatively weak inducers
of innate immunity and can eventually lead to a long-lasting
infection (7). The replication of
Brucella during infection is independent of toll-like
receptor (TLR)4, TLR3, TLR2, TLR5 or the TIR-domain-containing
adapter-inducing interferon-β adapter (TRIF), allowing the bacteria
to evade immune detection and adapt to intracellular conditions
(8–10). Brucella can evade phagocytic
lysosomes and prevent phagosomal-lysosomal maturation to avoid
degradation (11). Brucella
has been indicated to prevent macrophages from secreting cytokines,
undergoing apoptosis and presenting antigens to T cells (12). Macrophages are one of the primary
targets during Brucella infection, accounting for the
elimination of intracellular Brucella (13). Pro-inflammatory cytokines that are
secreted by activated macrophages, including tumor necrosis factor
(TNF)-α and interleukin (IL)-12, serve important roles in the
regulation of early Brucella infection (3). TNF-α amplifies and mediates
pro-inflammatory signalling pathways to enhance the host response
upon Brucella infection (14). IL-12 can stimulate T helper 1 (Th1)
and natural killer cells to participate in defence against
Brucella, and the depletion of IL-12 has been revealed to
impair the production of nitrous oxide (NO) and interferon (IFN)-γ
(15). TNF-α and IL-12 are positive
regulators of IFN-γ, which is the most potent inducer of
anti-Brucella activity in monocytes and macrophages, and
reduces the intracellular growth and replication of Brucella
by stimulating the production of reactive oxygen species, reactive
nitrogen species and TNF-α (16).
Furthermore, cytokines can activate macrophages and protect hosts
from prolonged infection (9).
Astragalus polysaccharide (APS) is a bioactive
component that is extracted from astragalus. A study has identified
APS as a bio-immunomodulator that promotes nonspecific and specific
immunity, and as a vaccine adjuvant that enhances vaccine antigens
(17). APS promotes the phagocytosis
of macrophages, the proliferation of dendritic cell (DC) precursors
and the ability of DCs to induce T cell proliferation and elevate
the secretion of cytokines, including TNF-α, IL-6, IL-8, IFN-γ and
IL-12 (18,19). The administration of APS to mice with
sepsis can downregulate regulatory T cells (Treg) and restore the
balance between Th1 and Th2 cells, leading to immunoregulation
(20). Additionally, APS exhibits a
number of additional biological activities, including anti-tumour,
antioxidant and antiviral activity (21,22).
In the current study, the inflammatory and immune
responses of mice and macrophages pretreated with APS were
determined during Brucella infection. Enhanced cytokine
production, macrophage activation and Brucella clearance
were observed, and these observations demonstrated that APS
improves the host response to Brucella in mice and is a
promising candidate that can be used to control and prevent
brucellosis.
Materials and methods
Preparation of APS
An APS injection (Qilu Animal Health Products Co.,
Ltd.) of 20 mg/ml was diluted with sterile normal saline solution
and mixed prior to use.
Animals and administration
A total of 160 male BALB/c mice (age, 6–8 weeks)
were supplied by Shanghai Laboratory Animal Centre. The animals
were maintained in a specific pathogen-free (SPF) environment and
kept under constant conditions, including 24±1°C temperature,
40–60% humidity and a 12 h light/dark cycle with access to food and
water ad libitum. After acclimatization for one week, the
mice were weighed and randomly divided into five groups. The
uninfected group was intraperitoneally injected with normal saline
once a day for 4 days. The infected control group was
intraperitoneally injected with normal saline once a day for 4 days
and infected with B. suis S2 (1×104 CFU live
bacteria per animal) on day 4 via intraperitoneal injection. The
other three groups (S2+0.3 mg/ml APS, S2+0.6 mg/ml APS and S2+1.2
mg/ml APS) were administered 0.3, 0.6 and 1.2 mg/ml APS by
intraperitoneal injection once a day for 4 days, respectively, and
infected intraperitoneally with B. suis S2 (1×104
CFU per animal) on day 4. The mice were randomly selected for
euthanasia at 1, 6, 24, 48 and 72 h after infection (n=6). Blood
samples, peritoneal fluids and spleens were collected. An
additional 10 mice were intraperitoneally injected with 2 ml 4%
sterile starch solution, and sacrificed one day subsequent to the
isolation of peritoneal macrophages.
Macrophage phagocytosis of bacteria
and morphological examination of macrophages
The peritoneal fluids were collected from 1 and 72
h-infected groups (n=6). The phagocytic rate of macrophages was
calculated using Wright-Giemsa staining (Sangon Biotech Co., Ltd.)
according to the manufacturer's protocol. Macrophages were
re-suspended in 0.5 ml phosphate buffer saline (PBS) and prepared
as cell smears. After fixation in 95% ethanol at room temperature
for 5 min, cells were stained with Wright-Giemsa for 8 min at room
temperature and then washed with PBS. Cell samples were air-dried,
and cell morphology was examined using a light microscope
(magnification, ×200). A total of 100 macrophages were counted
manually, and the phagocytic rate was calculated using the
following equation: Phagocytic rate = (the number of macrophages
containing bacteria/100) ×100%.
Determination of bacterial loads in
macrophages and spleens
Peritoneal macrophages were collected at 1, 6 and 24
h from infected groups and spleens were collected at 6, 24 and 48 h
from infected groups. A total of 2×104 macrophages were lysed with
l ml of 1% Triton X-100 (Beyotime Institute of Biotechnology) for
15 min. The lysate was diluted to 1:100 using sterile PBS. A total
of 100 µl lysate was spread on TSA solid medium (Hangzhou Microbial
Reagent Co., Ltd.), which was equivalent to the lysate of 20
macrophages, and then incubated for 72 h at 37°C. The spleens were
weighed and homogenized with 1 ml of 1% Triton X-100. A total of
100 µl homogenate, which was equivalent to the homogenate of 10% of
the whole spleen, was spread onto TSA solid medium and incubated
for 72 h at 37°C. The numbers of bacteria in the macrophages and
the spleens were determined by counting bacteria colonies. Log10
CFU/1,000 macrophages=Log10 (colony count ×500). Log10 CFU per gram
spleen=Log10 (colony count ×10)/spleen weight (g).
Peritoneal macrophage culture
Peritoneal macrophages were isolated from BALB/c
mice 1 day after intraperitoneal injection of 2 ml 4% sterile
starch solution. A total of 2×106 cells/ml were
suspended in RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 4 mM L-glutamine (Thermo Fisher
Scientific, Inc.), 1% penicillin/streptomycin (Thermo Fisher
Scientific, Inc.) and 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), incubated in 12 or 96-well culture plates in a humidified 5%
CO2 incubator at 37°C for further use.
Measurement of cytokines and NO
production
The levels of TNF-α, IL-12 and IFN-γ in mouse serum
collected at 6, 24 and 48 h time points were measured using ELISA
kits (NeoBioscience Technology Co., Ltd.). The serum was obtained
by centrifugation at 1200 × g, room temperature for 10 min. For the
in vitro assay, peritoneal macrophages were cultured in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 4 mM L-glutamine (Thermo Fisher Scientific,
Inc.), 1% penicillin/streptomycin (Thermo Fisher Scientific, Inc.)
and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in 12-well
plates in the presence of different concentrations of APS (0, 50,
100 and 200 µg/ml) for 24 h in a humidified 5% CO2
incubator at 37°C. B. suis S2 was then loaded at a
multiplicity of infection, at 100:1. The uninfected group was not
treated with APS or bacteria. After co-incubation in a humidified
5% CO2 incubator at 37°C for 1 h, extracellular bacteria
were washed away by removal of the medium and washing with PBS, and
previous concentrations of APS (0, 50, 100 and 200 µg/ml) in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 4 mM L-glutamine (Thermo Fisher Scientific,
Inc.), 1% penicillin/streptomycin (Thermo Fisher Scientific, Inc.)
and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) were added. The
cells then were incubated in a humidified 5% CO2
incubator at 37°C for 24 h. The levels of NO and TNF-α in the
culture supernatant were determined using a total nitric oxide
assay kit (Beyotime Institute of Biotechnology) and an ELISA kit
(NeoBioscience Technology Co., Ltd.), respectively, at 24 h after
the medium was replaced.
Cell viability
Murine peritoneal macrophages were seeded at a
density of 2×105 cells/well in a 96-well plate. After
attachment, the cells were treated with the indicated
concentrations of APS (0, 25, 50, 100, 200 or 400 µg/ml) and
incubated in a humidified 5% CO2 incubator at 37°C for
48 h. Cell viability was analysed using MTT as previously described
(23). Briefly, MTT solution was
added to the cells at a final concentration of 0.5 mg/ml for 4 h at
37°C in a humidified 5% CO2 atmosphere. The medium was
then removed and 150 µl of DMSO was added to each well. The optical
density of each well was measured at 570 nm.
Statistical analysis
The data were expressed as the mean ± SEM or mean ±
standard deviation. A one-way ANOVA followed by a Tukey's or
Dunnett's test was performed using GraphPad Prism 5 software
(GraphPad Software, Inc.) to determine statistical comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of APS on the morphology of
macrophages from B. suis S2-infected mice
Wright-Giemsa staining was used to examine
macrophage morphology. After the mice were infected with B.
suis S2 for 1 or 72 h, peritoneal macrophages were collected
and stained. As presented in Fig.
1A, at 1 h after infection, the macrophages from the
APS-treated groups ingested more bacteria than those from the
Brucella-infected control group. In the 72 h-infected
samples, bacteria were identified at very low levels inside the
macrophages from each group (Fig.
1B). The membrane integrity of macrophages from the S2-infected
group was destroyed, as the vacuoles were formed in the cytoplasm
and the nucleuses were broken (Fig.
1B). In the groups treated with 0.6 and 1.2 mg/ml APS
respectively and infected with B. suis S2 for 72 h, the
membranes and the nuclei were intact, and the cytoplasm was normal
(Fig. 1B). In the S2+0.3 mg/ml APS
group, the membranes and nuclei remained intact with prominent
nucleoli, but the size of the macrophage increased and the vacuoles
appeared around the nucleus in the cytoplasm (Fig. 1B). These results demonstrated that
APS promoted macrophage activation and protected the integrity of
macrophages during infection.
Effects of APS on macrophage
phagocytosis of B. suis S2 and bacterial loads in macrophages
To assess the phagocytosis of macrophages, the
phagocytic rate, which is the percentage of macrophages ingesting
Brucella, was measured at 1 h after infection. The
phagocytic rates of macrophages were increased in the groups
pretreated with APS compared with the mice infected with
Brucella alone, implying that APS might stimulate
macrophages to ingest bacteria (Fig.
2A). The bacterial loads in peritoneal macrophages from each
group were examined at 1, 6 and 24 h following infection. The
amount of Brucella engulfed by macrophages was relatively
high at 1 h after infection, and as time progressed, the load
markedly declined (Fig. 2B).
Compared with the macrophages from the B. suis S2-infected
mice without APS protection, the macrophages from the 1.2 mg/ml
APS-administered mice engulfed significantly more Brucella
at the 1 h time point, and no Brucella remained inside the
macrophages at 24 h following infection (Fig. 2B). Furthermore, a dose-dependent
decline in the number of intracellular bacteria was observed at the
6 and 24 h time points (Fig. 2B). In
conclusion, these results suggested that APS was able to promote
macrophage phagocytosis of B. suis S2 in a dose-dependent
manner and accelerate the removal of Brucella to avoid
long-term infection.
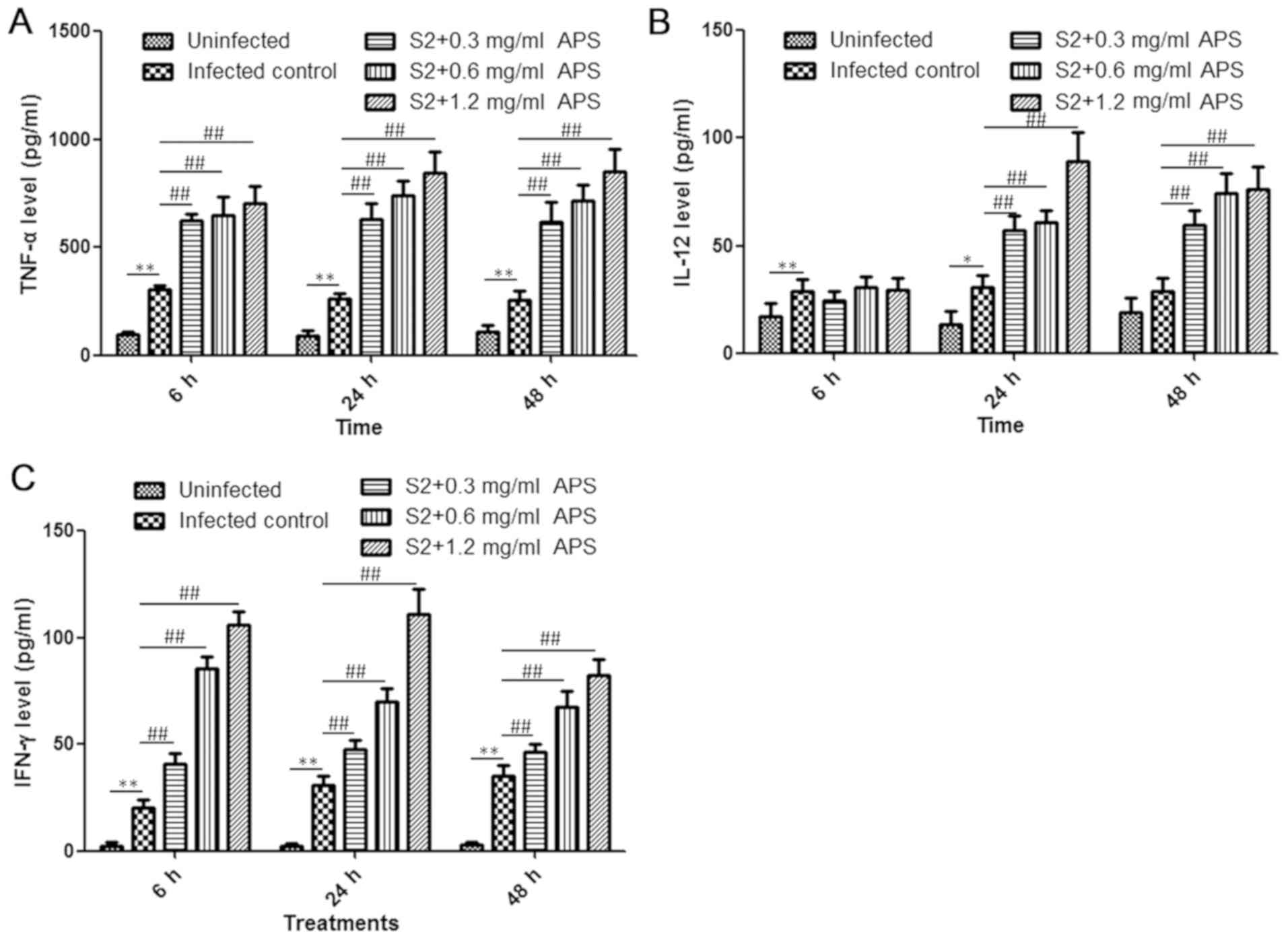
APS elevated the levels of serum
TNF-α, IL-12 and IFN-γ during B. suis S2 infection
Cytokines are critical effector molecules in the
control of Brucella growth and brucellosis treatment, and
the level of cytokines is associated with the intensity of the host
immune response (24). Consequently,
cytokine content in the serum from mice in each group was analysed
at the 6, 24 and 48 h time points. Compared with the uninfected
control, the B. suis S2-infected mice exhibited enhanced
production of TNF-α, IL-12 and IFN-γ at all the time points, and in
the APS-treated S2-infected groups, the levels of all the tested
cytokines were higher than those in the S2-infected control group,
except for the IL-12 levels at 6 h post-infection (Fig. 3A-C). At 24 h post-infection, the
secretion of TNF-α, IL-12 and IFN-γ in the S2+1.2 mg/ml APS group
increased by 3.25-, 2.93- and 3.62-fold, respectively, compared
with that of the S2-infected control group (Fig. 3A-C). IL-12 levels remained similar in
the S2-infected control group as the infection time increased,
while IL-12 levels in the APS-treated S2-infected mice was higher
at the 24 h time point compared with the 6 h time point (Fig. 3B). IFN-γ levels in the S2-infected
control group increased modestly in a time-dependent manner during
48 h infection and decreased in the 0.6 and 1.2 mg/ml APS-treated
S2-infected mice (Fig. 3C). However,
the serum concentration of IFN-γ was higher in the APS-administered
mice at all the time points (Fig.
3C). In conclusion, APS treatment can induce cytokine
production, facilitating the inflammatory reaction and
immunological response against Brucella infection.
Bacterial loads in the spleen were
affected by APS
The spleen is a major organ that serves an important
role in the immune response and contains a high number of bacteria
during brucellosis (25). Therefore,
spleens were collected from B. suis S2-infected mice at 6,
24 and 48 h post-infection and the number of bacteria in each
spleen was determined. The average weight of spleens from each
infected group collected at 24 h post-infection showed no
significant difference (Fig. 4A).
The bacterial loads in the spleen were increased in the S2-infected
control mice as the infection time increased, and in contrast, the
maximum CFU detected in the spleen from the 0.6 and 1.2 mg/ml
APS-treated S2-infected mice was at 6 h after infection (Fig. 4B). At 6 h post-infection, the number
of spleen bacteria in the 1.2 mg/ml APS-treated and S2-infected
mice was significantly higher than that in the S2-infected control
mice, and at 48 h after infection, the results were reversed
(Fig. 4B). The results revealed that
APS had a positive effect on the immune response in mice and
improved the clearance of Brucella in the spleen.
Enhanced production of TNF-α and NO by
APS following Brucella infection in vitro
A cell viability assay was performed to determine
the toxicity of APS and the suitable dose for in vitro
assay. The tested concentrations of APS exhibited no significant
influence on the viability of murine peritoneal macrophages
(Fig. 5A). For the cytokine assay,
200 µg/ml APS was selected as the highest concentration. After
attachment, peritoneal macrophages were treated with 0, 50, 100 or
200 µg/ml APS for 24 h and infected with B. suis S2 for 1 h.
After infection, the original medium was replaced with fresh medium
containing the same concentrations of APS that were present
previously. A period of 24 h later, the culture supernatant was
collected, and the content was detected. Consistent with the in
vivo results, the level of TNF-α in the cell culture medium was
significantly increased by APS after infection, indicating the
promotion of macrophage activation (Fig.
5B). NO production by activated macrophages has been indicated
to mediate the intracellular replication and killing of
Brucella (26). As
illustrated in Fig. 5C, APS-induced
macrophages were observed to produce a relatively large amount of
NO that may have suppressed the survival of intracellular
Brucella. In summary, APS facilitated the production of the
pro-inflammatory effectors TNF-α and NO to eradicate
Brucella.
Discussion
The aim of the current study was to examine the
potential effect of APS on the protection and treatment of
Brucella infection. Due to lab safety issues, the B.
suis S2 vaccine, which is of low virulence, was used instead of
virulent Brucella. The results demonstrated that APS
treatment in mice promoted the secretion of pro-inflammatory
cytokines and macrophage activation during Brucella
infection to enhance the inflammatory and immune responses of the
host, and ultimately accelerated Brucella clearance in
macrophages and spleens to avoid long-term infection. The induction
of TNF-α and NO by APS was observed in in vitro experiments.
The present study demonstrated that the protective role of APS in
Brucella infection is at least partially due to the
enhancement of host defence reactions.
The mouse model is widely used to study the
interaction between Brucella and the immune system due to
the practicality of this model and the avoidance of economic loss
and ethical issues. Innate immune and adaptive immune systems
participate in the resistance to brucellosis. During infection,
macrophages are the first line of defence in the innate immune
response. The current study indicated that at the early stages of
infection, the phagocytic activity of macrophages was intensified
in the APS-treated groups, and at the late stages of infection, the
APS-treated mice had a decreased number of bacteria inside the
peritoneal macrophages compared with the infected control group,
suggesting that APS promotes the killing of Brucella via
macrophage activation. Consistently, a rapid clearance of
Brucella was observed in the spleens of the APS-treated
mice, while in the infected control group, the bacterial loads in
spleens were increased as infection time increased, indicating that
a number of bacteria evaded the bactericidal function of phagocytic
cells and had successfully survived and multiplied inside the
cells. Typically, within the first few hours after infection, the
majority of bacteria are eliminated by macrophages (27). However, some pathogens may survive
due to the Brucella species exhibiting strategies to evade
innate immunity. The mechanism of evasion includes the inhibition
of TLR signalling and suppression of the complement system and is
associated with the specific structures of Brucella species,
including lipid A, modified LPS with longer fatty acid residue and
flagellin (28).
TNF-α, IL-12 and IFN-γ are cytokines that initiate
host defence responses and modulate the activity of
immune-associated cells in brucellosis (3,14,24). In
the present study, the serum levels of TNF-α, IL-12 and IFN-γ were
determined in mice to assess the production of cytokines from
spleen cells (3). APS treatment
enhanced the secretion of TNF-α, IL-12 and IFN-γ during infection,
indicating a stronger potential for macrophage activation and
bactericidal function. In support of in vivo research, the
current in vitro study using mouse peritoneal macrophages
demonstrated that APS increased the production of TNF-α and NO
following Brucella infection, suggesting that APS suppressed
bacterial intracellular replication. Pro-inflammatory cytokines,
including TNF-α and IFN-γ can induce the killing ability of
macrophages, which is responsible for Brucella elimination
in infected mice (14,24). The failed induction of TNF-α is
associated with reduced toxicity of host cells and favours the
replication of intracellular Brucella (29). An in vitro study has
demonstrated that macrophages activated by TNF-α can inhibit
Brucella replication (30).
IFN-γ is produced by CD4+, CD8+ and T lymphocytes and serves an
important role in the clearance of Brucella. IFN-γ induces
macrophages to degrade intracellular bacteria and CD8+ and T cells
to exert cytotoxic effects on infected macrophages (3). Furthermore, IFN-γ mediates the type I
immune response and prompts the expression of MHC-I and MHC-II
against brucellosis (28,31). Brucella species exhibit the
ability to repress the secretion of the aforementioned cytokines to
interfere with the activity of phagocytic cells and the protective
Th1 immune response (32). Following
Brucella invasion and internalization, the production of
pro-inflammatory cytokines is often reduced due to TLR inhibition,
incomplete antigen presentation and the inhibition of DCs and naïve
T cell maturation (32,33).
The present study demonstrated that APS enhanced
host defences during Brucella infection, and provide novel
insight into the use of APS in vaccine immunization. Zhou et
al reported that APS increases NO, TNF-α, IL-1b and IL-6
concentration through TLR4- and MyD88-dependent pathways to
modulate host immunity (34). As a
vaccine adjuvant, APS has been indicated to improve the immune
response against H5N1 avian influenza virus (AIV) and reduce the
replication of H9N2 subtype AIV in chickens (35,36).
Additionally, APS has been revealed to alleviate ochratoxin
A-induced immune stress by reducing pro-inflammatory cytokine
expression, cell apoptosis and spleen damage (37). A mechanism study indicated that APS
activates the AMPK/SIRT-1 pathway and inhibits NF-κB against immune
stress (37). Previous studies have
indicated that APS can modulate the immune response, and can
ameliorate over-reactive immune stress and boost the host immune
response during pathogen invasion, providing balance in the immune
system (34,36).
In conclusion, the current study highlights the
potential use of APS for the control of Brucella infection.
Future studies should determine whether APS can improve the
protective efficacy of the S2 vaccine and its underlying mechanisms
to further verify its therapeutic efficacy and safety when used
against virulent Brucella.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
QS performed the experiments and wrote the
manuscript. L. Zhao collected and analyzed the data. L. Zhang
designed and performed the experiments. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
The experiments were approved by the Institutional
Animal Care and Use Committee of Zhejiang Ocean University and
adhered to the code of the World Medical Association (Declaration
of Helsinki).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoon H, Moon OK, Lee SH, Lee WC, Her M,
Jeong W, Jung SC and Kim DS: Epidemiology of brucellosis among
cattle in Korea from 2001 to 2011. J Vet Sci. 15:537–543. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reyes AW, Hop HT, Arayan LT, Huy TX, Min
W, Lee HJ, Chang HH and Kim S: Nocodazole treatment interrupted
Brucella abortus invasion in RAW 264.7 cells, and successfully
attenuated splenic proliferation with enhanced inflammatory
response in mice. Microb Pathog. 103:87–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reyes AWB, Arayan LT, Hop HT, Ngoc Huy TX,
Vu SH, Min W, Lee HJ and Kim S: Effects of gallic acid on signaling
kinases in murine macrophages and immune modulation against
Brucella abortus 544 infection in mice. Microb Pathog. 119:255–259.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou H, Liu X and Peng Q: The advances in
brucellosis vaccines. Vaccine. 37:3981–3988. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu L, Feng Y, Zhang G, Jiang H, Zhang Z,
Wang N, Ding J and Suo X: Brucella suis strain 2 vaccine is
safe and protective against heterologous Brucella spp. infections.
Vaccine. 34:395–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abbassi-Daloii T, Yousefi S, Sekhavati MH
and Tahmoorespur M: Impact of heat shock protein 60KD in
combination with outer membrane proteins on immune response against
Brucella melitensis. APMIS. 126:65–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barquero-Calvo E, Chaves-Olarte E, Weiss
DS, Guzmán-Verri C, Chacón-Díaz C, Rucavado A, Moriyón I and Moreno
E: Brucella abortus uses a stealthy strategy to avoid activation of
the innate immune system during the onset of infection. PLoS One.
2:e6312007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki A and Medzhitov R: Toll-like
receptor control of the adaptive immune responses. Nat Immunol.
5:987–995. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu N, Wang L, Sun C, Yang L, Tang B, Sun
W and Peng Q: Macrophage activation induced by Brucella DNA
suppresses bacterial intracellular replication via enhancing NO
production. Microb Pathog. 89:177–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Snyder GA, Deredge D, Waldhuber A,
Fresquez T, Wilkins DZ, Smith PT, Durr S, Cirl C, Jiang J, Jennings
W, et al: Crystal structures of the Toll/Interleukin-1 receptor
(TIR) domains from the Brucella protein TcpB and host adaptor TIRAP
reveal mechanisms of molecular mimicry. J Biol Chem. 289:669–679.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lalsiamthara J and Lee JH: Development and
trial of vaccines against Brucella. J Vet Sci. 18:281–290. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Hanniffy S, Arce-Gorvel V,
Conde-Alvarez R, Oh S, Moriyón I, Mémet S and Gorvel JP:
Immunomodulatory properties of Brucella melitensis
lipopolysaccharide determinants on mouse dendritic cells in vitro
and in vivo. Virulence. 9:465–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo X, Zhang X, Wu X, Yang X, Han C, Wang
Z, Du Q, Zhao X, Liu SL, Tong D and Huang Y: Brucella downregulates
tumor necrosis factor-α to promote intracellular survival via Omp25
regulation of different microRNAs in porcine and murine
macrophages. Front Immunol. 8:20132018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hop HT, Reyes AWB, Huy TXN, Arayan LT, Min
W, Lee HJ, Rhee MH, Chang HH and Kim S: Activation of
NF-kB-mediated TNF-induced antimicrobial immunity is required for
the efficient brucella abortus clearance in RAW 264.7 cells. Front
Cell Infect Microbiol. 7:4372017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui B, Liu W, Wang X, Chen Y, Du Q, Zhao
X, Zhang H, Liu SL, Tong D and Huang Y: Brucella Omp25 upregulates
miR-155, miR-21-5p, and miR-23b to inhibit interleukin-12
production via modulation of programmed Death-1 signaling in human
monocyte/macrophages. Front Immunol. 8:7082017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elfaki MG, Alaidan AA and Al-Hokail AA:
Host response to Brucella infection: Review and future perspective.
J Infect Dev Ctries. 9:697–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun B, Yu S, Zhao D, Guo S, Wang X and
Zhao K: Polysaccharides as vaccine adjuvants. Vaccine.
36:5226–5234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chu X, Liu XJ, Qiu JM, Zeng XL, Bao HR and
Shu J: Effects of Astragalus and Codonopsis pilosula
polysaccharides on alveolar macrophage phagocytosis and
inflammation in chronic obstructive pulmonary disease mice exposed
to PM2.5. Environ Toxicol Pharmacol. 48:76–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Ma W, Zhang J, Song X, Sun W and
Fan Y: The immunoregulatory activities of astragalus polysaccharide
liposome on macrophages and dendritic cells. Int J Biol Macromol.
105:852–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou YC, Wu JM, Wang MY, Wu MH, Chen KY,
Yeh SL and Lin MT: Modulatory effects of astragalus polysaccharides
on T-cell polarization in mice with polymicrobial sepsis. Mediators
Inflamm. 2015:8263192015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Liu X, Liu H, Wang W, Liu X, Li X
and Wu X: Astragalus polysaccharides inhibit avian infectious
bronchitis virus infection by regulating viral replication. Microb
Pathog. 114:124–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Liu Z, Long T, Zhou L and Bao Y:
Immunomodulatory effects of herbal formula of astragalus
polysaccharide (APS) and polysaccharopeptide (PSP) in mice with
lung cancer. Int J Biol Macromol. 106:596–601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Shi Q, Zhang L and Zhao H: High
molecular weight hyaluronan attenuates fine particulate
matter-induced acute lung injury through inhibition of
ROS-ASK1-p38/JNK-mediated epithelial apoptosis. Environ Toxicol
Pharmacol. 59:190–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Snyder DT, Hedges JF and Jutila MA:
Getting ‘Inside’ type I IFNs: Type I IFNs in intracellular
bacterial infections. J Immunol Res. 2017:93618022017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Li Z, Li B, Chi H, Li J, Fan H,
Yao R, Li Q, Dong X, Chen M, et al: Bioluminescence imaging of
colonization and clearance dynamics of brucella suis vaccine
strain S2 in mice and guinea pigs. Mol Imaging Biol. 18:519–526.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei P, Lu Q, Cui G, Guan Z, Yang L, Sun C,
Sun W and Peng Q: The role of TREM-2 in internalization and
intracellular survival of Brucella abortus in murine macrophages.
Vet Immunol Immunopathol. 163:194–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watarai M, Makino S, Fujii Y, Okamoto K
and Shirahata T: Modulation of Brucella-induced macropinocytosis by
lipid rafts mediates intracellular replication. Cell Microbiol.
4:341–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed W, Zheng K and Liu ZF: Establishment
of chronic infection: Brucella's stealth strategy. Front Cell
Infect Microbiol. 6:302016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dorneles EM, Teixeira-Carvalho A, Araújo
MS, Sriranganathan N and Lage AP: Immune response triggered by
Brucella abortus following infection or vaccination. Vaccine.
33:3659–3666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang K, Wang H, Guo F, Yuan L, Zhang W,
Wang Y and Chen C: OMP31 of Brucella melitensis 16M impairs the
apoptosis of macrophages triggered by TNF-α. Exp Ther Med.
12:2783–2789. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barrionuevo P, Delpino MV, Pozner RG,
Velásquez LN, Cassataro J and Giambartolomei GH: Brucella abortus
induces intracellular retention of MHC-I molecules in human
macrophages down-modulating cytotoxic CD8(+) T cell responses. Cell
Microbiol. 15:487–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salcedo SP, Marchesini MI, Lelouard H,
Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O,
Alexopoulou L, et al: Brucella control of dendritic cell maturation
is dependent on the TIR-containing protein Btp1. PLoS Pathog.
4:e212008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Billard E, Dornand J and Gross A:
Brucella suis prevents human dendritic cell maturation and
antigen presentation through regulation of tumor necrosis factor
alpha secretion. Infect Immun. 75:4980–4989. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou
X and Bao Y: Astragalus polysaccharides exerts immunomodulatory
effects via TLR4-mediated MyD88-dependent signaling pathway in
vitro and in vivo. Sci Rep. 7:448222017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdullahi AY, Kallon S, Yu X, Zhang Y and
Li G: Vaccination with astragalus and ginseng polysaccharides
improves immune response of chickens against H5N1 Avian influenza
virus. Biomed Res Int. 2016:15102642016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kallon S, Li X, Ji J, Chen C, Xi Q, Chang
S, Xue C, Ma J, Xie Q and Zhang Y: Astragalus polysaccharide
enhances immunity and inhibits H9N2 avian influenza virus in vitro
and in vivo. J Anim Sci Biotechnol. 4:222013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu D, Su J, Lin J, Qian G, Chen X, Song S
and Huang K: Activation of AMPK-dependent SIRT-1 by astragalus
polysaccharide protects against ochratoxin A-induced immune stress
in vitro and in vivo. Int J Biol Macromol. 120:683–692. 2018.
View Article : Google Scholar : PubMed/NCBI
|